Abstract
Previous studies have demonstrated that when the temperature of hippocampal brain slices is increased, there is a corresponding depression of synaptic potentials mediated by an increased activation of presynaptic adenosine A1 receptors. The present experiments demonstrate that when the temperature of hippocampal slices is raised from 32.5°C to either 38.5°C or 40.0°C there is a marked, temperature-dependent increase in the efflux of endogenous adenosine and a corresponding decrease in excitatory synaptic responses. The increase in efflux is rapidly reversible on lowering the slice temperature and the temperature-induced efflux is repeatable. Control experiments suggest that this increased efflux of adenosine is not the result of hypoxia or ischemia secondary to a temperature-induced increase in the metabolic rate of the slice. The increase in adenosine efflux was not accompanied by any significant change in the ATP levels in the brain slice, whereas a hypoxic stimulus sufficient to produce a comparable depression of excitatory transmission produced an ~75% decrease in ATP levels. These experiments indicate that changes in brain slice temperature can alter purine metabolism in such a way as to increase the adenosine concentration in the extracellular space, as well as adenosine efflux from hippocampal slices, in the absence of significant changes in ATP levels.
Keywords: adenosine, adenosine receptors, hippocampus, hypoxia, hyperthermia
INTRODUCTION
A number of studies have established that metabolic stress will increase extracellular adenosine in the brain, both in vivo, and in brain slices. Increased efflux of adenosine from hippocampal slices can be induced by hypoxia, ischemia, field electrical stimulation, or application of excitatory amino acids (Ambrósio et al., 1997; Fredholm and Lloyd, 1991; Lloyd et al., 1993; Pedata et al., 1991, 1993). Because extracellular adenosine acting at A1 receptors can abolish excitatory synaptic transmission in the hippocampus (primarily by presynaptic inhibition of transmitter release; Corradetti et al., 1984; Fredholm and Dunwiddie, 1988; Wu and Saggau, 1997), the release of adenosine under hypoxic or ischemic conditions is accompanied by a profound decrease in synaptic transmission (Latini et al., 1998). This inhibition can be largely blocked by adenosine receptor antagonists (Fowler, 1989, 1990; Latini et al., 1999; Lucchi et al., 1996).
Recent studies have demonstrated that a modest increase in the temperature of rat hippocampal slices results in a large decrease in excitatory transmission, and that this effect is mediated by adenosine A1 receptors (Gabriel et al., 1998; Masino and Dunwiddie, 1999). The most likely explanation for these results is that there is an increase in the extracellular concentration of adenosine but this has not been demonstrated directly. Thus, one objective of the present experiments was to use biochemical methods to determine whether these types of manipulations increase the efflux of adenosine from hippocampal slices.
The mechanisms that regulate the extracellular concentration of adenosine in brain are not completely understood. Extracellular ATP is rapidly broken down by ecto-nucleotidases (Dunwiddie et al., 1997), so the release of ATP (or other nucleotides) might lead to local increases in adenosine. Under conditions where there is a net breakdown of intracellular ATP, the increase in 5′-AMP formed from ATP, and its subsequent dephosphorylation by 5′ nucleotidase to adenosine, are thought to lead directly to increased adenosine release from the cell. Such a mechanism could underlie adenosine release induced by exposure to ischemia-like conditions in vitro, which decreases ATP content and reduces the energy charge (Latini et al., 1995). ATP levels are also compromised during hypoxia (Lipton and Whittingham, 1982), anoxia (Yoneda and Okada, 1989), ischemia (Nabetani et al., 1997) and by high levels of electrical activity (Folbergrova et al., 1981; Marichich and Nasello, 1973), all conditions that also release adenosine. Although adenosine release may be a direct consequence of the increased intracellular formation of adenosine from the breakdown of ATP, it is also possible that other aspects of reduced ATP levels serve as a signal to release adenosine.
The mechanism underlying adenosine release induced by increased temperature is also unclear, although there is suggestive evidence that adenosine is formed intracellularly and released from cells via nucleoside transporters, rather than being generated in the extracellular space by degradation of adenine nucleotides (Masino and Dunwiddie, 1999). It is possible that increased temperature increases the metabolic rate of brain slices sufficiently that there is a net breakdown of ATP, which leads to increased adenosine release (Laptook et al., 1995). However, unlike many other manipulations that induce adenosine release (and also induce a strong depression of synaptic transmission), there is a full recovery of the synaptic response following increased temperature even when the higher temperature is maintained for prolonged periods of time (≥1 h; Masino and Dunwiddie, 1999). Nevertheless, determining whether or not temperature changes that induce substantial adenosine release also produce corresponding changes in intracellular ATP levels should provide insights into whether such a mechanism might contribute to temperature-induced adenosine release.
