Abstract
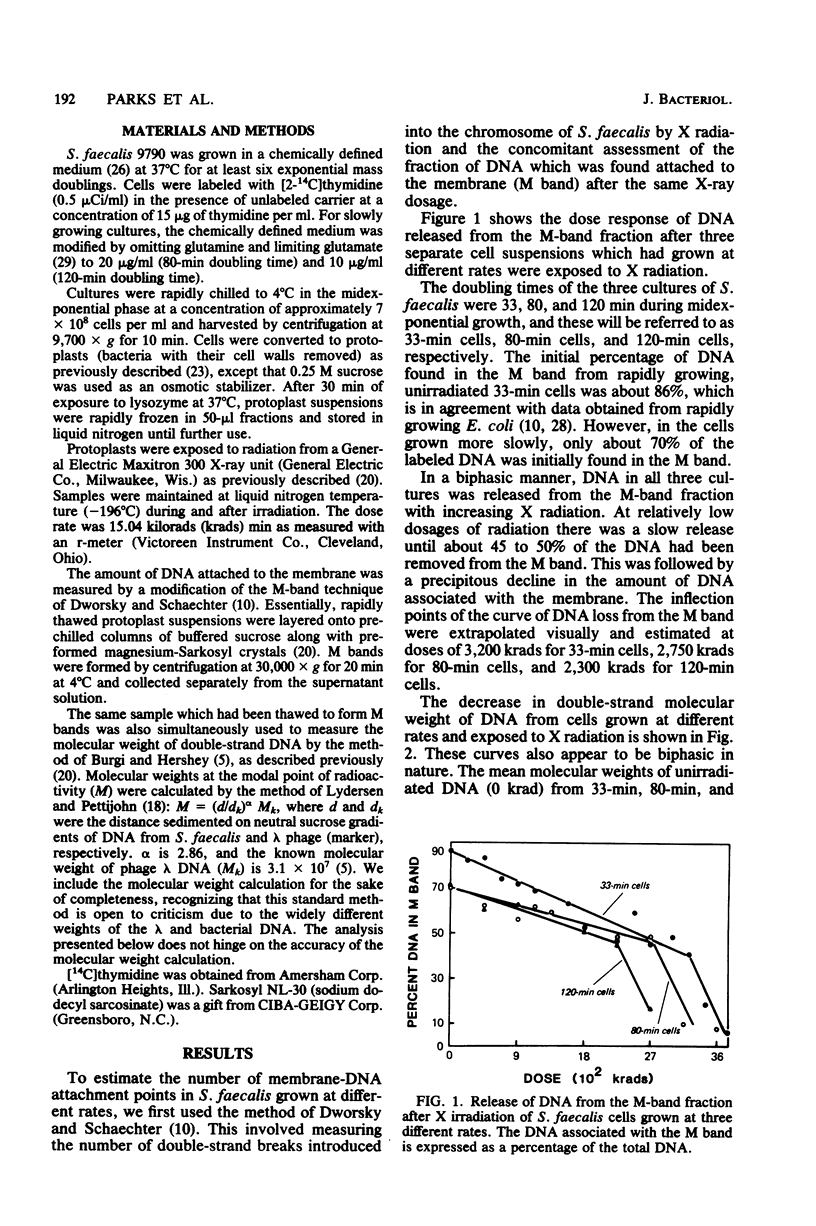
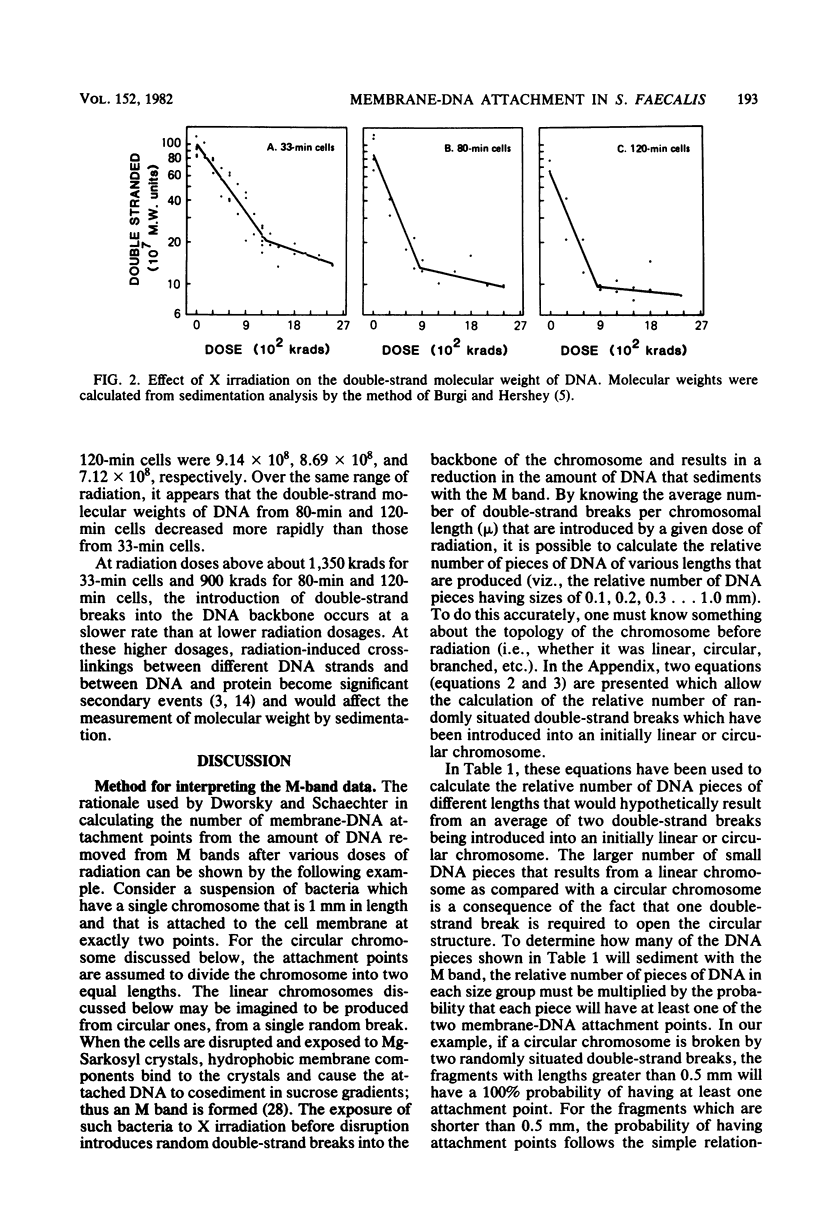
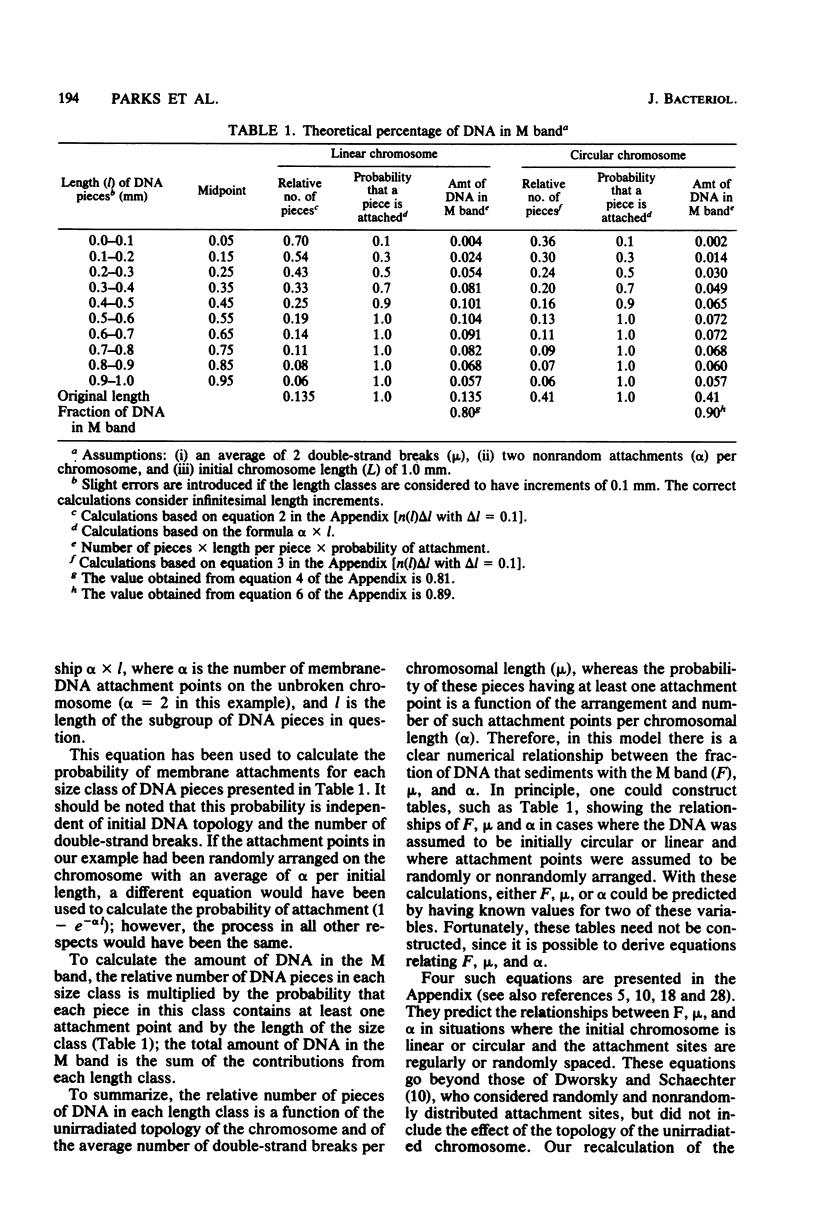
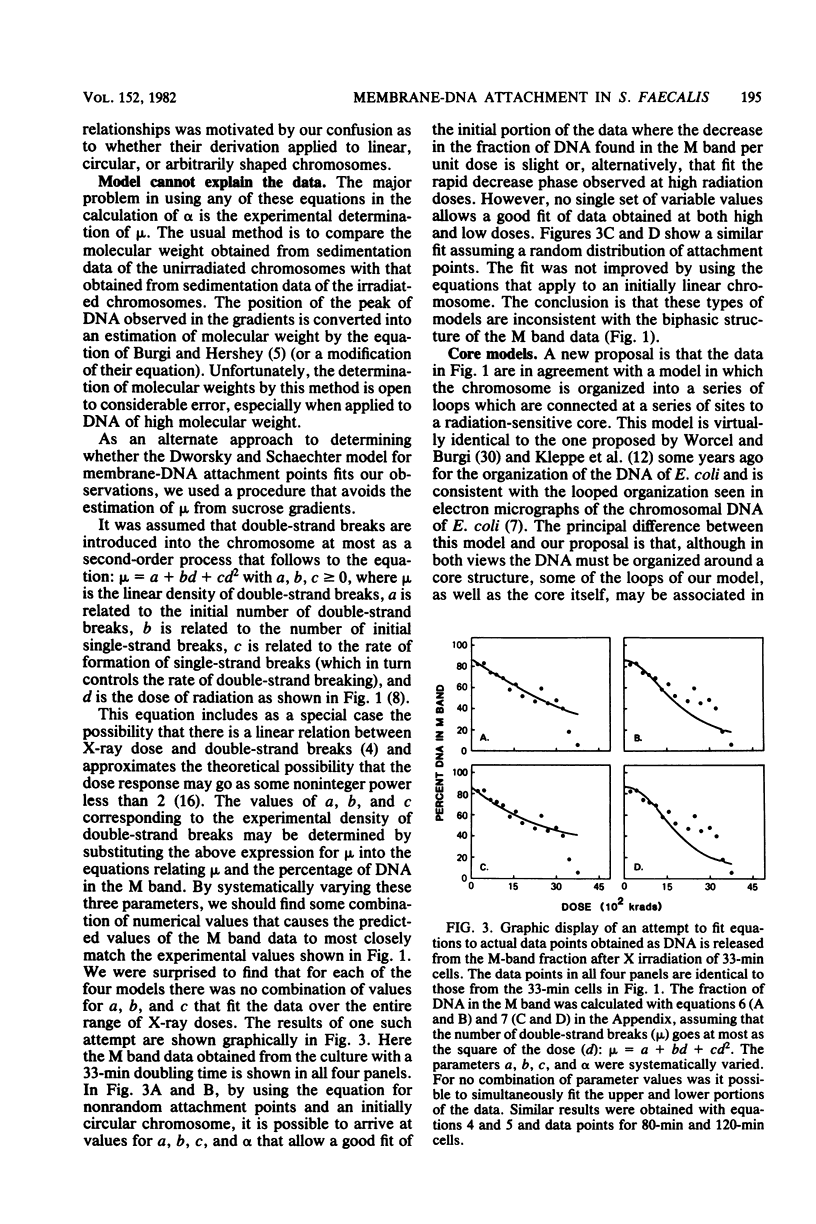
The M-band technique was used to assess the number of attachment points of DNA to the cell membrane of Streptococcus faecalis grown at three different rates. Cells were X irradiated in liquid nitrogen and then analyzed simultaneously for the introduction of double-strand breaks into the chromosome and the degree of removal of DNA from the cell membrane (M band). Consideration of the data from these experiments and of the topology of the bacterial chromosome resulted in a reevaluation of former quantitative models. Our results are consistent with a semiquantitative model in which the bacterial chromosome is organized around a core structure. We interpret our data to mean that the core is attached to the membrane and that the complexity of the core changes more drastically with growth rate than does the number of membrane-DNA attachment points. An alternative model in which RNA hybridizes with DNA containing single- and double-strand breaks is also discussed. In any event, the complexity of these interactions precludes a reliable estimate of the number of membrane-DNA attachment sites.