Abstract
In this paper we present the finding that lovastatin arrests cells by inhibiting the proteasome, which results in the accumulation of p21 and p27, leading to G1 arrest. Lovastatin is an inhibitor of hydroxymethyl glutaryl (HMG)-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. Previously, we reported that lovastatin can be used to arrest cultured cells in the G1 phase of the cell cycle, resulting in the stabilization of the cyclin-dependent kinase inhibitors (CKIs) p21 and p27. In this report we show that this stabilization of p21 and p27 may be the result of a previously unknown function of the pro-drug, β-lactone ring form of lovastatin to inhibit the proteasome degradation of these CKIs. The lovastatin mixture used in this study is 80% open-ring form and 20% pro-drug, β-lactone form. We show that while the lovastatin open-ring form and pravastatin (a lovastatin analogue, 100% open ring) inhibit the HMG-CoA reductase enzyme, lovastatin pro-drug inhibits the proteasome but does not inhibit HMG-CoA reductase. In addition, many of the properties of proteasome inhibition by the pro-drug are the same as the specific proteasome inhibitor lactacystin. Lastly, mevalonate (used to rescue cells from lovastatin arrest) unexpectedly abrogates the lactacystin and lovastatin pro-drug inhibition of the proteasome. Mevalonate increases the activity of the proteasome, which results in degradation of the CKIs, allowing lovastatin- and lactacystin-arrested cells to resume cell division. The lovastatin-mediated inhibition of the proteasome suggests a unique mechanism for the chemopreventative effects of this agent seen in human cancer.
Metabolic and cellular processes that require exquisite temporal precision, like the cell cycle, often involve selective proteolytic degradation of regulated proteins (1). One major degradative pathway capable of such activity is the proteasome pathway (2, 3). This pathway is involved in the regulation of diverse processes including embryogenesis, signal transduction, and cell cycle progression (2, 4). For example, degradation of several proteins involved in cell cycle regulation such as Clns, Clbs, cyclins A, B, D, E, p53, and pRb are via ubiquitin-mediated proteolysis (2, 4). The ubiquitin pathway also regulates the levels of cyclin-dependent kinase inhibitors (CKIs) p27 and p21 (5–7).
Proteasome activity is inhibited by several peptide aldehydes (e.g., LLnL) and compounds like 3,4-dichloroisocoumarin and lactacystin (8). Lactacystin, a Streptomyces metabolite containing a β-lactone ring, selectively inhibits proteolytic activities of the proteasome (8, 9). The moiety crucial for inhibition of the proteasome activity is the β-lactone electrophilic carbonyl, which targets enzymes containing a catalytic nucleophile such as a protease. In contrast, the dihydroxy acid form of lactacystin is essentially inert to nucleophilic attack and is incapable of inhibiting the proteasome (10). These findings suggest that the pro-drug form of another β-lactone, lovastatin, similar in structure to lactacystin, may inhibit the ubiquitin-mediated proteolysis of key regulatory proteins such as the cyclins and CKIs.
Lovastatin is used for the treatment of hypercholesterolemia (11) because it inhibits hydroxymethyl-glutaryl (HMG)-CoA reductase, and thus prevents HMG-CoA’s conversion into mevalonic acid (12, 13). When mevalonate levels decrease as a response to lovastatin, isoprenylation of key signal transduction proteins (e.g., Ras, Rap, etc.) is prevented, their subcellular localization is disrupted, and they are inactivated as signal transducers (14). Administration of lovastatin to cells in culture impacts cell cycle progression. We have reported that lovastatin effectively synchronizes both tumor and normal cells (15) and arrests cells in G1 (16, 17). The cell cycle pathways perturbed by lovastatin have been shown by several laboratories to result in the induction of CKIs p21 and/or p27 (16–21) independent of other standard G1-arresting agents/conditions such as serum starvation or double thymidine block (17). Additionally, the lovastatin-mediated G1 arrest and p21/p27 induction occur independently of the ras signaling pathway/function (22, 23).
How lovastatin induces G1 arrest and simultaneously increases p21 and/or p27 currently is undefined. One simple explanation is that decreased cholesterol and/or its intermediary metabolites prevent cell cycle progression and that the induction of p21/p27 is a secondary event. Indeed because mevalonate releases arrested cells from G1 block, it has been assumed that the target of lovastatin action is within the mevalonate/cholesterol pathway and that mevalonate or one of its downstream products is essential for cell division (20, 24).
The studies presented here suggest that alternative pathways may be targeted by lovastatin; these pathways may mediate the lovastatin effects on cell proliferation. We show that lovastatin in its pro-drug form (β-lactone) is entirely responsible for the effects on p21 and p27 leading to G1 arrest. We identified the ubiquitin-proteasome pathway as an alternative, HMG-CoA independent, pathway and show that the pro-drug inhibits the proteasome both in vitro and in vivo. Our studies also suggest that an additional role for mevalonate is to abrogate the effects of both lactacystin and the pro-drug form of lovastatin by increasing the activity of the proteasome.