In the present experiments we have characterized the changes in synaptic transmission, adenosine efflux, and intracellular ATP induced by a temperature increase in hippocampal slices. The objectives of these studies were to determine whether the inhibition of synaptic transmission induced by increased temperature is paralleled by changes in adenosine efflux, and whether there are changes in ATP levels during increased temperature which account for, or are a signal for, increased extracellular adenosine during the increased temperature.
MATERIALS AND METHODS
Simultaneous measurement of adenosine efflux and synaptic transmission
Preparation of hippocampal slices
Hippocampal slices (400 μm thick) were prepared from male Wistar rats (100–150 g; Charles River, Fortage, MI) as previously described (Corradetti et al., 1984) and kept at room temperature for at least 1 h in oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) containing (in mM): NaCl 124, KCl 3.33, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 25, and D-glucose 10. Five slices were then placed on a nylon mesh, completely submerged in a small chamber, and superfused using a peristaltic pump with oxygenated aCSF at 32°C, at a constant flow rate of 1.5 ml/min.
Electrophysiological recording
The extracellular field excitatory postsynaptic potential (fEPSP) was recorded from one of the five slices in the perfusion chamber. Test pulses (80 μs, 0.06 Hz) were delivered through a bipolar nichrome electrode positioned in the stratum radiatum. Evoked extracellular potentials were recorded with glass microelectrodes (2–10 MΩ) filled with 3 M NaCl, placed in the CA1 region of the stratum radiatum. Responses were amplified (Neurolog NL 104, Digitimer Ltd), digitized (sample rate, 33.33 kHz), and stored for later analysis using pCLAMP 6 software facilities (Axon Instruments, Burlingame, CA). Stimulus–response curves were obtained by gradual increases in stimulus strength. The test stimulus pulse was then adjusted to produce an fEPSP whose slope was 40–50% of the maximum and was kept constant throughout the experiment. The fEPSP amplitude was routinely measured and expressed as the percentage of the average amplitude of the potentials measured during the 10-min preceding exposure of the hippocampal slices to the temperature increase.
Temperature manipulation
The temperature of the superfusing aCSF was measured with a thermocouple placed in the recording chamber. The temperature was regulated by a thermostatic controller (±0.5°C). After a 30-min period of baseline stabilization at 32°C, the temperature was increased to a higher temperature (38.5°C in most experiments). The higher temperature was reached in about 7 min and was maintained for 7–8 min, then decreased back to baseline (see Fig. 1C).
Fig. 1.
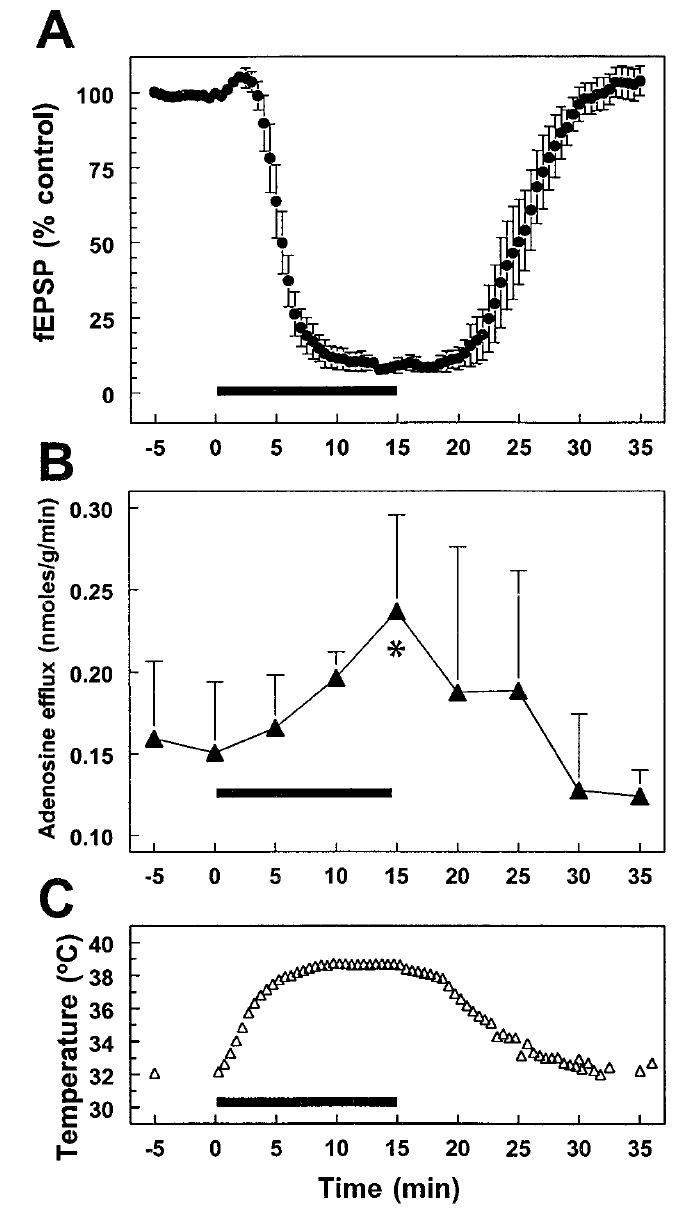
Effect of changes in brain slice temperature on synaptic potentials and adenosine efflux. The effect of an increase in temperature (slice chamber temperature indicated in C; chamber heating indicated by thick bar on abscissa in each panel) on synaptically evoked fEPSP responses in the Schaffer collateral/commissural pathway to CA1 is illustrated in A. Increasing temperature produced a rapid and completely reversible decrease in the fEPSP response. There was a concurrent increase in the concentration of adenosine measured in the effluent from the slice chamber. Net adenosine efflux averaged 0.15 ± 0.04 nmoles/g/minute during the control period and increased to a peak of 0.24 ± 0.06 nmoles/g/min after 15 min at increased temperature (*P < 0.02 compared with time zero) (B). The measurements of adenosine efflux shown in B are derived from the same brain slices tested electrophysiologically in A.