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Brown C., Hendrickson W. G., Boyd D. H., Clifford P., Cote R. H., Schaechter M. Release of Escherichia coli DNA from membrane complexes by single-strand endonucleases. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2756–2760. doi: 10.1073/pnas.74.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achey P. M., Billen D., Beltranena H. P. Single-strand breaks in gamma-irradiated phi-X174 DNA induced by exposure to alkali. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20(5):501–504. doi: 10.1080/09553007114551411. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne L., Coquerelle T., Hagen U. Radiation sensitivity of bacteriophage DNA. II. Breaks and cross-links after irradiation in vivo. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):205–215. doi: 10.1080/09553007014550241. [DOI] [PubMed] [Google Scholar]
- Bonura T., Town C. D., Smith K. C., Kaplan H. S. The influence of oxygen on the yield of DNA double-strand breaks in x-irradiated Escherichia coli K-12. Radiat Res. 1975 Sep;63(3):567–577. [PubMed] [Google Scholar]
- Churchward G., Estiva E., Bremer H. Growth rate-dependent control of chromosome replication initiation in Escherichia coli. J Bacteriol. 1981 Mar;145(3):1232–1238. doi: 10.1128/jb.145.3.1232-1238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Streips U. N., Imada S., Fan V. S., Brown W. C. Genetic transformation with cell wall-associated deoxyribonucleic acid in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):957–966. doi: 10.1128/jb.144.3.957-966.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe K., Ovrebö S., Lossius I. The bacterial nucleoid. J Gen Microbiol. 1979 May;112(1):1–13. doi: 10.1099/00221287-112-1-1. [DOI] [PubMed] [Google Scholar]
- LETT J. T., STACEY K. A., ALEXANDER P. Crosslinking of dry deoxyribonucleic acids by electrons. Radiat Res. 1961 Apr;14:349–362. [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydersen B. K., Pettijohn D. E. Interactions stabilizing DNA tertiary structure in the Escherichia coli chromosome investigated with ionizing radiation. Chromosoma. 1977 Jul 8;62(3):199–215. doi: 10.1007/BF00286044. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hendrickson W., Balakrishnan R., Yamaki H., Boyd D., Schaechter M. Isolation of a replication origin complex from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):262–266. doi: 10.1073/pnas.77.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Dicker D. T., Conger A. D., Daneo-Moore L., Higgins M. L. Effect of chromosomal breaks induced by x-irradiation on the number of mesosomes and the cytoplasmic organization of Streptococcus faecalis. J Mol Biol. 1981 Mar 15;146(4):413–431. doi: 10.1016/0022-2836(81)90040-1. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Prokaryotic DNA in nucleoid structure. CRC Crit Rev Biochem. 1976 Nov;4(2):175–202. doi: 10.3109/10409237609105458. [DOI] [PubMed] [Google Scholar]
- Robev S., Marinova Z. X-radiation sensitivity of DNA--ability to form specific molecular hybrids with isologous mRNA. Nature. 1967 Mar 4;213(5079):935–935. doi: 10.1038/213935a0. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOENNIES G., ISZARD L., ROGERS N. B., SHOCKMAN G. D. Cell multiplication studied with an electronic particle counter. J Bacteriol. 1961 Dec;82:857–866. doi: 10.1128/jb.82.6.857-866.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]