METHODS
Materials, Cell Lines, and Culture Conditions.
Lovastatin was provided by William Henckler (Merck, Sharp, and Dohme Research Pharmaceuticals, Rahway, NJ) and was converted from its pro-drug form to its dihydroxy-open acid form as described (15). The pro-drug was soluble in 60% ethanol, and pravastatin was soluble in water. Treatments of MDA-MB-157 with lovastatin, its pro-drug form, pravastatin, LLnL, or lactacystin and Western blot analysis were performed as described (15, 16).
HPLC Analysis of Pro-Drug, Lovastatin, and Pravastatin.
RP-HPLC using a Waters 625 chromatography system was performed as described (25). Briefly, a C 18 Novopac column was equilibrated with the stationary phase of 0.1% trifluoroacetic acid (TFA) and 90% acetonitrile as the mobile phase. Ten micrograms of lovastatin mixture or β-lactone (diluted 0.1% TFA) was analyzed by HPLC, and the eluted fractions were subjected to mass spectrometry as described (26).
Assay for HMG-CoA Reductase Activity.
The HMG-CoA reductase assays in MDA-MB-157 cells were performed as described (27). Briefly, cells were homogenized by sonication in the reaction buffer (200 mM phosphate buffer pH 7.4/20 mM DTT/40 mM glucose-6-phosphate/3 mM β-NADPH/2 units/ml glucose-6-phosphatase), and the 20,000 × g supernatant was incubated with the pro-drug, lovastatin, or pravastatin at 37°C, followed by the addition of the [14C] HMG-CoA. Samples were subjected to TLC with [14C] mevalolactone (MVA) as standard and analyzed by PhosphorImager scanning. The efficiency of the enzymatic reaction is a measure of conversion of [14C] HMG-CoA into [14C] MVA formed and expressed as percent conversion per mg of microsomal protein.
Proteasome Activity Assay.
For measurement of the proteasome chymotrypsin peptidase activity, 10 μl of cellular extract (100 μg, prepared by brief sonication of cells and fractionation at 16,000 × g) was diluted in a cuvette containing 2 ml of 20 mM Hepes, 0.5 M EDTA, pH 8, 0.035% SDS with the indicated concentrations of lactacystin, pro-drug, lovastatin, and pravastatin. The cell extracts contain a mixture of 26S and 20S proteasomes. (The proteasome assays of the pretreated cells were performed after treatment of MDA-MB-157 cells with the indicated drugs, and the medium was removed and the cells were washed several times before they were lysed to removed excess inhibitor). The above mixture was incubated at 37°C before the addition of the fluorogenic substrate, 10 μM succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin. Substrate hydrolysis was measured by continuous monitoring of fluorescence (emission at 440 nm, excitation at 380 nM) of the liberated 7-amido-4-methylcoumarin for 15 min as described (9).
35S Metabolic Labeling for MDA-MB-157 Cells.
Pulse–chase experiments with MDA-MB-157 were performed as described (18) with the following modifications. Cells were incubated in DMEM without methionine and cysteine and with β-lactone or no drug for 2 hr. EXPRESS [35S]protein labeling mix (0.2 mCi; NEN/DuPont) was added, and the cells were pulse-labeled for 4 hr.
RESULTS
Lovastatin-Mediated Induction of p21 and p27 Is Independent of the HMG-CoA Reductase Block.
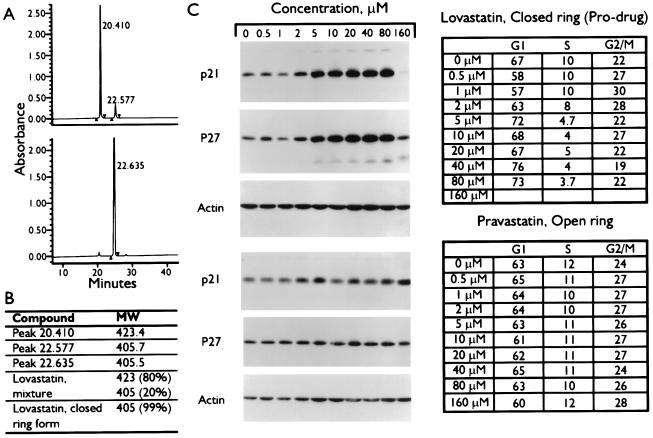
Our hypothesis is that the induction of CKIs by lovastatin is not solely through inhibition of the HMG-CoA reductase pathway, but also through the inhibition of the proteasome pathway. We base this hypothesis on the structural similarities between the pro-drug form of lovastatin and lactacystin, a proteasome inhibitor. The chemical form of lovastatin likely to inhibit the proteasome pathway is the closed β-lactone ring (referred to herein as pro-drug) form, whereas the open-ring form inhibits the HMG-CoA reductase. The pro-drug form of lovastatin is routinely modified to its open dihydroxy acid form chemically as described (15) before treatment of cells. By using RP-HPLC we found that the chemical conversion of lovastatin from its pro-drug to open-ring form was 80% efficient; 20% of the lovastatin mixture after preparation remained in the unmodified pro-drug form (Fig. 1A, Upper). The pro-drug form of lovastatin elutes with the same HPLC retention time as the second peak of the modified lovastatin (Fig. 1A, Lower), showing that the actual prepared form of lovastatin used in our studies contains both forms. Electrospray ionization mass spectrometry analysis confirmed the identity of the eluted HPLC peaks (Fig. 1B). The first peak of the lovastatin mixture is the open-ring form, with a mass of 423 (and was 80% of the mixture), whereas the second peak had an identical mass as the pro-drug (i.e., 405), suggesting that they are the same compound. The ratio of the two forms is constant over time and not affected by the medium used (data not shown).
