Abstract
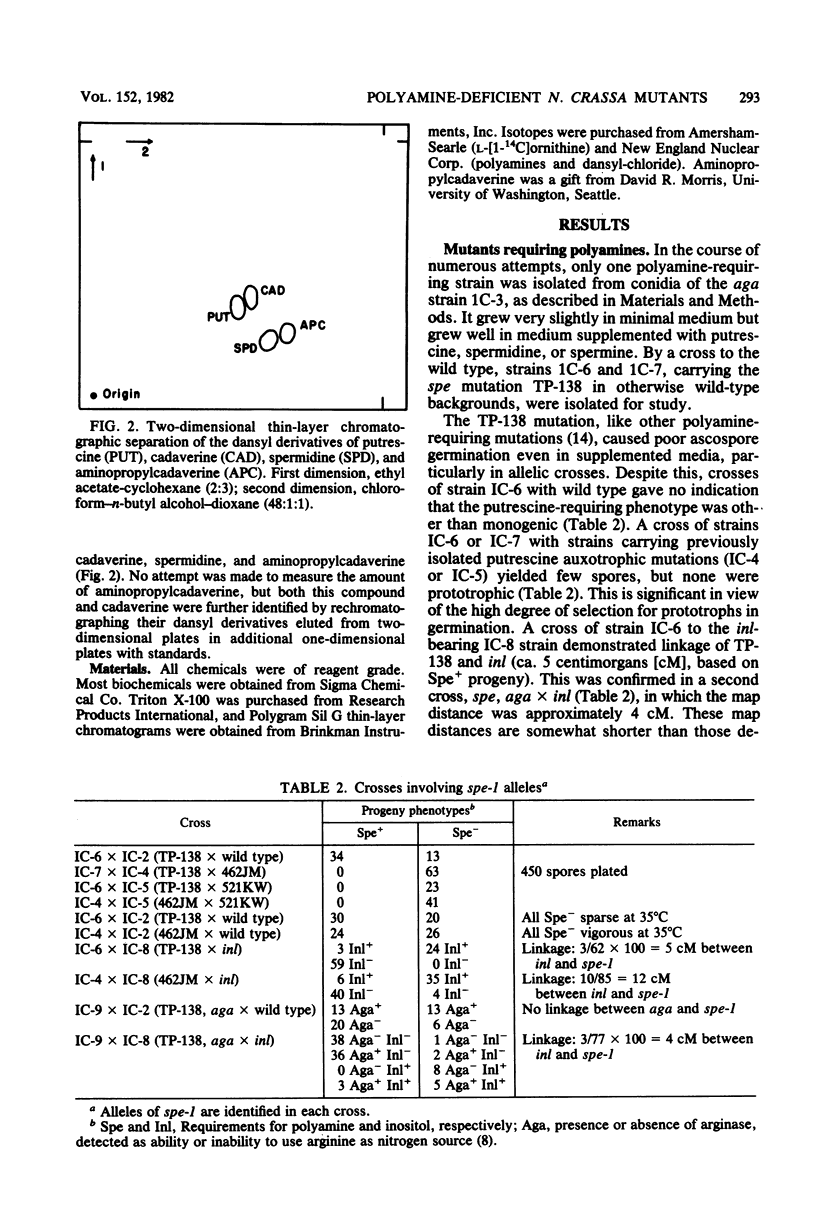
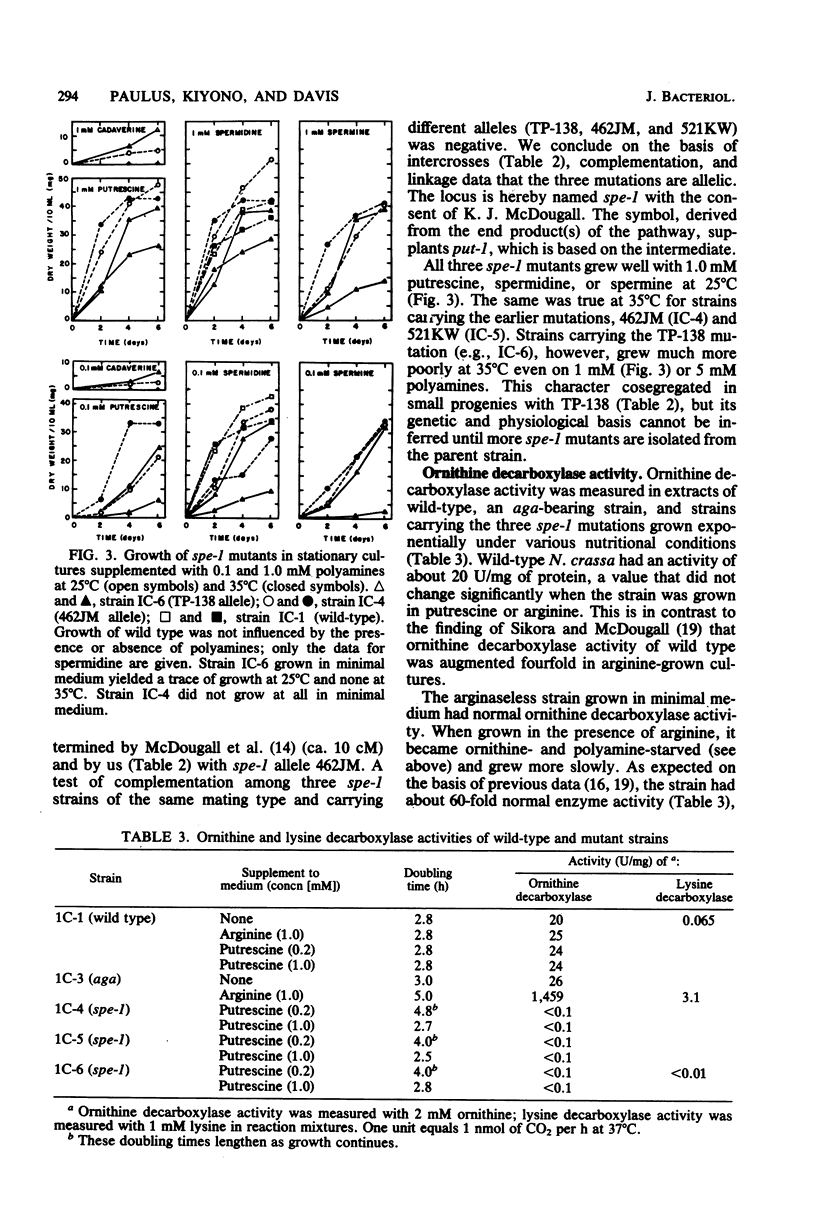
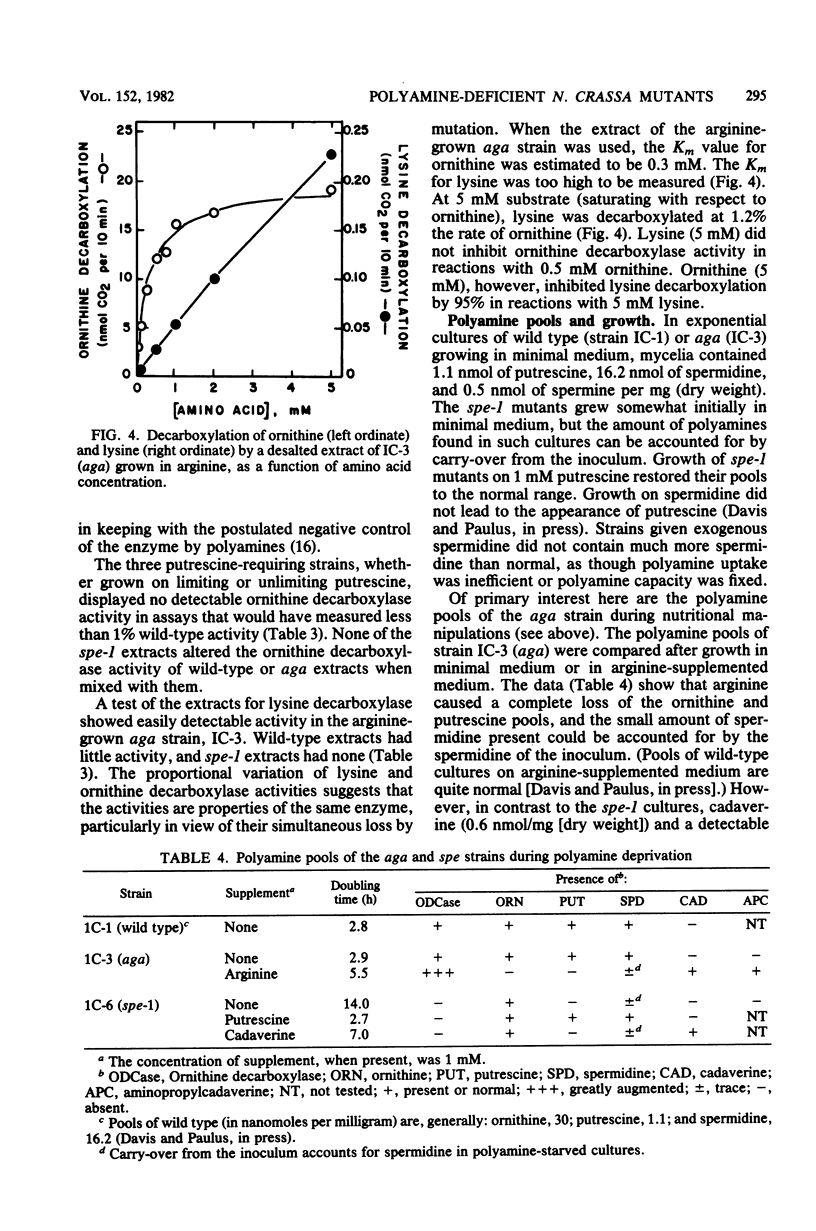
The polyamine path of Neurospora crassa originates with the decarboxylation of ornithine to form putrescine (1,4-diaminobutane). Putrescine acquires one or two aminopropyl groups to form spermidine or spermine, respectively. We isolated an ornithine decarboxylase-deficient mutant and showed the mutation to be allelic with two previously isolated polyamine-requiring mutants. We here name the locus spe-1. The three spe-1 mutants form little or no polyamines and grow well on medium supplemented with putrescine, spermidine, or spermine. Cadaverine (1,5-diaminopentane), a putrescine analog, supports very slow growth of spe-1 mutants. An arginase-deficient mutant (aga) can be deprived of ornithine by growth in the presence of arginine, because arginine feedback inhibits ornithine synthesis. Like spe-1 cultures, the ornithine-deprived aga culture failed to make the normal polyamines. However, unlike spe-1 cultures, it had highly derepressed ornithine decarboxylase activity and contained cadaverine and aminopropylcadaverine (a spermidine