Sample collection and adenosine measurement
During the experiment, perfusate samples (7.5 ml of aCSF) were collected from the recording chamber every 5 min. In each experiment the time that the aCSF solution needed to cover the distance between the chamber and the test tube in which the sample was collected (60 sec) was controlled and taken into consideration for sample collection; in this way, samples collected corresponded temporally to the synaptic potentials recorded from the CA1 area. Perfusate samples and adenosine standards, prepared in the same volume of aCSF, were freeze-dried overnight, resuspended in 1.3 ml of methanol, and centrifuged at 1,200g for 10 min at 4°C. The supernatant was evaporated under nitrogen, resuspended in 100 μl of distilled water, and analyzed for adenosine using HPLC coupled with fluorimetric detection, according to the method previously described (Pedata et al., 1993). Adenosine outflow is expressed as nanomoles per gram of wet weight of the slices per minute of superfusion.
Measurement of tissue ATP and synaptic transmission
Preparation of hippocampal slices
Hippocampal slices (400 μm) were prepared and used for extracellular recording as previously described (Masino and Dunwiddie, 1999). The superfusion buffer was saturated with 95% O2/5% CO2 at 38°C and circulated through a closed tubing system before entering the recording chamber to superfuse the slice. To achieve the desired temperature in the recording chamber, aCSF was reheated with an in-line heater (Warner Instruments, Hamden, CT) just before entering the recording chamber and measured with a thermistor placed in the recording chamber along with the slice. After physiological recording, the ATP concentration was determined in a subset of slices divided into three groups: control (32.5°C) slices, 32.5°C slices raised to 38.5°C, and 32.5°C slices made hypoxic by superfusion with buffer equilibrated with 95% N2/5% CO2. In all slices the total recording time was 20–25 min. The recording during hypoxia was continued until the fEPSP dropped to approximately the average degree of inhibition observed during the temperature increase. An 80% inhibition of the fEPSP during hypoxia was extremely rapid—less than 4 min after switching to the oxygen-free superfusion medium and within a minute of a detectable fEPSP decrease. For all three groups each slice was carefully removed from the recording chamber with a paintbrush and immediately frozen in dry ice-cooled perchloric acid (12%). After snap freezing, each sample was defrosted on ice, homogenized by hand with a tissue homogenizer, and centrifuged (4°C, 10,000g for 10 min). The supernatant and the protein pellet were individually frozen at −80°C. Subsequently, the protein content of each slice was determined with a bicinchoninic acid protein assay kit (Sigma, St. Louis, MO). The supernatants were defrosted on ice, neutralized to pH 7.4–8.0 with 7.5 N KOH/50 mM NaH2PO4, centrifuged to remove the precipitate, and the ATP concentration in the final supernatant was assayed using the luciferin-luciferase method (Kimmich et al., 1975) (ATP assay, Calbiochem, LaJolla, CA).