Figure 1.
Induction of CKIs by the β-lactone form of lovastatin. (A) Chromatographic separation of lovastatin mixture (Upper) and closed-ring form (Lower) by HPLC analysis as described (15, 25). (B) Fractions corresponding to each HPLC peak were collected and subjected to mass determination by electrospray ionization quadrupole mass spectrometry analysis as described (26). The lovastatin mixture and closed-ring forms also were subjected to mass spectrometry analysis to determine the components of each reagent. (C) MDA-MB-157 tumor cells were treated with the indicated concentrations of lovastatin pro-drug or pravastatin for 36 hr. Cells were harvested and subjected to either flow cytometry (Right) or Western blot analysis with the indicated antibodies (Left).
To determine which form of lovastatin (open or closed) is responsible for CKI induction cells were treated with either the pro-drug form of lovastatin or an analogue of lovastatin, called pravastatin, present only in its open-ring form. (Lovastatin cannot be prepared entirely as the open-ring form.) Pravastatin is similar in structure and potency to the open-ring form of lovastatin and does not require modification to inhibit HMG-CoA reductase activity. MDA-MB-157 cells were treated with the indicated concentrations of these two agents for 36 hr and analyzed by flow cytometry and Western blot (Fig. 1C). Treatment of cells with the pro-drug form of lovastatin resulted in inhibition of cell proliferation and pronounced CKI induction in a dose-dependent manner (Fig. 1C). The flow cytometric data shows that after treatment of cells with only 5 μM pro-drug the cells accumulate in the G1 phase with a concomitant reduction in S phase. To achieve the same degree of growth inhibition, 40 μM of the chemically modified lovastatin (a mixture of open and closed forms) had to be used (data not shown and ref. 16). The highest concentrations of the pro-drug, i.e., 160 μM, resulted in apoptosis of most of the cells. Pravastatin, on the other hand, does not induce the CKIs or arrest cells at any concentration examined (Fig. 1C). Collectively these studies suggest that the pro-drug, closed-ring form of lovastatin, but not the open-ring form is responsible for the induction of p21 and p27. These observations also raise the hypothesis that the mechanism of CKI induction by lovastatin is not through the inhibition of the HMG-CoA reductase enzyme.
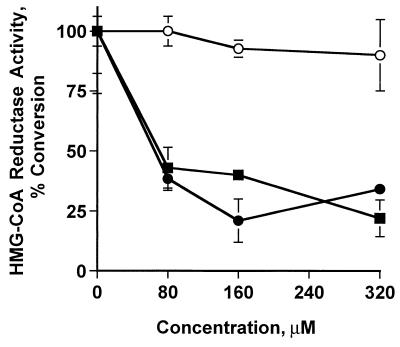
To directly determine the abilities of the lovastatin mixture, lovastatin pro-drug, and pravastatin to inhibit HMG-CoA reductase we prepared cell extracts from MDA-MB-157 cells and assayed for HMG-CoA reductase as described (27). The results of this assay revealed that while both pravastatin and the lovastatin mixture inhibited the HMG-CoA reductase in a dose-dependent manner the pro-drug form of lovastatin was incapable of inhibiting the activity of HMG-CoA reductase over the concentration range examined (Fig. 2). The data strongly suggest that the pro-drug form of lovastatin induces the CKIs through a pathway independent of HMG-CoA reductase and that inhibition of this enzyme may not be essential for CKI induction.
Figure 2.
Inactivation of HMG-CoA reductase enzyme by lovastatin. Microsomes (100 μg) prepared from subconfluent cultures of MDA-MB-157 were incubated at 37°C in the presence of the indicated concentrations of lovastatin, pro-drug, or pravastatin followed by the addition of 14C-HMG-CoA. After the lactonization of the reaction with HCL, the samples were resolved by TLC and analyzed by PhosphorImaging. The activity of HMG-CoA reductase is a measure of the percent conversion of 14C-HMG-CoA into mevalolactone, the end product of the HMG-CoA reductase. ●, lovastatin mixture; ○, pro-drug; ■, pravastatin.