analog), especially when lysine was added to cells. Moreover, the ornithine-deprived aga culture was capable of indefinite growth. It is likely that the continued growth is due to the presence of cadaverine and its derivatives and that ornithine decarboxylase is responsible for cadaverine synthesis from lysine. In keeping with this, an inefficient lysine decarboxylase activity (Km greater than 20 mM) was detectable in N. crassa. It varied in constant ratio with ornithine decarboxylase activity and was wholly absent in the spe-1 mutants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L., Jänne J. Polyamine depletion induces enhanced synthesis and accumulation of cadaverine in cultured Ehrlich ascites carcinoma cells. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1005–1013. doi: 10.1016/0006-291x(80)90589-6. [DOI] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Veijalainen P., Ek-Kommonen C., Jänne J. Polyamines in mycoplasmas and in mycoplasma-infected tumour cells. Biochem J. 1982 Jan 15;202(1):267–270. doi: 10.1042/bj2020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catcheside D. G. Fungal genetics. Annu Rev Genet. 1974;8:279–300. doi: 10.1146/annurev.ge.08.120174.001431. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Regulatory mutations affecting ornithine decarboxylase activity in Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):791–799. doi: 10.1128/jb.142.3.791-799.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Stimulation of deoxyribonucleic acid replication fork movement by spermidine analogs in polyamine-deficient Escherichia coli. J Bacteriol. 1980 Mar;141(3):1192–1198. doi: 10.1128/jb.141.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- McDougall K. J., Deters J., Miskimen J. Isolation of putrescine-requiring mutants of Neurospora crassa. Antonie Van Leeuwenhoek. 1977;43(2):143–151. doi: 10.1007/BF00395669. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Sojka W. J. Anionic and cationic components of the K99 surface antigen from Escherichia coli B41. J Gen Microbiol. 1978 Jul;107(1):173–175. doi: 10.1099/00221287-107-1-173. [DOI] [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Metabolic sequestration of putrescine in Neurospora crassa. Biochem Biophys Res Commun. 1982 Jan 15;104(1):228–233. doi: 10.1016/0006-291x(82)91963-5. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



