Abstract
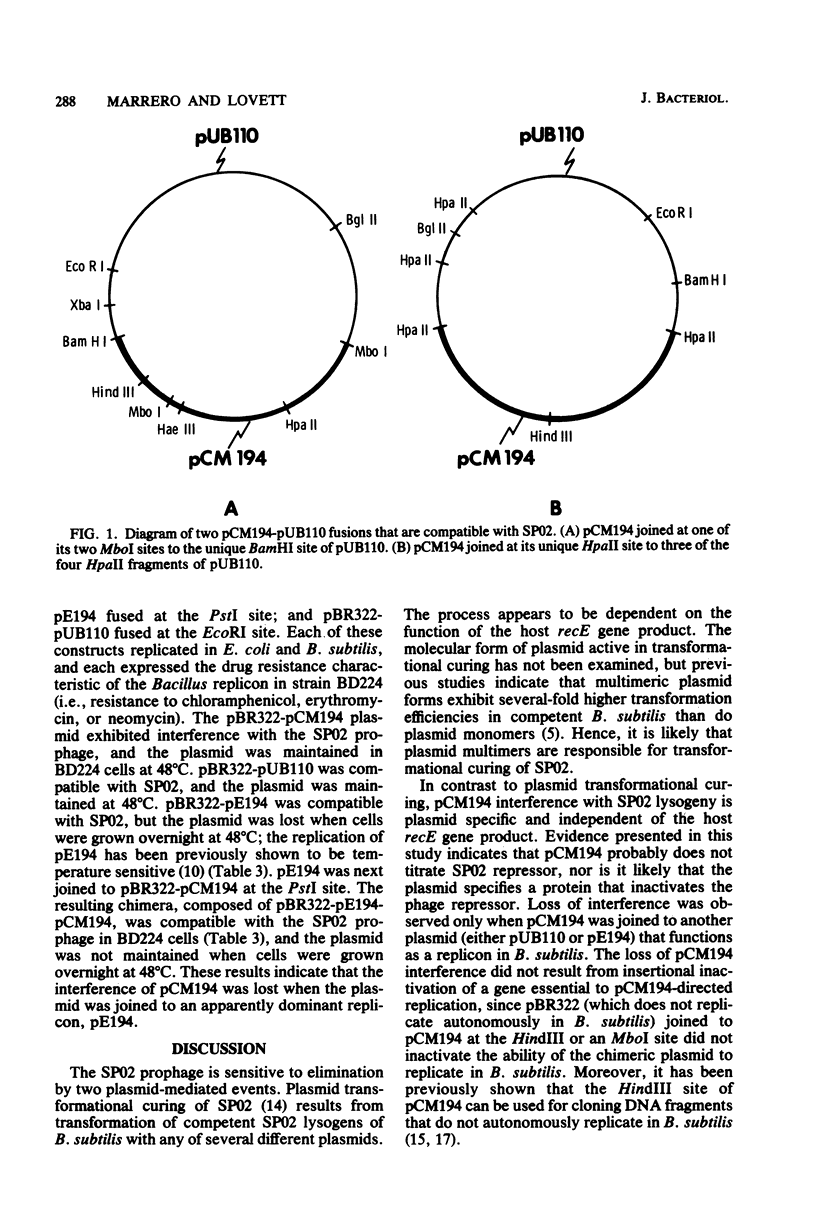
Three observations indicated that the 2-megadalton chloramphenicol resistance plasmid pCM194 interferes with SP02 lysogeny of Bacillus subtilis. SP02 plaques formed on B. subtilis(pCM194) appeared almost clear, whereas plaques produced on plasmid-free or pUB110-containing cells contained large turbid centers. The number of phages spontaneously liberated by B. subtilis(SP02) was increased 10-fold or more when pCM194 was also present in the lysogens. Lastly, growth of B. subtilis(SP02, pCM194) for approximately 20 to 25 generations resulted in essentially complete loss of the prophage. This interference was not observed with pUB110 or pE194, and the pCM194 interference was not directed against B. subtilis temperate phage phi 105, which is unrelated to SP02. Lytic replication of SP02 appeared to be unaffected by pCM194. pCM194 interference with SP02 lysogeny was demonstrable in recombination-proficient strains and a recE mutant of B. subtilis. SP02 prophage which were noninducible due to the phage ind mutation were resistant to pCM194 interference. pCM194 interference was lost when the entire pCM194 molecule was joined at its unique HpaII site or at one of the two MboI sites to pUB110 or pUB110 derivatives. pBR322 joined to pCM194 at the same MboI site or at the HindIII site produced chimeras that retained the ability to interfere with SP02 lysogeny. A three-part plasmid constructed by joining pBR322 to pCM194 (at HindIII sites) and to pE194 (at PstI sites) was compatible with the SP02 prophage and showed a temperature-sensitive replication phenotype characteristic of the pE194 replicon. One explanation for the interference involves competition for a host component between an SP02 genome attempting to establish lysogeny and plasmids whose replication is directed by the pCM194 replicon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci M. G., Lovett P. S. Temperate bacteriophage infectious for asporogenic variants of Bacillus pumilus. J Virol. 1974 Nov;14(5):1281–1287. doi: 10.1128/jvi.14.5.1281-1287.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Garro A. J., Law M. F. Relationship between lysogeny, spontaneous induction, and transformation efficiencies in Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1256–1259. doi: 10.1128/jb.120.3.