Inhibition of the Proteasome by Lovastatin.
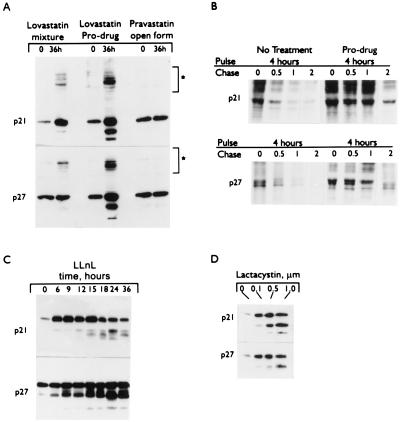
Previous reports have shown that both p21 and p27 are substrates for ubiquitination and proteasome-dependent degradation (5, 6, 28). Western blot analysis of cell extracts treated with lovastatin mixture and the pro-drug shows that antibodies to both p21 and p27 recognize specific proteins of higher molecular masses (i.e., 70–90 kDa) that are likely to correspond to ubiquitinated forms of these CKIs (Fig. 3A). Pravastatin does not cause similar results. The pro-drug affects the stability of p21 and p27 by altering the turnover rates of these CKIs. Compared with untreated cells, the pro-drug-treated cells degraded both p21 and p27 much more slowly (Fig. 3B). The increased half-life (0.13 versus 1.5 hr for both p21 and p27) accounts for the net increase of protein observed from drug treatment (Fig. 3B). Furthermore, treatment of cells with two different proteasome inhibitors (LLnL and lactacystin) resulted in a dramatic induction of p21 and p27 in a dose- and time-dependent fashion (Fig. 3 C and D). This finding suggests that the proteasome pathway has a role in the lovastatin-mediated stabilization of the CKIs.
Figure 3.
Induction of p21 and p27 by proteasome inhibitors. MDA-MB-157 tumor cells were treated with (A) 40 μM of either lovastatin mixture (open and closed rings), lovastatin closed β-lactone ring (pro-drug), or pravastatin for 36 hr, (C) 10 μM LLnL for 0–36 hr, or (D) indicated concentrations of lactacystin for 24 hr and subjected (50 μg/lane) to Western blot analysis with the indicated antibodies. Brackets in A indicate the high molecular weight laddering of p21 and p27, diagnostic for poly-ubiquitination. (B) For turnover studies, cells were treated with 40 μM pro-drug (or no drug) for 36 hr at which point the cells were incubated for 4 hr with [35S]methionine and [35S]cysteine (pulse) and subsequently incubated in the presence of an excess of nonradioactive methionine and cysteine for the additional times indicated (chase). p21 and p27 were precipitated from nondenatured protein extracts with polyclonal antibodies (p27-C19 and p21-C19-G, Santa Cruz Biotechnology), separated by SDS/PAGE, and detected by autofluorography.
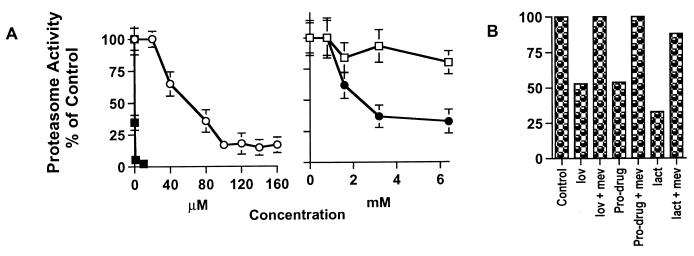
We next evaluated the kinetics of proteasome inhibition by the pro-drug form of lovastatin (Fig. 4). The ability of lactacystin, the pro-drug, lovastatin mixture, and pravastatin to inhibit the proteasome complex (a mixture of 26S and 20S proteasomes) in MDA-MB-157 cell extracts was measured directly by using a fluorescence assay containing a fluorogenic peptide substrate for the chymotrypsin-like activity of this complex (29, 30). Lactacystin was capable of inhibiting the peptidase activity of the proteasome completely at 1 μM (Fig. 4A). The pro-drug also inhibits the proteasome activity in a dose-dependent fashion with half-maximal inhibition occurring at 40 μM. The lovastatin mixture, which contains only 20% of the pro-drug form, also inhibited the proteasome activity but at much higher concentrations, reflecting the low percentage of the pro-drug form and that in the mixture, lovastatin may block the peptidase mediated inhibition by the pro-drug. Pravastatin, on the other hand, was incapable of inhibiting the proteasome over the concentration ranges examined (i.e., up to 6.4 mM) (Fig. 4A). This analysis clearly revealed that the pro-drug form of lovastatin inhibits the proteasome activity in vitro. Because the concentration required to inhibit the proteasome in vitro is higher than that used to arrest cells, we also performed the proteasome experiments in vivo by first treating cells with the inhibitors, followed by measurement of the peptidase activity of the proteasome (Fig. 4B). These analyses revealed that lovastatin mixture, its pro-drug form, and lactacystin inhibited the activity of the proteasome at concentrations similar to those required to achieve G1 arrest in vivo. Differences in drug potency in vivo and in vitro are likely caused by different rates of uptake and metabolism. Furthermore, mevalonate was able to abrogate the inhibitory activity of these agents on the proteasome (Fig. 4B and see below). These studies (Figs. 1–4) suggest that a specific proteasome-mediated pathway contributes to increased expression, via stabilization, of p21 and p27 in cells treated with the lovastatin mixture or the pro-drug.