Analysis
The ATP concentration was compared between the three groups of slices using a Kruskal-Wallis one-way ANOVA. Other statistical analyses included linear regression analysis and Student’s two-tailed t-tests, paired or unpaired where appropriate. All data are expressed as mean ± SEM.
RESULTS
An increase in the temperature of hippocampal slices from 32.5–38.5°C caused a decrease in synaptic transmission (to 9.0 ± 2.6% of baseline, n = 4 slices, P < 0.0001, paired t-test; Fig. 1A) as well as an increase in adenosine efflux (to 142.8 ± 12.2% of baseline, n = 4 experiments, P < 0.02, paired t-test; Fig. 1B) from the same group of slices. In these experiments the adenosine efflux was significantly correlated with temperature when the two variables were compared before, during, and after the temperature increase (r = 0.83, n = 9, P < 0.01). As expected, the fEPSP and the temperature showed a significant negative correlation (r = − 0.86, n = 9, P < 0.005). The increase in adenosine efflux appeared to lag the decrease in the fEPSP, which probably corresponds to the time required for increased extracellular adenosine concentrations in the slice to be reflected in increased efflux from the superfusion chamber. These results demonstrate that both a decrease in synaptic transmission and an increase in adenosine efflux occur during the temperature increase, and each of these responses fully recovers when the temperature is reduced (Fig. 1). Previous work has shown that the inhibition of synaptic responses induced by a temperature increase is repeatable (Masino and Dunwiddie, 1999), so a second group of slices was tested to determine whether the increased efflux of adenosine was also repeatable within the same slices. In this paradigm, the temperature was increased to 38.5°C (see Fig. 1), returned to baseline for approximately 20 min, and then increased again to 38.5°C. As with the depression of the fEPSP, the increase in adenosine efflux was also repeatable with a second temperature increase. There was no difference in the magnitude of the increase in adenosine efflux calculated at the peak of an initial vs. a subsequent temperature increase in the same set of slices (+34.3 ± 12.4% vs. +35.2 ± 3.3%, respectively, n = 3, paired t-test, Fig. 2). In an additional set of experiments the temperature was raised to 40.0°C, rather than 38.5°C. The adenosine efflux in these slices was not greater than what was observed with the lower temperature increase (+47.1 ± 9.1%, n = 4, vs. +42.8 ± 12.2%, n = 4, unpaired t-test, P = n.s.).
Fig. 2.
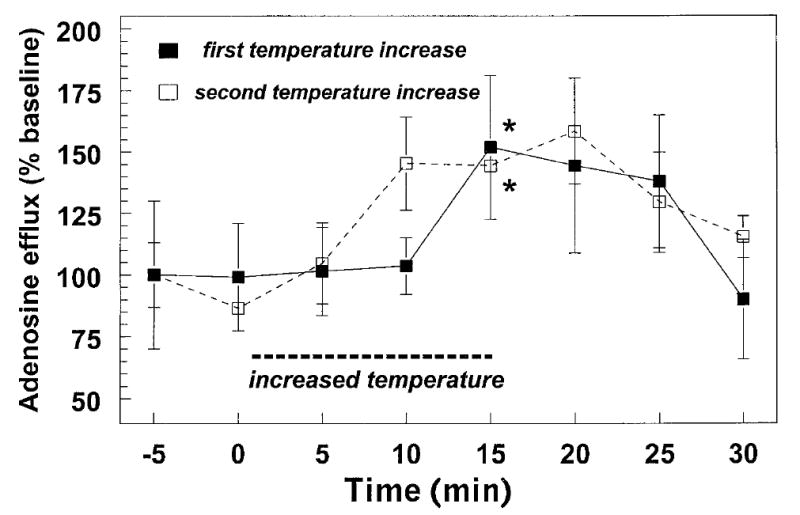
Repeatability of stimulated efflux of adenosine from hippocampal brain slices. This figure illustrates the increased efflux of endogenous adenosine induced by two successive 6°C increases in slice temperature. Efflux was measured from groups of five slices that were incubated simultaneously in the same slice chamber (three replications). The magnitude of the increase in adenosine induced by the second temperature step was not different from the increase induced by the first stimulus (*P < 0.05).
