Abstract
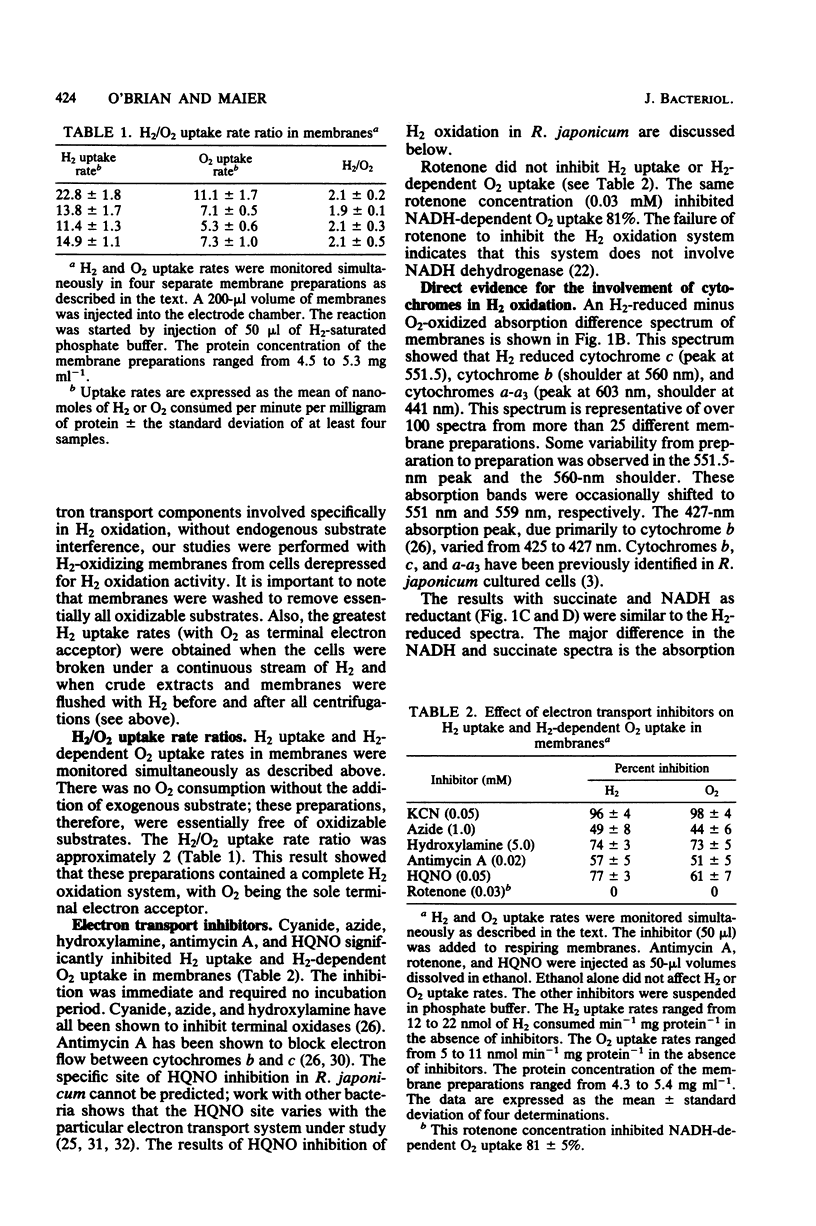
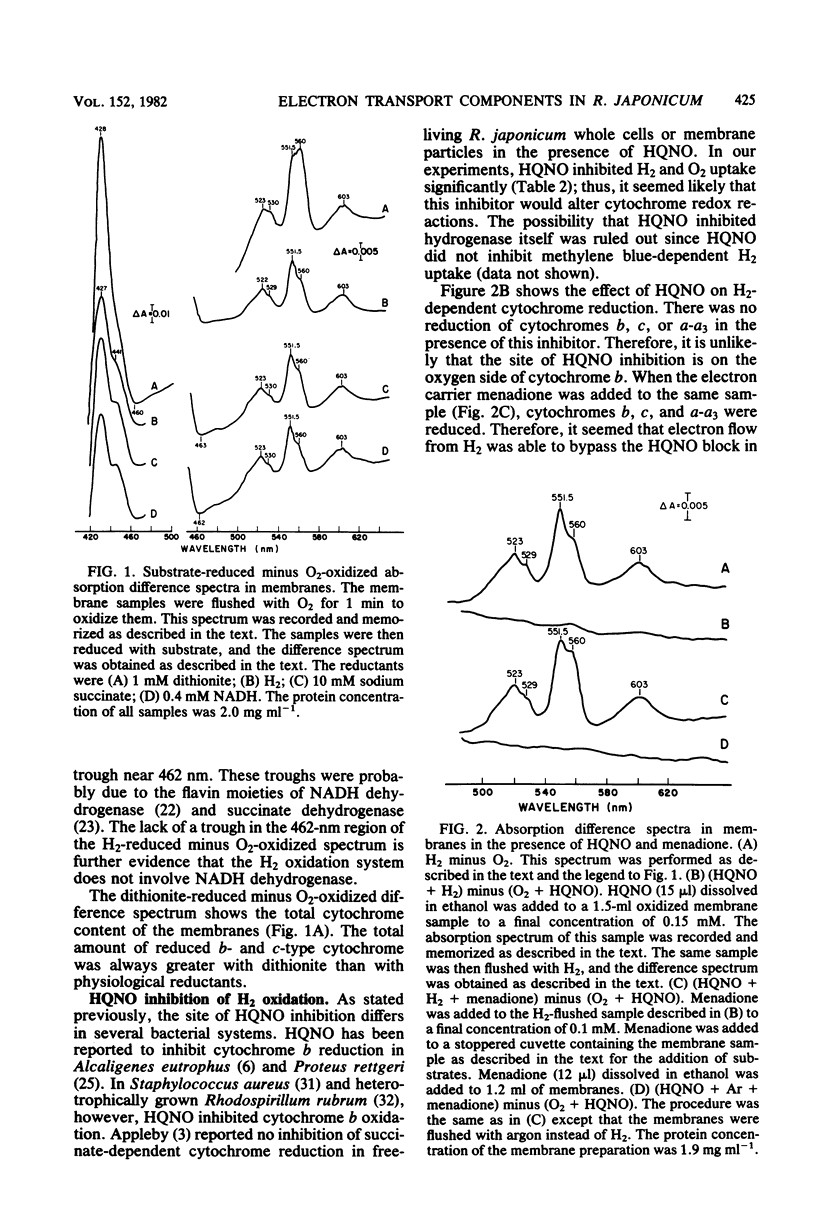
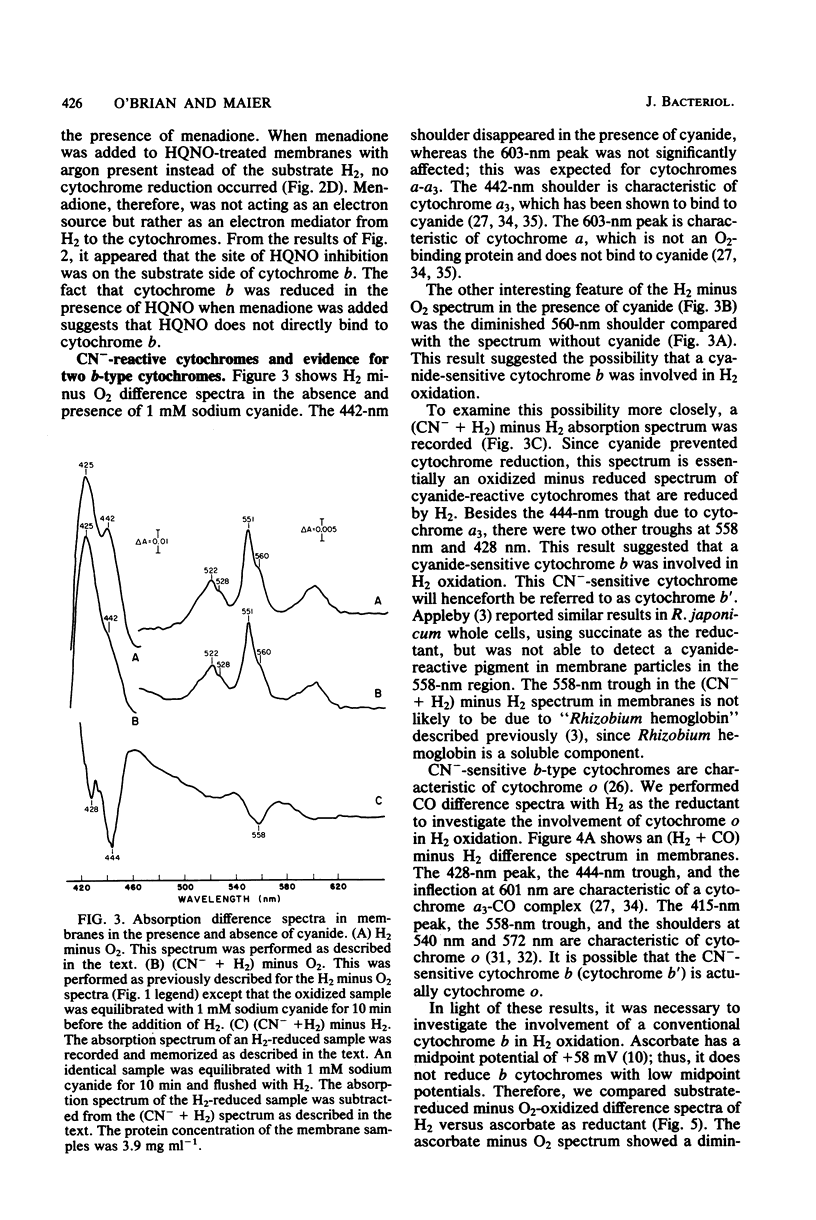
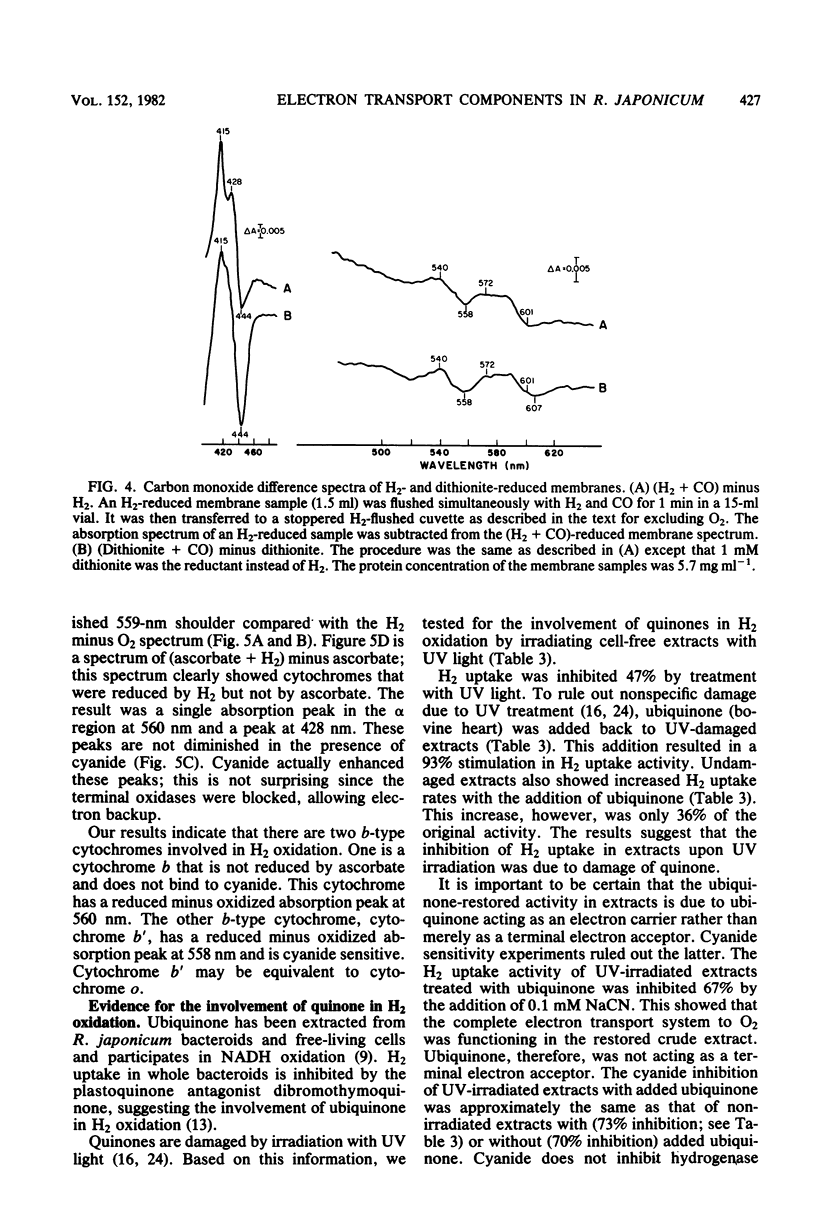
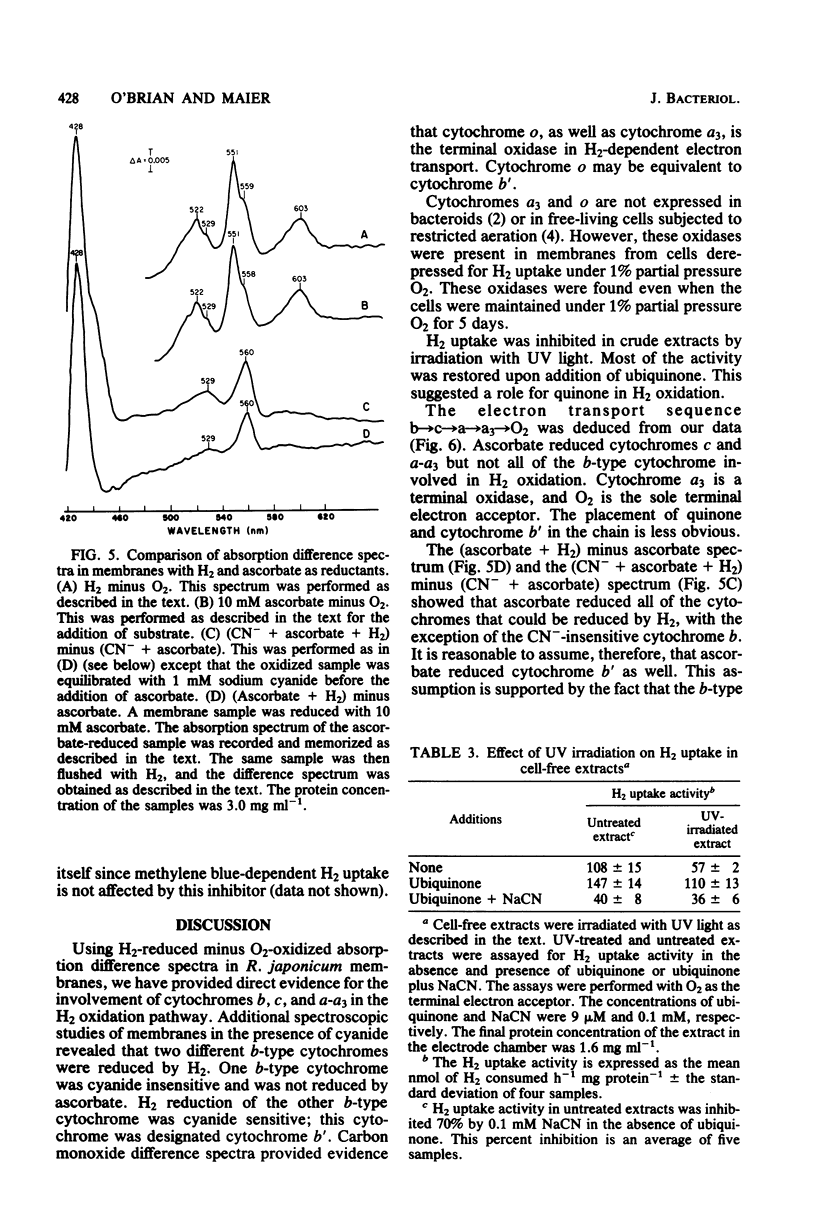
Membranes from free-living Rhizobium japonicum were isolated to study electron transport components involved in H2 oxidation. The H2/O2 uptake rate ratio in membranes was approximately 2. The electron transport inhibitors antimycin A, cyanide, azide, hydroxylamine, and 2-n-heptyl-4-hydroxyquinoline-N-oxide (HQNO) inhibited H2 uptake and H2-dependent O2 uptake significantly. H2-reduced minus O2-oxidized absorption difference spectra revealed peaks at 551.5, 560, and 603 nm, indicating the involvement of cytochromes c, b, and a-a3, respectively. H2-dependent cytochrome reduction was completely inhibited in the presence of 0.15 mM HQNO. This inhibition was relieved by the addition of 0.1 mM menadione. Evidence