Figure 4.
Inhibition of the proteasome activity by the pro-drug form of lovastatin and lactacystin. Cell extracts were prepared from either MDA-MB-157 cells (A) without drug treatment or (B) treated with either lovastatin (lov) (40 μM), pro-drug (40 μM), or lactacystin (lact) (1 μM) in the presence or absence of mevalonate (mev) (5 mM) for 36 hr and assayed for proteasome enzyme activity. The extracts in A were incubated in the presence of the indicated concentrations of the drugs for 20 min at 37°C at which point the fluorogenic peptide substrate (100 μM final concentration) for the chymotrypsin-like activity of the proteasome (i.e., Suc-LLVY-AMC) was added to extracts. The fluorescence assays (excitation/emission 380/440 nm) were conducted at 37°C for 750 sec, and n = 3. The pretreated extracts in B were not further incubated in the presence of the inhibitors and were directly assayed for the proteasome activity. ■, lactacystin; ○, pro-drug; ●, lovastatin; □, pravastatin.
Mevalonate Abrogates the Lovastatin- and Lactacystin-Mediated Inhibition of the Proteasome.
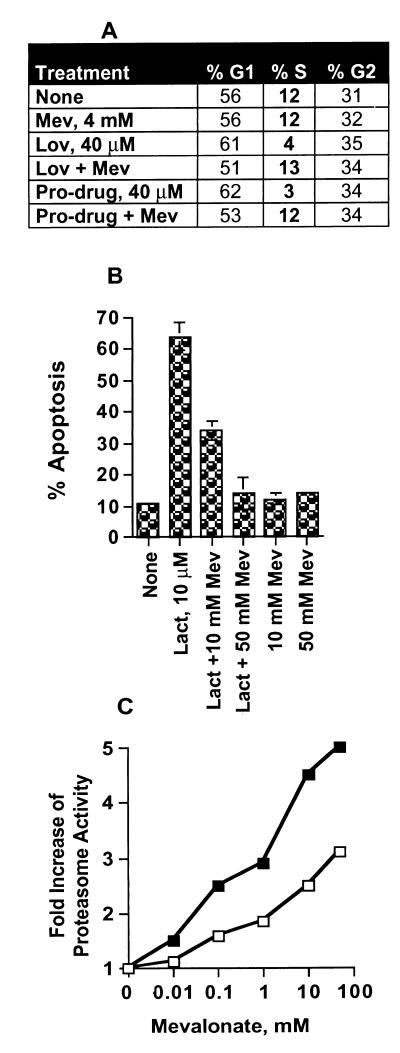
In this study we are proposing that mevalonate also has a dual function. Mevalonate reverses the lovastatin inhibition of HMG-CoA reductase and restores cholesterol biosynthesis. Unexpectedly, mevalonate also abrogates the lactacystin/pro-drug inhibition of the proteasome. Fig. 5A shows that the G1 arrest mediated by both lovastatin and its pro-drug form can be abrogated by mevalonate. Because the pro-drug form of lovastatin does not inhibit HMG-CoA reductase (Fig. 2), it follows that mevalonate must have another role in reversing the pro-drug mediated G1 arrest. If our hypothesis is correct and the lovastatin-mediated inhibition of the proteasome leads to G1 arrest, then mevalonate should modulate proteasome activity. We directly addressed the ability of mevalonate to abrogate the effects of lactacystin on cells (Fig. 5B). Treatment of cells with 10 μM lactacystin resulted in significant (i.e., 70%) apoptosis of cells (Fig. 5B). [Lactacystin (10 μM) also completely inhibited the proteasome activity as shown in Fig. 4.] Intriguingly, when cells were treated with lactacystin in the presence of increasing concentrations of mevalonate, the lactacystin-mediated apoptosis was completely abrogated. However, addition of mevalonate after lactacystin does not reverse the apoptotic effects of lactacystin (data not shown). These results show that mevalonate abrogates the effects of lactacystin inhibition of the proteasome in vivo.
Figure 5.