In another group of slices levels of tissue ATP were measured under control conditions, at increased temperature, or during a brief period of hypoxia. All slices included in the assay were initially incubated at 32.5°C and fEPSP recordings were made throughout the experiment from every slice. The protocols for the three experimental groups are illustrated in Figure 3. Control slices were recorded without any manipulation for 20–25 min at 32.5°C, whereas increased temperature slices were incubated at 32.5°C for 10 min, the temperature was increased to 38.5°C, and the tissue was collected 15 min after beginning the temperature increase. Hypoxic slices were incubated at 32.5°C for 15–20 min, then superfused with aCSF saturated with 95% N2/5% CO2. Hypoxic slices were collected at the time when the inhibition of the fEPSP (−72 ± 1.8%) was equivalent to the depression caused by the increased temperature (−67 ± 5.0%; P = n.s.). In every case this criterion was reached <4 min after initiating superfusion with oxygen-free aCSF. Examples of fEPSPs recorded from each group of slices are illustrated in Figure 3. ATP levels were not significantly affected by increased temperature, but were significantly reduced in hypoxic slices when compared with either increased temperature or control slices (P = 0.006, one-way ANOVA). Thus, the temperature increase did not cause a significant decrease in ATP levels, but a hypoxic stimulus sufficient to induce a comparable inhibition of synaptic transmission (approximately 70% decrease in the fEPSP) resulted in a profound decrease in hippocampal ATP levels.
Fig. 3.
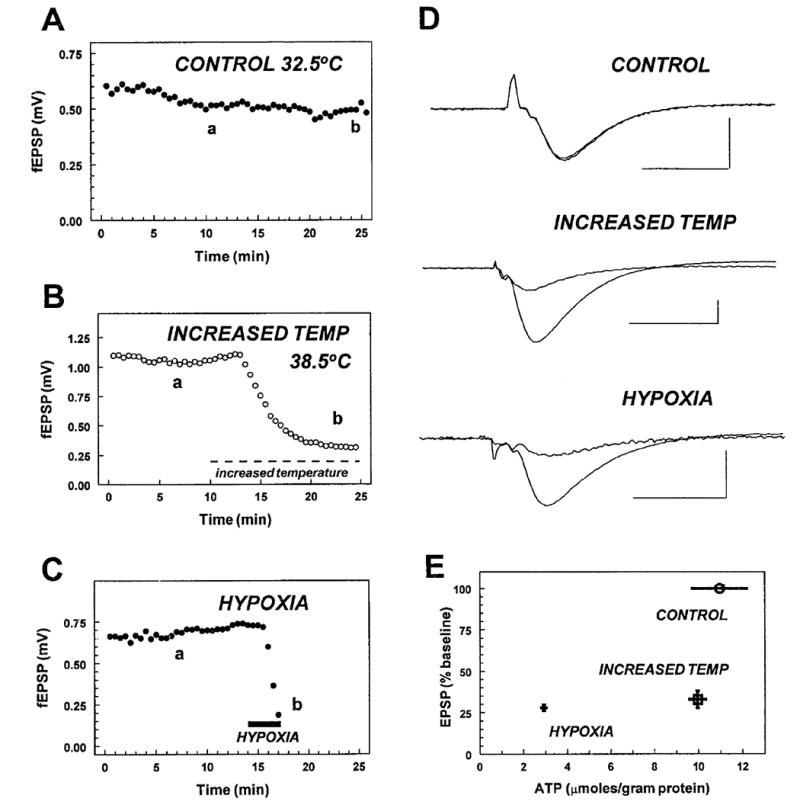
Effect of changes in brain slice temperature and hypoxia on evoked fEPSPs and ATP levels. Slices were either incubated under control conditions (32.5°C, 95% O2) throughout the experiment (A; control), at 32.5°C and then increased to 38.5°C (B; increased temp), or with 95% O2 and then switched to 95% N2 to induce hypoxia (C; hypoxia). Panels A, B, and C show the amplitude of the fEPSP response up until the time when the slices were collected for ATP measurements. D illustrates averaged fEPSP responses during baseline and at the end of the recording under each condition. In all cases (a) indicates the synaptic response sampled during baseline recording and (b) indicates the synaptic response obtained just prior to tissue collection. Scale bars = 10 ms and 0.5 mV. Each record is an average of 4–6 responses, with the exception of the synaptic response during hypoxia where a single response is shown. E illustrates relationship between the fEPSP and ATP levels during control, increased temperature, and hypoxic conditions. The temperature increase and the brief hypoxic episode caused a comparable inhibition of the fEPSP, whereas control responses were stable throughout the experiment. ATP levels measured during the increased temperature were not significantly different from control (9.95 ± 0.85 μmol/g protein, n = 9, vs. 10.9 ± 2.57 μmol/g, n = 5, in controls at 32.5°C; n.s.), but ATP levels measured after hypoxia (2.92 ± 0.84 μmol/g, n = 6) were significantly reduced when compared with both the control and the increased temperature slices (P = 0.006, one-way ANOVA).