is presented for the involvement of two b-type cytochromes in H2 oxidation. One b-type cytochrome was not reduced by ascorbate and had an absorption peak at 560 nm. The reduction of this cytochrome by H2 was not inhibited by cyanide. A second b-type cytochrome, cytochrome b', was not reduced by H2 in the presence of cyanide. This cytochrome had an absorption peak at 558 nm. Carbon monoxide difference spectra with H2 as reductant provided evidence for the involvement of cytochrome o as well as cytochrome a3 in H2 oxidation. H2 uptake activity in cell-free extracts was inhibited by UV light irradiation. Most of the activity of the UV-treated extracts was restored with the addition of ubiquinone. The restored activity was inhibited by cyanide. A branched electron transport pathway from H2 to O2 is proposed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. II. Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim Biophys Acta. 1969 Jan 14;172(1):88–105. doi: 10.1016/0005-2728(69)90094-2. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers L. Phosphorylation in hydrogen bacteria. J Bacteriol. 1967 May;93(5):1615–1623. doi: 10.1128/jb.93.5.1615-1623.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B. Sequence of b cytochromes relative to ubiquinone in the electron transport chain of Escherichia coli. J Bacteriol. 1978 Feb;133(2):477–484. doi: 10.1128/jb.133.2.477-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisbrenner G., Evans H. J. Carriers in electron transport from molecular hydrogen to oxygen in Rhizobium japonicum bacteroids. J Bacteriol. 1982 Mar;149(3):1005–1012. doi: 10.1128/jb.149.3.1005-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Russell S. A., Evans H. J. Investigation of the H(2) Oxidation System in Rhizobium japonicum 122 DES Nodule Bacteroids. Plant Physiol. 