Mevalonate reversal of lovastatin, lovastatin pro-drug, and lactacystin. (A) MDA-MB-157 cells were treated with the indicated concentration of lovastatin (lov) or the pro-drug in the presence or absence of 4 mM mevalonate (Mev) for 36 hr and subjected to flow cytometric measurements of DNA content. (B) MDA-MB-157 cells were treated with 10 μM lactacystin (lact) in the presence or absence of 10 mM or 50 mM mevalonate for 36 hr. At the end of treatment cells were subjected to flow cytometric measurement of DNA content. Percent apoptosis reflects the accumulation of cells with sub-G1 DNA content. (Apoptosis also was measured by chromosome condensation/4′,6-diamidino-2-phenylindole staining; data not shown.) (C) Induction of the proteasome activity by mevalonate. MDA-MB-436 (closed symbols) and MDA-MB-157 (open symbols) were treated with the indicated concentrations of mevalonate for 36 hr. After treatment, crude cell extracts were prepared and assayed for proteasome enzyme activity by measuring the chymotrypsin-like activity of the proteasome (described for Fig. 4B). Values are expressed as fold increase over no treatment controls. n = 3, and the averages of the values are indicated.
The reversal of lovastatin-mediated inhibition of HMG-CoA reductase by mevalonate is biochemically sound and documented extensively. However, the abrogation of proteasome inhibition by mevalonate represents an exciting enigma, because it suggests a unique function for mevalonate. One way mevalonate might abrogate proteasome inhibition is through activation of the proteasome itself. To examine whether mevalonate could modulate the proteasome activity we pretreated cells with increasing concentrations of mevalonate for 36 hr and measured the proteasome activity (29, 30). These studies in two breast cancer cell lines (MDA-MB-157 and MDA-MB-436) revealed that when cells were treated with increasing concentrations of mevalonate, the peptidase activity of the proteasome increased 300–500% over the concentration range examined (Fig. 5C). The mevalonate-mediated increase in the peptidase activity occurred if cells were pretreated with mevalonate for the indicated times, not when mevalonate was added to cell extracts (data not shown). We believe that mevalonate abrogates the action of both lactacystin and the pro-drug form of lovastatin on the proteasome by either increasing the activity of the proteasome complex, increasing the assembly of the active complexes, or unmasking inactive complexes. Alternatively, mevalonate also may either prevent the cellular uptake of the pro-drug or inactivate the β-lactone. Although the mechanism of mevalonate abrogation of proteasome inhibition currently is not known, our studies suggest that in addition to being an intermediate of cholesterol biosynthesis, mevalonate has a role in facilitating proteolytic degradation.
DISCUSSION
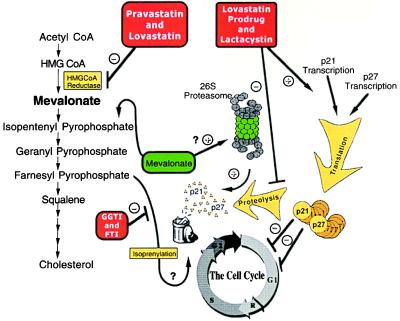
The hypothesis we present here is that lovastatin induces CKIs p21 and p27 in breast cancer cells by modulation of the ubiquitin-proteasome pathway, independent of inhibition of the HMG-CoA reductase enzyme. The model for this hypothesis is presented in Fig. 6. The left portion of this diagram illustrates the traditional role of HMG-CoA reductase inhibitors that block mevalonate synthesis, preventing the isoprenylation of key proteins implicated in cell division. The right side of the diagram illustrates our hypothesis that the proteasome is inhibited by both the pro-drug form of lovastatin and lactacystin, leading to accumulation of p21 and p27 and subsequent G1 arrest or apoptosis. Central to our hypothesis is the unusual discovery that mevalonate, in addition to its known ability to rescue HMG-CoA reductase inhibition, unexpectedly abrogates inhibition of the proteasome by lactacystin and the pro-drug form of lovastatin. This process leads to the degradation of the CKIs and resumption of cell division.
Figure 6.
Cell cycle regulation by inhibitors of HMG-CoA reductase and proteasome.
Although our results and hypothesis do not dispute the role of lovastatin (open-ring form) in the cholesterol biosynthesis pathway, they do describe alternative roles for the pro-drug form of lovastatin and mevalonate. On examination of the lovastatin literature we found that our previously unrecognized “side effects” of lovastatin and mevalonate might provide insights for numerous studies, which seemed unrelated to the cholesterol biosynthesis pathway. For example, a number of studies indicate that lovastatin and lactacystin share common ground in their biological effects (Fig. 6). Inhibitors of both HMG-CoA reductase and the proteasome have similar stimulatory effects on the differentiation of PC12 neuronal cells. Simvastatin, a lovastatin analogue, causes neurite-like outgrowth and inhibition of cell proliferation in PC12 cells, which was completely reversible by mevalonate. Lactacystin also causes neurite outgrowth of PC12 cells and results in neurogenesis and neurite outgrowth in a murine cell line (29, 31, 32). In contrast, pravastatin, which resembles only the active form of lovastatin, had no effect on these cells (33).
