Abstract
Phospholipase C (heat-labile hemolysin) was purified from Pseudomonas aeruginosa culture supernatants to near homogeneity by ammonium sulfate precipitation followed by a novel application of DEAE-Sephacel chromatography. Enzymatic activity remained associated with DEAE-Sephacel even in the presence of 1 M NaCl, but was eluted with a linear gradient of 0 to 5% tetradecyltrimethylammonium bromide. Elution from DEAE-Sephacel was also obtained with 2% lysophosphatidylcholine, and to a lesser extent with 2% phosphorylcholine, but not at all with choline. The enzyme was highly active toward phospholipids possessing substituted ammonium groups (e.g., phosphatidycholine, lysophosphatidylcholine, and sphingomyelin); however, it had little if any activity toward phospholipids lacking substituted ammonium groups (e.g., phosphatidylethanolamine, phosphatidylserine, and phosphaditylglycerol). Collectively, these data suggest that phospholipase C from P. aeruginosa exhibits high affinity for substituted ammonium groups, but requires an additional hydrophobic moiety for optimum binding. The specific activity of the purified enzyme preparation increased 1,900-fold compared with that of culture supernatants. The molecular weight of the phospholipase C was estimated to be 78,000 by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Sephacryl S-200 column chromatography and was 76,000 by high-performance size exclusion chromatography. The isoelectric point was 5.5. Amino acid analysis showed that phospholipase C was rich in glycine, serine, threonine, aspartyl, glutamyl, and aromatic amino acids, but was cystine free.
