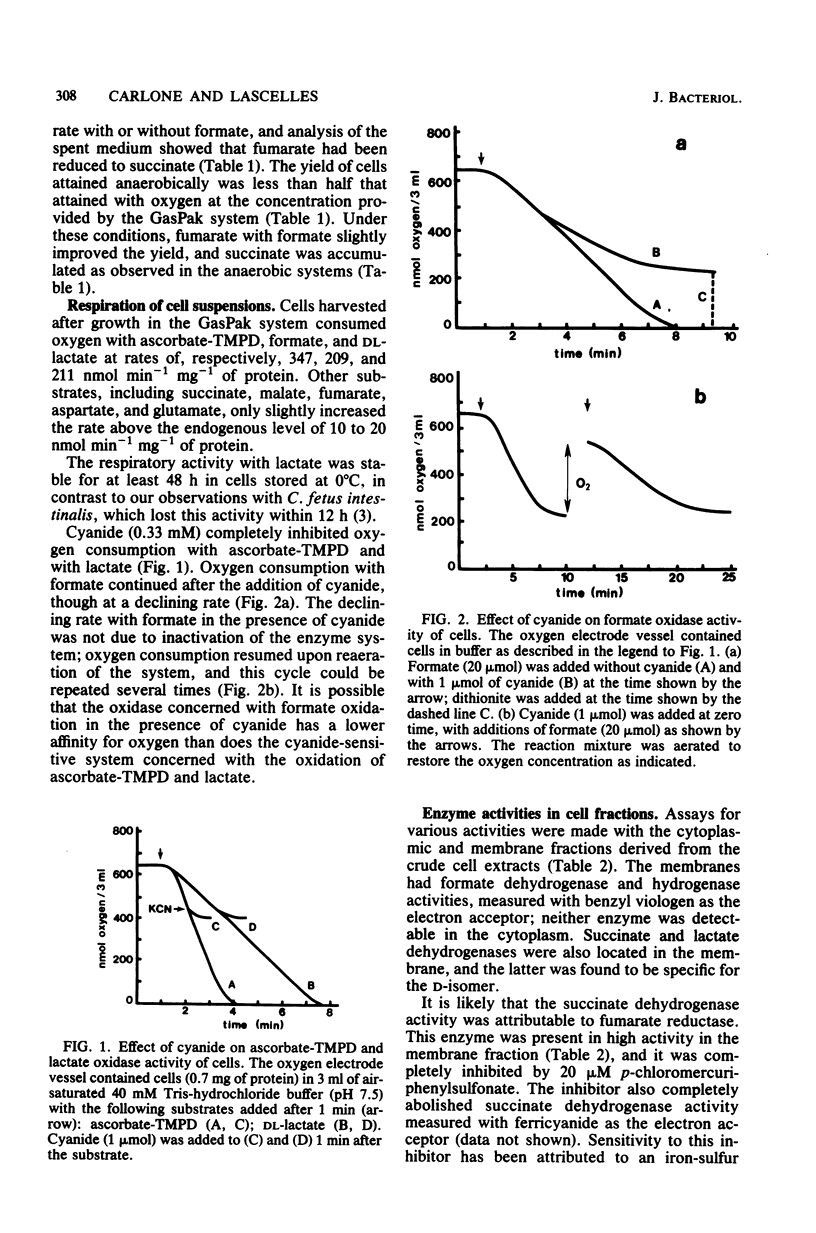
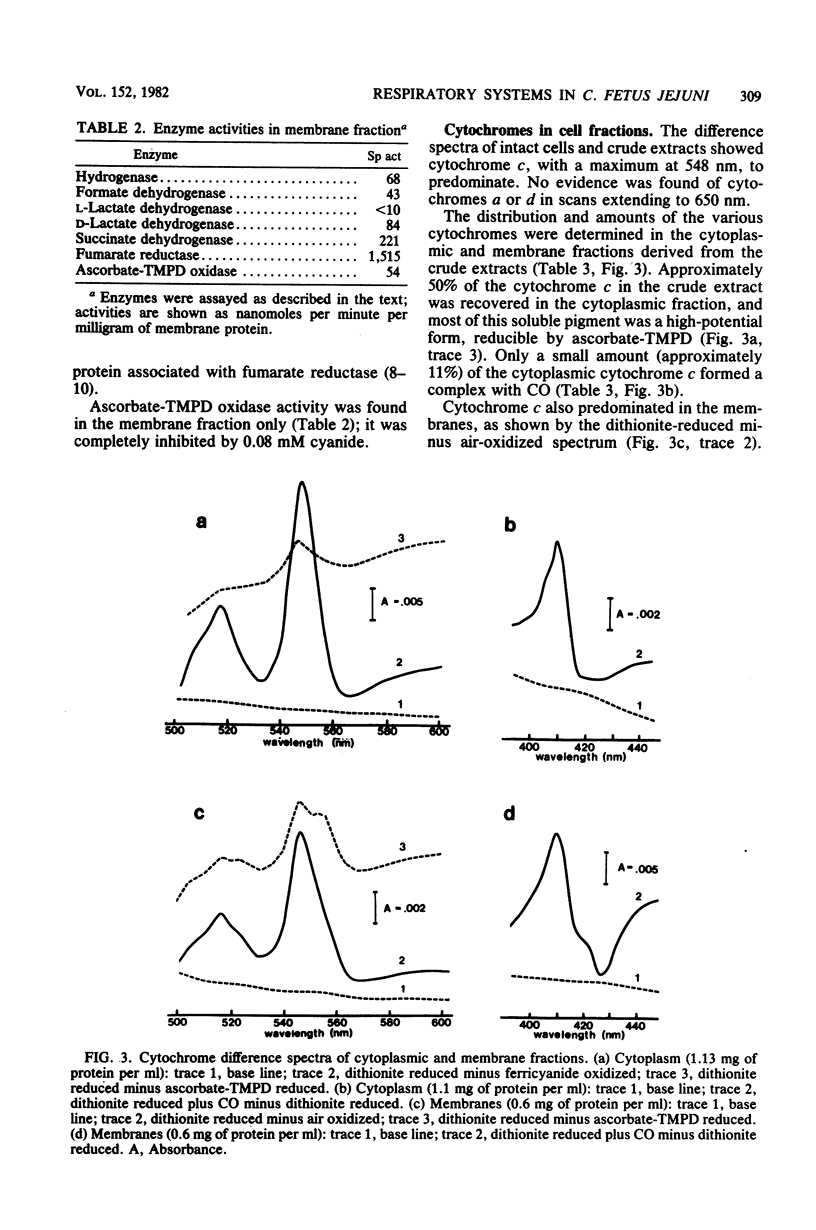
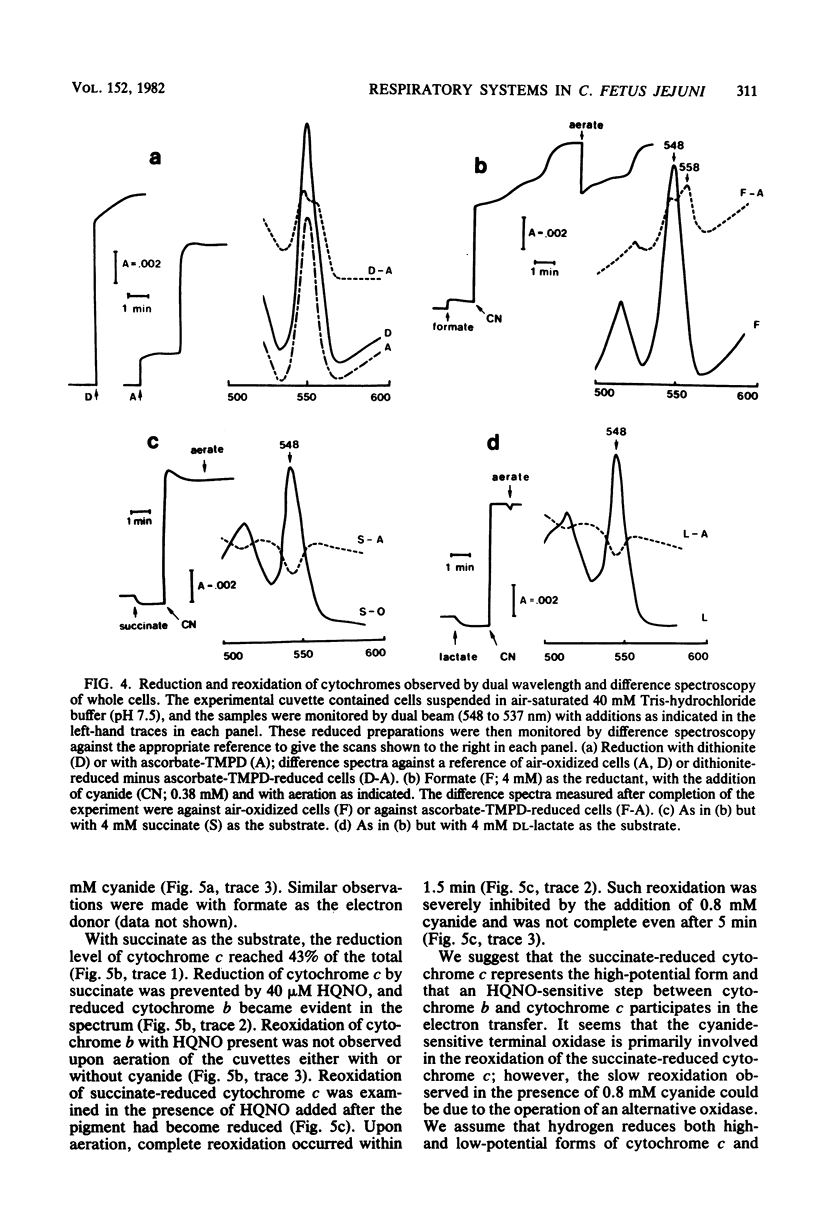
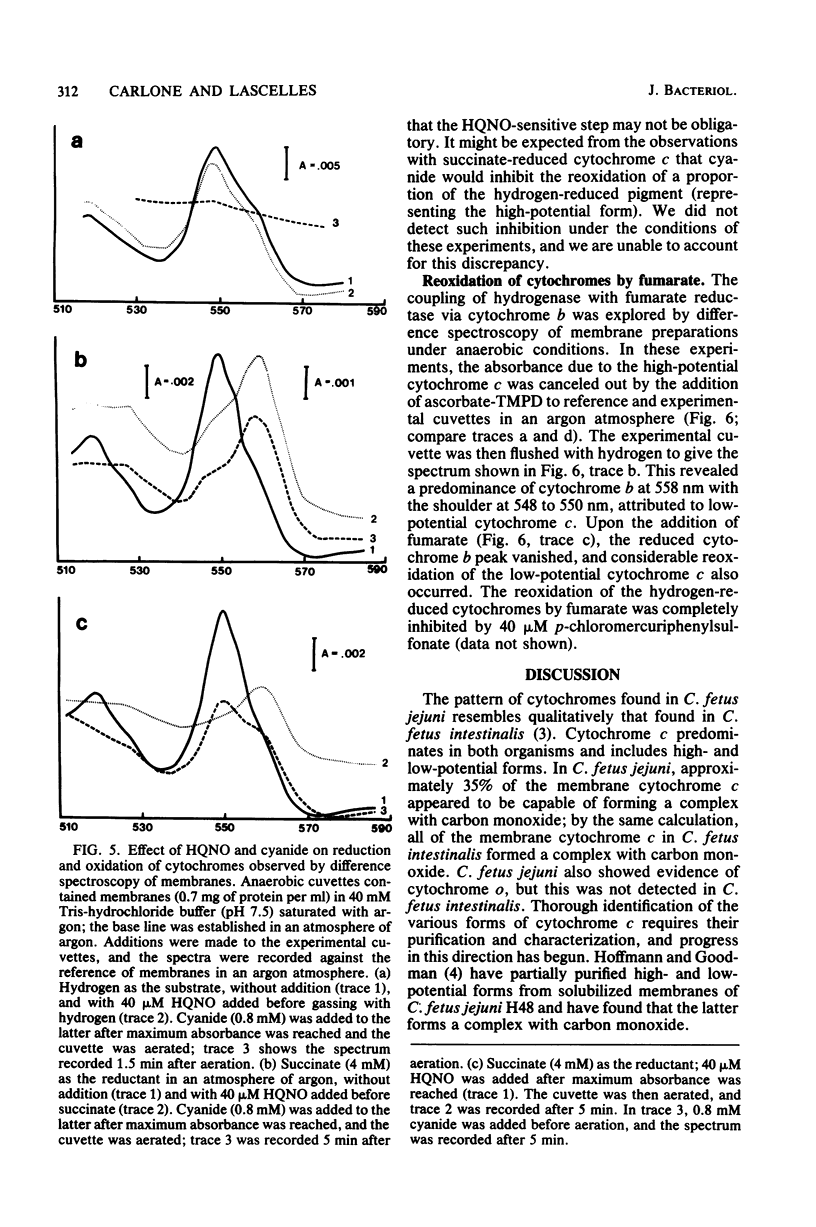
Abstract
Maximum growth of Campylobacter fetus subsp. jejuni, strain C-61, occurred when the cultures were incubated with shaking in atmospheres containing approximately 30% hydrogen, 5% oxygen, and 10% CO2. Suspensions of cells grown under these conditions consumed oxygen with formate as the substrate in the presence of 0.33 mM cyanide, which completely inhibited respiration with ascorbate-N,N,N',N'-tetramethyl-p-phenylenediamine and with lactate. Spectroscopic evidence with intact cells suggested that a form of cytochrome c, reducible with formate but not with lactate or ascorbate-N,N,N',N'-tetramethyl-p-phenylenediamine, can be