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Shivakumar A. G., Dubnau D. Characterization of chimeric plasmid cloning vehicles in Bacillus subtilis. J Bacteriol. 1980 Jan;141(1):246–253. doi: 10.1128/jb.141.1.246-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Duvall E. J., Keggins K. M. Bacillus pumilus plasmid pPL10: properties and insertion into Bacillus subtilis 168 by transformation. J Bacteriol. 1976 Aug;127(2):817–828. doi: 10.1128/jb.127.2.817-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Keggins K. M. Bacillus subtilis as a host for molecular cloning. Methods Enzymol. 1979;68:342–357. doi: 10.1016/0076-6879(79)68025-4. [DOI] [PubMed] [Google Scholar]
- Marrero R., Chiafari F. A., Lovett P. S. High-frequency elimination of SP02 prophage from Bacillus subtilis by plasmid transformation. J Virol. 1981 Jul;39(1):318–320. doi: 10.1128/jvi.39.1.318-320.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Chiafari F. A., Lovett P. S. SP02 particles mediating transduction of a plasmid containing SP02 cohesive ends. J Bacteriol. 1981 Jul;147(1):1–8. doi: 10.1128/jb.147.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Lovett P. S. Transductional selection of cloned bacteriophage phi 105 and SP02 deoxyribonucleic acids in Bacillus subtilis. J Bacteriol. 1980 Aug;143(2):879–886. doi: 10.1128/jb.143.2.879-886.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Rådén B., Flock J. I. Cloning and expression of bacteriophage SP02 DNAZ polymerase gene L in Bacillus subtilis, using the Staphylococcus aureus plasmid pC194. J Virol. 1981 Aug;39(2):407–412. doi: 10.1128/jvi.39.2.407-412.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schoner R. G., Duvall E. J., Preis L. H., Lovett P. S. Expression of Escherichia coli trp genes and the mouse dihydrofolate reductase gene cloned in Bacillus subtilis. Gene. 1981 Dec;16(1-3):199–206. doi: 10.1016/0378-1119(81)90076-7. [DOI] [PubMed] [Google Scholar]
- Yasunaka K., Tsukamoto H., Okubo S., Horiuchi T. Isolation and properties of suppressor-sensitive mutants of Bacillus subtilis bacteriophage SP02. J Virol. 1970 Jun;5(6):819–821. doi: 10.1128/jvi.5.6.819-821.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