The above studies provide evidence that the common biological effects of lovastatin and lactacystin may be through modulation of the proteasome pathway. Other studies highlight properties of lovastatin that seem unrelated to cholesterol biosynthesis. For example, treatment of cells with the lovastatin analogue, simvastatin (more potent than lovastatin) resulted in regeneration of cultured rat skeletal muscle cells with a toxic effect on growth and differentiation, without influencing the cholesterol and phospholipid content of the cells (34). Inhibition of the HMG-CoA reductase also induces differentiation of human monocytic cells associated with growth retardation and expression of differentiation markers (35). Lovastatin also inhibited experimental lung metastasis of the highly metastatic B16F10 mouse melanoma in nude mice (36). Lastly, data from a large clinical trial of lovastatin produced the unexpected finding that lovastatin also may have chemoprevention abilities. When patients with severe hypercholesterolemia were treated with lovastatin, a decreased incidence (14 patients) of cancer of all types was observed in these patients compared with the expected rates (21 patients) during the 5-year period of the study (37). This clinical study emphasizes the significance of our finding in terms of the observed biological effects of lovastatin. We suggest that the mechanism by which lovastatin functions as a chemopreventative agent may be through the inhibition of the proteasome, resulting in increased stabilization of p21 and p27, which have tumor suppressive abilities.
The ability of mevalonate to reverse the effects of lovastatin fits well with the idea that activity of HMG-CoA reductase is needed to provide precursors vital to cell division. However, our data suggests another role for mevalonate is to abrogate the effects of both lactacystin and the pro-drug form of lovastatin. On one hand, mevalonate acts as cornerstone of cholesterol biosynthesis, and on the other, it is an allosteric effector of the proteasome (Fig. 6). We show that mevalonate abrogates the inhibitory action of both the pro-drug and lactacystin by the up-regulation of proteasome activity. There is precedent for this hypothesis. It has been reported that whereas HMG-CoA reductase is normally a stable enzyme with an extended half-life, downstream products such as mevalonate, sterols, and their derivatives like 25-hydroxycholesterol will promote the rapid and specific degradation of this enzyme (38, 39). Because HMG-CoA reductase is degraded through the proteasome pathway (40) and mevalonate increases the activity of the proteasome (this study), it follows that addition of mevalonate could promote the degradation of this enzyme (38).
In summary, we have provided evidence that lovastatin suppresses cell proliferation through inhibition of proteasome-mediated degradation of p21 and p27, and mevalonate can abrogate this effect by activation of the proteasome. These additional effects of lovastatin and mevalonate, not only provide insights into the biochemical pathways disturbed by these agents, but also provide explanation for numerous studies documenting their unrecognized effects. One such unrecognized effect of lovastatin is its chemopreventative abilities that may be mediated through inhibition of the proteasome.
Acknowledgments
We gratefully acknowledge Dr. Katherine Henrickson and Mr. Christopher G. Danes for the critical reading of this manuscript, Dr. Julie Gray-Bablin for helpful discussions during the course of this work, and the use of Wadsworth Center’s Immunology, Biochemistry, Mass Spectrometry, Tissue Culture, and Photography/Graphics core facilities. S.R. is a fellow of the Cancer Research Foundation of America. This research was supported in part by Grant DAMD-17-94-J-4081 from the U.S. Army Medical Research Acquisition Activity and by Grant No. R29-CA666062 from the National Cancer Institute (both to K.K.)