Because there was a small (albeit clearly not significant) decrease in the ATP concentration in brain slices incubated at 38.5°C, this suggested that there may have been some decrease in ATP, which could have been responsible for the increased adenosine efflux observed. Increased metabolic rate at the higher temperature could have resulted in a small degree of hypoxia/ischemia, which might account for the increased release of adenosine and small decline in ATP. It has been argued that there is a “hypoxic core” in brain slices where the oxygen tension is reduced (Bingmann and Kolde, 1982; Jiang et al., 1991), and this might expand at increased temperature, with a corresponding increase in the release of adenosine. Therefore, several experiments were conducted to determine whether this was the case. In one set of experiments, the flow rate of the incubation buffer was increased from 2.0 to 4.0 ml/min in order to enhance the oxygen/glucose supply to the slice. However, this manipulation had no effect on the inhibition of synaptic transmission induced by an increase in slice temperature (−71 ± 5.0%; n = 3). Second, experiments were conducted on slices that were 200 μm thick instead of the normal 400 μm, which would reduce or eliminate a central hypoxic region in the slice (Fujii et al., 1982); again, there was no effect of this manipulation on the magnitude or time course of the depression of synaptic responses (−68 ± 2.1%; n = 2) observed with increased temperature. Finally, we have observed that slices that are maintained at a gas/liquid interface typically show more robust electrophysiological responses than do fully submerged slices (Masino and Dunwiddie, unpublished), and such slices have been shown to have higher oxygen tensions near the gas/slice interface as opposed to the slice/liquid interface (Bingmann and Kolde, 1982), presumably because the rate of oxygen transfer is faster from a 95% oxygen phase than from saturated buffer. Therefore, a final set of experiments were conducted in “interface slices” in which only one side of the slice was exposed to oxygenated buffer and the other was in contact with humidified, warmed 95% O2/5% CO2. This also had no effect on the time course or amplitude of synaptic depression (−72 ± 4.0%; n = 3) mediated by increased brain slice temperature.
DISCUSSION
Previous studies have demonstrated that there is an adenosine-mediated decrease in excitatory synaptic transmission that occurs on increasing the temperature of hippocampal slices (Gabriel et al., 1998; Masino and Dunwiddie, 1999), but is it unclear whether this is due to an increased sensitivity to adenosine or, perhaps more likely, to an increase in the extracellular concentration of adenosine. The present experiments demonstrate that the concentration of adenosine in the effluent from hippocampal slices is elevated during a temperature increase and returns to baseline when the temperature is reduced. Although temperature-dependent changes in receptor or effector systems cannot be completely excluded, the magnitude of the effect of temperature on agonist binding to adenosine receptors suggests that such changes are unlikely to account for the effects that we observed (Borea et al., 1996). The time course of the change in adenosine efflux corresponds roughly to the time course of the change in the synaptic potentials, although there did appear to be some differences. Most notable was the lag in the rise in adenosine efflux (Fig. 1A,B), which may reflect the time required for adenosine to diffuse out of the interior of the slice, where presumably most of the adenosine is being generated. The falling phase of the adenosine efflux corresponded much more closely to the electrophysiological response; this could reflect the fact that both diffusion out of the slice as well as adenosine uptake are acting to clear the elevated extracellular concentrations of adenosine, so this occurs more rapidly than the increase in efflux.