reoxidized by a cyanide-insensitive system. Analysis of membranes from the cells showed high- and low-potential forms of cytochrome c, cytochrome b, and various enzymes, including hydrogenase, formate dehydrogenase, and fumarate reductase. The predominant carbon monoxide-binding pigment appeared to be a form of cytochrome c, but the spectra also showed evidence of cytochrome o. The membrane cytochromes were reduced by hydrogen in the presence of 2-heptyl-4-hydroxyquinoline-N-oxide at concentrations which prevented the reduction of cytochrome c with succinate as the electron donor. Reoxidation of the substrate-reduced cytochromes by oxygen was apparently mediated by cyanide-sensitive and cyanide-insensitive systems. The membranes also had hydrogen-fumarate oxidoreductase activity mediated by cytochrome b. We conclude that C. fetus jejuni has high- and low-potential forms of cytochrome which are associated with a complex terminal oxidase system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross A. R., Anthony C. The purification and properties of the soluble cytochromes c of the obligate methylotroph Methylophilus methylotrophus. Biochem J. 1980 Nov 15;192(2):421–427. doi: 10.1042/bj1920421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Harvey S., Lascelles J. Respiratory systems and cytochromes in Campylobacter fetus subsp. intestinalis. J Bacteriol. 1980 Dec;144(3):917–922. doi: 10.1128/jb.144.3.917-922.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Goodman T. G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982 Apr;150(1):319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Edwards C. The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol. 1977 Oct 24;115(1):85–93. doi: 10.1007/BF00427850. [DOI] [PubMed] [Google Scholar]
- Niekus H. G., van Doorn E., Stouthamer A. H. Oxygen consumption by Campylobacter sputorum subspecies Bubulus with formate as substrate. Arch Microbiol. 1980 Sep;127(2):137–143. doi: 10.1007/BF00428017. [DOI] [PubMed] [Google Scholar]
- O'Keeffe D. T., Anthony C. The two cytochromes c in the facultative methylotroph Pseudomonas am1. Biochem J. 1980 Nov 15;192(2):411–419. doi: 10.1042/bj1920411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. Bacterial cytochromes and their spectral characterization. Methods Enzymol. 1978;53:202–212. doi: 10.1016/s0076-6879(78)53025-5. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