ABBREVIATIONS
- HMG
hydroxymethyl glutaryl
- CKI
cyclin-dependent kinase inhibitor
References
- 1.Hershko A, Ciechanover A. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- 2.Hochstrasser M. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 4.Pagano M. FASEB J. 1997;11:1067–1075. doi: 10.1096/fasebj.11.13.9367342. [DOI] [PubMed] [Google Scholar]
- 5.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew R P, Draetta G F, Rolfe M. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 6.Blagosklonny M V, Wu G S, Mura S, Eldeiry W S. Biochem Biophys Res Commun. 1996;227:564–569. doi: 10.1006/bbrc.1996.1546. [DOI] [PubMed] [Google Scholar]
- 7.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 8.Lee D H, Goldberg A L. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 9.Dick L R, Cruikshank A, Destree T, Grenier L, McCormack T A, Melandri F D, Nunes S L, Palombella V J, Parent L A, Plamondon L, Stein R L. J Biol Chem. 1997;272:182–188. doi: 10.1074/jbc.272.1.182. [DOI] [PubMed] [Google Scholar]
- 10.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 11.Rettersol K, Stuggard M, Gorbitz C, Ose L. Am J Cardiol. 1996;78:1369–1374. doi: 10.1016/s0002-9149(96)00649-2. [DOI] [PubMed] [Google Scholar]
- 12.Corsini A, Maggi F M, Catapano A L. Pharmacol Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 13.Endo A, Kuroda M, Tansawa K. FEBS Lett. 1976;72:323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 14.Maltese W A. FASEB J. 1990;4:3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- 15.Keyomarsi K, Sandoval L, Band V, Pardee A B. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 16.Rao S, Lowe M, Herliczek T, Keyomarsi K. Oncogene. 1998;17:2393–2402. doi: 10.1038/sj.onc.1202322. [DOI] [PubMed] [Google Scholar]
- 17.Gray-Bablin J, Rao S, Keyomarsi K. Cancer Res. 1997;57:604–609. [PubMed] [Google Scholar]
- 18.Hengst L, Reed S I. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 19.Terada Y, Inoshita S, Nakashima O, Yamada T, Kuwahara M, Sasaki S, Marumo F. J Am Soc Nephrol. 1998;9:2235–2243. doi: 10.1681/ASN.V9122235. [DOI] [PubMed] [Google Scholar]
- 20.Lee S J, Ha M J, Lee J, Nguyen P M, Choi Y H, Pirnia F, Kang W-K, Wang X-F, Kim S-J, Trepel J B. J Biol Chem. 1998;273:10618–10623. doi: 10.1074/jbc.273.17.10618. [DOI] [PubMed] [Google Scholar]
- 21.Vogt A, Sun J, Qian Y, Hamilton A D, Sebti S M. J Biol Chem. 1997;272:27224–27229. doi: 10.1074/jbc.272.43.27224. [DOI] [PubMed] [Google Scholar]
- 22.Muller C, Bockhorn A G, Klusmeier S, Kiehl M, Roeder C, Kalthoff H, Koch O M. Int J Oncol. 1998;12:717–723. doi: 10.3892/ijo.12.3.717. [DOI] [PubMed] [Google Scholar]
- 23.DeClue J E, Vass W C, Papageorge A G, Lowy D R, Willumsen B M. Cancer Res. 1991;51:712–717. [PubMed] [Google Scholar]
- 24.Bradfute D L, Silva C J, Simoni R D. J Biol Chem. 1992;267:18308–18314. [PubMed] [Google Scholar]
- 25.Stubbs R J, Schwartz M, Bayne W F. J Chromat. 1986;383:438–443. doi: 10.1016/s0378-4347(00)83492-1. [DOI] [PubMed] [Google Scholar]
- 26.Hall M, Bates S, Peters G. Oncogene. 1995;11:1581–1588. [PubMed] [Google Scholar]
- 27.Larsson O, Barrios C, Latham C, Ruiz J, Zetterberg A, Zickert P, Wejde J. Cancer Res. 1989;49:5605–5610. [PubMed] [Google Scholar]
- 28.Maki C G, Howley P M. Mol Cell Biol. 1997;17:355–363. doi: 10.1128/mcb.17.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenteany G, Standaert R F, Reichard G A, Corey E J, Schreiber S L. Proc Natl Acad Sci USA. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maupin-Furlow J A, Ferry J G. J Biol Chem. 1995;270:28617–28622. doi: 10.1074/jbc.270.48.28617. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Tsubuki S, Ito H, Kawashima S. Neurosci Lett. 1990;120:1–4. doi: 10.1016/0304-3940(90)90153-z. [DOI] [PubMed] [Google Scholar]
- 32.Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. J Biochem. 1996;119:572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- 33.Sato-Suzuki I, Murota S-I. Neurosci Lett. 1996;220:21–24. doi: 10.1016/s0304-3940(96)13221-3. [DOI] [PubMed] [Google Scholar]
- 34.Veerkamp J H, Smit J W A, Benders A A G M, Oosterhof A. Biochem Biophys Acta. 1996;1315:217–222. doi: 10.1016/0925-4439(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 35.Weber C, Erl W, Weber P C. Cell Biochem Funct. 1995;13:273–277. doi: 10.1002/cbf.290130408. [DOI] [PubMed] [Google Scholar]
- 36.Jani J P, Specht S, Stemmler N, Blanock K, Singh S V, Gupta V, Katoh A. Invasion Metastasis. 1993;13:314–324. [PubMed] [Google Scholar]
- 37.Stein E A, Lazkarzewski P, Steiner P The Lovastatin Study Groups I–IV. Arch Intern Med. 1993;153:1079–1087. [PubMed] [Google Scholar]
- 38.McGee T P, Cheng H H, Kumagai H, Omura S, Simoni R D. J Biol Chem. 1996;271:25630–25638. doi: 10.1074/jbc.271.41.25630. [DOI] [PubMed] [Google Scholar]
- 39.Correll C C, Ng L, Edwards P A. J Biol Chem. 1994;269:17390–17393. [PubMed] [Google Scholar]
- 40.Moriyama T, Sather S K, McGee T P, Simoni R D. J Biol Chem. 1998;273:22037–22043. doi: 10.1074/jbc.273.34.22037. [DOI] [PubMed] [Google Scholar]