The mechanism underlying the increased efflux of adenosine at elevated temperatures is unclear. Previous experiments have shown that an adenosine transport inhibitor slows the rate (but ultimately enhances the magnitude) of the inhibition of the fEPSP response induced by a temperature increase (Masino and Dunwiddie, 1999), which suggests that adenosine is released from cells via purine transporters, rather than being formed extracellularly. If the latter were the case, a transport inhibitor would be expected to increase the rate of onset of the adenosine inhibition, rather than delay it. Thus, it appears that the release of adenosine induced by increased temperature results from the intracellular conversion of purine nucleotides to adenosine and subsequent transport out of the cell via facilitated diffusion transport. A somewhat puzzling aspect of this type of adenosine release is the notable lack of correspondence between the breakdown of ATP and adenosine efflux during the temperature increase and hypoxia. In a similar manner, electrical stimulation of hippocampal slices can produce an equivalent, or even a greater amount of adenosine efflux than does ischemia, but has less effect on ATP levels (Latini et al., 1995), and other studies have also suggested that decreases in ATP levels are not tightly coupled to the production of adenosine (Cox et al., 1985; Yoneda and Okada, 1989; Doolette, 1997; Nabetani et al., 1997). We did observe a nonsignificant decrease in ATP when slice temperature was raised from 32.5–38.5°C, but because intracellular ATP concentrations are so high (~3 mM), conversion of an undetectable amount of ATP to adenosine could produce very large changes in adenosine. It is also worth noting that ATP measurements reflect levels in the entire slice, and it is possible that a subpopulation of cells (e.g., glial cells) might show larger changes in ATP, or contribute disproportionately to the extracellular pool of adenosine. Moreover, electrophysiological measurements only reflect the adenosine levels at the synaptic terminals, not in other extracellular regions. Lipton and Whittingham (1982) demonstrated that during hypoxia, ATP levels in synaptic regions can change more rapidly than do whole-slice ATP concentrations and that there is a closer correspondence between ATP decreases in synaptic regions and the adenosine-mediated decrease in synaptic transmission. Thus, some of the disparities between changes in ATP and extracellular adenosine may reflect the fact that they are linked to somewhat different purine pools within the slice.
Although the mechanism underlying the temperature-induced increase in adenosine efflux is obscure, it appears highly unlikely that hypoxia induced by a temperature-related increase in metabolic rate is responsible. As noted above, a hypoxic stimulus that produced a comparable change in extracellular adenosine produced a much larger decrease in ATP than did the temperature increase, suggesting that the two stimuli were not equivalent. Furthermore, a variety of manipulations (decreased slice thickness, increased buffer flow rate, and “interface” slices) that would improve oxygenation of the slice had no effect on the temperature-induced decrease in the response. Finally, although the present experiments characterized the changes in adenosine induced by 6–8°C changes in temperature, a 2°C change in temperature is sufficient to induce a statistically significant change in adenosine inhibition (Masino and Dunwiddie, 1999); it seems highly unlikely that this small a change would be sufficient to induce a significant change in the energy status of the slice preparation.
In conclusion, these studies demonstrate that large increases in extracellular adenosine can be induced by an increase in brain temperature that, unlike hypoxia, has no significant effect on the levels of tissue ATP. These experiments provide further evidence that large changes in extracellular adenosine can occur with minimal changes in ATP concentrations. In addition, this type of temperature manipulation might be an effective tool for investigating the cellular mechanisms that regulate extracellular adenosine concentrations in brain, because the temperature changes are not accompanied by the gross disruption of cellular metabolism that is associated with stimuli such as hypoxia or ischemia.
Acknowledgments
Contract grant number: RO1 NS 29173; Contract grant sponsors: the Veterans Administration Medical Research Service, MURST ex 40% 1999.
