Abstract
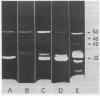
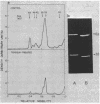
The membrane penicillinases of Bacillus licheniformis and Bacillus cereus are lipoproteins with N-terminal glyceride thioether modification identical to that of the Escherichia coli outer membrane lipoprotein. They are readily labeled with [3H]palmitate present during exponential growth. At the same time, a few other proteins in each organism become labeled and can be detected by fluorography after sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total membrane proteins. We distinguish these proteins from the O-acyl proteolipids by demonstrating the formation of glyceryl cysteine sulfone after performic acid oxidation and hydrolysis of the protein. By this criterion, B. licheniformis and B. cereus contain sets of lipoproteins larger in average molecular weight than that of E. coli. Members of the sets probably are under a variety of physiological controls, as indicated by widely differing relative labeling intensity in different media. The set in B. licheniformis shares with membrane penicillinase a sensitivity to release from protoplasts by mild trypsin treatment, which suggests similar orientation on the outside of the membrane. At least one protein is the membrane-bound partner of an extracellular hydrophilic protein, the pair being related as membrane and exopenicillinases are. We propose that the lipoproteins of gram-positive organisms are the functional equivalent of periplasmic proteins in E. coli and other gram-negative bacteria, prevented from release by anchorage to the membrane rather than by a selectively impermeable outer membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bettinger G. E., Lampen J. O. Further evidence for a partially folded intermediate in penicillinase secretion by Bacillus licheniformis. J Bacteriol. 1975 Jan;121(1):83–90. doi: 10.1128/jb.121.1.83-90.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Nielsen J. B., Izui K., Blobel G., Lampen J. O. Identification of the signal peptidase cleavage site in Bacillus licheniformis prepenicillinase. J Biol Chem. 1982 Apr 25;257(8):4340–4344. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Crane L. J., Bettinger G. E., Lampen J. O. Affinity chromatography purification of penicillinase of Bacillus licheniformis 749-C and its use to measure tuurnover of the cell bound enzyme. Biochem Biophys Res Commun. 1973 Jan 23;50(2):220–227. doi: 10.1016/0006-291x(73)90829-2. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Wouters J. T., Lampen J. O. Distribution of the sites of alkaline phosphatase(s) activity in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):928–937. doi: 10.1128/jb.108.2.928-937.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Izui K., Nielsen J. B., Caulfield M. P., Lampen J. O. Large exopenicillinase, initial extracellular form detected in cultures of Bacillus licheniformis. Biochemistry. 1980 Apr 29;19(9):1882–1886. doi: 10.1021/bi00550a023. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kuhn H., Fietzek P. P., Lampen J. O. N-terminal amino acid sequence of Bacillus licheniformis alpha-amylase: comparison with Bacillus amyloliquefaciens and Bacillus subtilis Enzymes. J Bacteriol. 1982 Jan;149(1):372–373. doi: 10.1128/jb.149.1.372-373.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Sarvas M., Brammar W. J., Neugebauer K., Wu H. C. Bacillus licheniformis penicillinase synthesized in Escherichia coli contains covalently linked fatty acid and glyceride. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3506–3510. doi: 10.1073/pnas.78.6.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. H., Philbrick W. M., Wu H. C. Acyl moieties in phospholipids are the precursors for the fatty acids in murein lipoprotein of Escherichia coli. J Biol Chem. 1980 Jun 10;255(11):5384–5387. [PubMed] [Google Scholar]
- Nielsen J. B., Caulfield M. P., Lampen J. O. Lipoprotein nature of Bacillus licheniformis membrane penicillinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3511–3515. doi: 10.1073/pnas.78.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Membrane-bound penicillinases in Gram-positive bacteria. J Biol Chem. 1982 Apr 25;257(8):4490–4495. [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Simons K., Sarvas M., Garoff H., Helenius A. Membrane-bound and secreted forms of penicillinase from Bacillus licheniformis. J Mol Biol. 1978 Dec 25;126(4):673–690. doi: 10.1016/0022-2836(78)90015-3. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tsugita A. Phosphoesterases of Bacillus subtilis. II. Crystallization and properties of alkaline phosphatase. J Biochem. 1967 Feb;61(2):231–241. doi: 10.1093/oxfordjournals.jbchem.a128535. [DOI] [PubMed] [Google Scholar]
- WEINTRAUB M., RAYMOND S. ANTISERUMS PREPARED WITH ACRYLAMIDE GEL USED AS ADJUVANT. Science. 1963 Dec 27;142(3600):1677–1678. doi: 10.1126/science.142.3600.1677. [DOI] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Crystallization and properties of alpha-amylase from five strains of Bacillus amyloliquefaciens. Biochemistry. 1967 Dec;6(12):3681–3689. doi: 10.1021/bi00864a010. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]