Full text
PDF

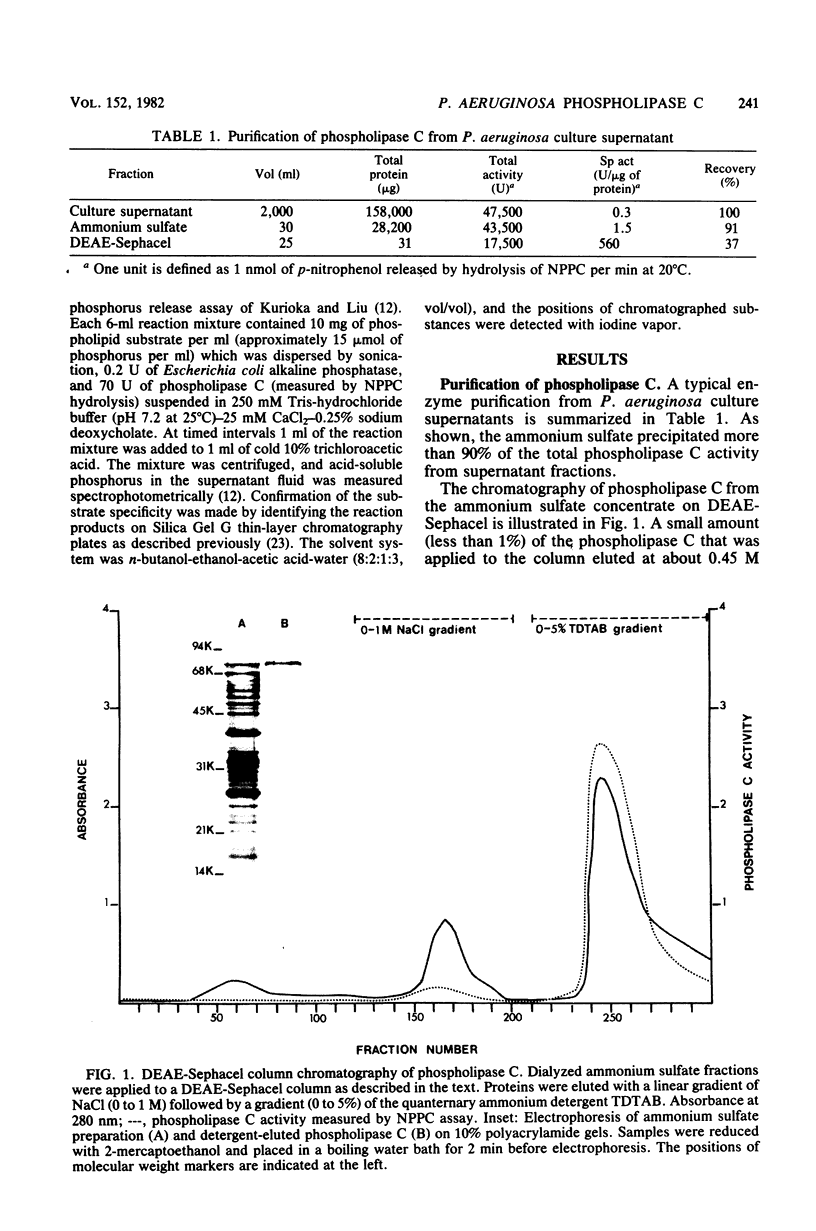
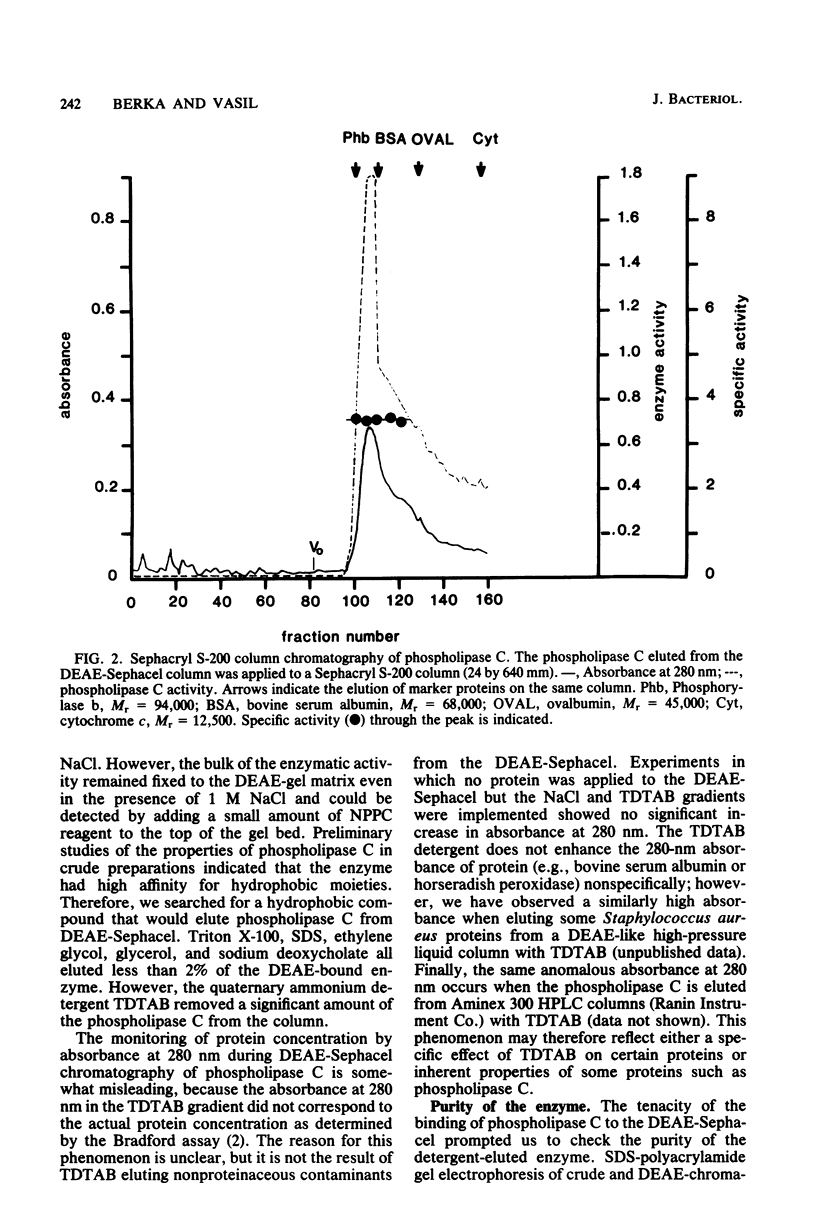
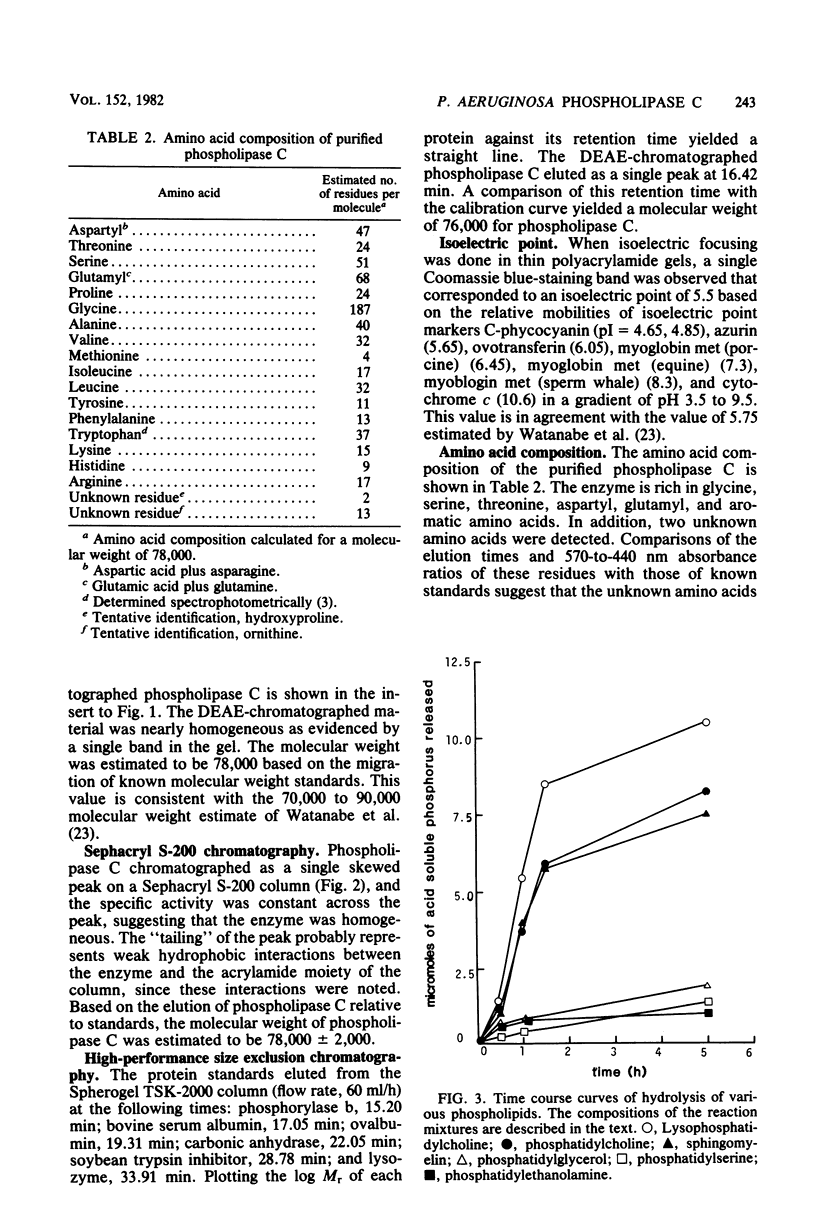


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- ESSELMANN M. T., LIU P. V. Lecithinase production by gramnegative bacteria. J Bacteriol. 1961 Jun;81:939–945. doi: 10.1128/jb.81.6.939-945.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellims P. H., Kao A. Y., Chabner B. A. Deoxycytidylate deaminase. Purification and some properties of the enzyme isolated from human spleen. J Biol Chem. 1981 Jun 25;256(12):6335–6340. [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. A Pseudomonas aeruginosa mutant non-derepressible for orthophosphate-regulated proteins. J Bacteriol. 1981 Aug;147(2):675–678. doi: 10.1128/jb.147.2.675-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. Phospholipase C regulatory mutation of Pseudomonas aeruginosa that results in constitutive synthesis of several phosphate-repressible proteins. J Bacteriol. 1982 Jun;150(3):1221–1226. doi: 10.1128/jb.150.3.1221-1226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Vasil M. L. Isolation and genetic characterization of toxin-deficient mutants of Pseudomonas aeruginosa PAO. J Bacteriol. 1981 Aug;147(2):275–281. doi: 10.1128/jb.147.2.275-281.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Vasil M. L. Mapping of a gene controlling the production of phospholipase C and alkaline phosphatase in Pseudomonas aeruginosa. Mol Gen Genet. 1981;183(2):403–405. doi: 10.1007/BF00270648. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol. 1967 Feb;93(2):670–674. doi: 10.1128/jb.93.2.670-674.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Improved assay method for phospholipase C. Appl Microbiol. 1967 May;15(3):551–555. doi: 10.1128/am.15.3.551-555.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Matsuda M. Phospholipase C assay using p-nitrophenylphosphoryl-choline together with sorbitol and its application to studying the metal and detergent requirement of the enzyme. Anal Biochem. 1976 Sep;75(1):281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- LIU P. V. FACTORS THAT INFLUENCE TOXIGENICITY OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Nov;88:1421–1427. doi: 10.1128/jb.88.5.1421-1427.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercenier A., Stalon V., Simon J. P., Haas D. Mapping of the arginine deiminase gene in Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):787–788. doi: 10.1128/jb.149.2.787-788.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Hayden C. Secretion of phospholipase C by Pseudomonas aeruginosa. Infect Immun. 1979 Aug;25(2):558–564. doi: 10.1128/iai.25.2.558-564.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Murata R., Homma J. Y. Partial purification of heat-labile hemolysin from Pseudomonas aeruginosa. Jpn J Exp Med. 1978 Oct;48(5):449–453. [PubMed] [Google Scholar]