References
- Ambrósio AF, Malva JO, Carvalho AP, Carvalho CM. Inhibition of N-, P/Q- and other types of Ca2+ channels in rat hippocampal nerve terminals by the adenosine A1 receptor. Eur J Pharmacol. 1997;340:301–310. doi: 10.1016/s0014-2999(97)01451-9. [DOI] [PubMed] [Google Scholar]
- Bingmann D, Kolde G. PO2-profiles in hippocampal slices of the guinea pig. Exp Brain Res. 1982;48:89–96. doi: 10.1007/BF00239575. [DOI] [PubMed] [Google Scholar]
- Borea PA, Dalpiaz A, Varani K, Gessi S, Gilli G. Binding thermodynamics at A1 and A2A adenosine receptors. Life Sci. 1996;59:1373–1388. doi: 10.1016/0024-3205(96)00311-6. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Lo CG, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol. 1984;104:19–26. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- Cox DW, Drower J, Bachelard HS. Effects of metabolic inhibitors on evoked activity and the energy state of hippocampal slices superfused in vitro. Exp Brain Res. 1985;57:464–470. doi: 10.1007/BF00237833. [DOI] [PubMed] [Google Scholar]
- Doolette DJ. Mechanism of adenosine accumulation in the hippocampal slice during energy deprivation. Neurochem Int. 1997;30:211–223. doi: 10.1016/s0197-0186(96)00055-1. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao LH, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrova J, Ingvar M, Siesjo BK. Metabolic changes in cerebral cortex, hippocampus, and cerebellum during sustained bicuculline-induced seizures. J Neurochem. 1981;37:1228–1238. doi: 10.1111/j.1471-4159.1981.tb04673.x. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Adenosine antagonists delay hypoxia-induced depression of neuronal activity in hippocampal brain slice. Brain Res. 1989;490:378–384. doi: 10.1016/0006-8993(89)90258-8. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Adenosine antagonists alter the synaptic response to in vitro ischemia in the rat hippocampus. Brain Res. 1990;509:331–334. doi: 10.1016/0006-8993(90)90560-x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lloyd HG. Sources of adenosine released from hippocampal slices following electrical and hypoxic/hypoglycemic stimulation. Ann NY Acad Sci. 1991;603:497–499. [Google Scholar]
- Fujii T, Baumgartl H, Lubbers DW. Limiting section thickness of guinea pig olfactory cortical slices studied from tissue pO2 values and electrical activities. Pflugers Arch Eur J Physiol. 1982;393:83–87. doi: 10.1007/BF00582396. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Klussmann FW, Igelmund P. Rapid temperature changes induce adenosine-mediated depression of synaptic transmission in hippocampal slices from rats (non-hibernators) but not in slices from golden hamsters (hibernators) Neuroscience. 1998;86:67–77. doi: 10.1016/s0306-4522(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Jiang C, Agulian S, Haddad GG. O2 tension in adult and neonatal brain slices under several experimental conditions. Brain Res. 1991;568:159–164. doi: 10.1016/0006-8993(91)91392-e. [DOI] [PubMed] [Google Scholar]
- Kimmich GA, Randles J, Brand JS. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975;69:187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Corbett RJ, Sterett R, Garcia D, Tollefsbol G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res. 1995;38:919–925. doi: 10.1203/00006450-199512000-00015. [DOI] [PubMed] [Google Scholar]
- Latini S, Corsi C, Pedata F, Pepeu G. The source of brain adenosine outflow during ischemia and electrical stimulation. Neurochem Int. 1995;27:239–244. doi: 10.1016/0197-0186(95)00042-7. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Temporal correlation between adenosine outflow and synaptic potential inhibition in rat hippocampal slices during ischemia-like conditions. Brain Res. 1998;794:325–328. doi: 10.1016/s0006-8993(98)00304-7. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, Corradetti R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P, Whittingham TS. Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol (Lond) 1982;325:51–65. doi: 10.1113/jphysiol.1982.sp014135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd HGE, Lindström K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Lucchi R, Latini S, de Mendonca A, Sebastiao AM, Ribeiro JA. Adenosine by activating A1 receptors prevents GABAA-mediated actions during hypoxia in the rat hippocampus. Brain Res. 1996;732:261–266. doi: 10.1016/0006-8993(96)00748-2. [DOI] [PubMed] [Google Scholar]
- Marichich ES, Nasello AG. Epilepsy and adenosinetriphosphate (ATP): effect of electrical stimulation and high potassium perfusion on hippocampal ATP contents. Brain Res. 1973;57:409–416. doi: 10.1016/0006-8993(73)90146-7. [DOI] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabetani M, Okada Y, Takata T, Takada S, Nakamura H. Neural activity and intracellular Ca2+ mobilization in the CA1 area of hippocampal slices from immature and mature rats during ischemia or glucose deprivation. Brain Res. 1997;769:158–162. doi: 10.1016/s0006-8993(97)00819-6. [DOI] [PubMed] [Google Scholar]
- Pedata F, Pazzagli M, Pepeu G. Endogenous adenosine release from hippocampal slices: excitatory amino acid agonists stimulate release, antagonists reduce the electrically-evoked release. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:538–543. doi: 10.1007/BF00170649. [DOI] [PubMed] [Google Scholar]
- Pedata F, Latini S, Pugliese AM, Pepeu G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Okada Y. Effects of anoxia and recovery on the neurotransmission and level of high-energy phosphates in thin hippocampal slices from the guinea-pig. Neuroscience. 1989;28:401–407. doi: 10.1016/0306-4522(89)90187-5. [DOI] [PubMed] [Google Scholar]