1980 Dec;66(6):1061–1066. doi: 10.1104/pp.66.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson S. K., Parker G. L. The electron-transport system of Micrococcus lutea (Sarcina lutea). Biochim Biophys Acta. 1969 May;180(1):56–62. doi: 10.1016/0005-2728(69)90193-5. [DOI] [PubMed] [Google Scholar]
- Ernster L., Lee I. Y., Norling B., Persson B. Studies with ubiquinone-depleted submitochondrial particles. Essentiality of ubiquinone for the interaction of succinate dehydrogenase, NADH dehydrogenase, and cytochrome b. Eur J Biochem. 1969 Jun;9(3):299–310. doi: 10.1111/j.1432-1033.1969.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. Flavoproteins of the electron transport system and the site of action of amytal, rotenone, and piericidin A. Proc Natl Acad Sci U S A. 1968 Jun;60(2):733–740. doi: 10.1073/pnas.60.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Planta R. J. Restoration of electron transport in ultraviolet-irradiated membranes of Aerobacter aerogenes. FEBS Lett. 1971 Apr 12;14(1):54–56. doi: 10.1016/0014-5793(71)80273-9. [DOI] [PubMed] [Google Scholar]
- Kröger A., Dadák V., Klingenberg M., Diemer F. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. Eur J Biochem. 1971 Aug 16;21(3):322–333. doi: 10.1111/j.1432-1033.1971.tb01472.x. [DOI] [PubMed] [Google Scholar]
- LEMBERG R., PILGER T. B., NEWTON N., CLARKE L. CYTOCHROME OXIDASE AND ITS DERIVATIVES. I. CARBON MONOXIDE AND CYANIDE COMPOUNDS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:405–428. doi: 10.1098/rspb.1964.0010. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., KAMEN M. D. THE OXIDASE SYSTEM OF HETEROTROPHICALLY-GROWN RHODOSPIRILLUM RUBRUM. Biochim Biophys Acta. 1965 Mar 22;96:395–428. doi: 10.1016/0005-2787(65)90560-5. [DOI] [PubMed] [Google Scholar]
- VANNESTE W. H., VANNESTE M. T. ABSOLUTE AND DIFFERENCE SPECTRA OF CYTOCHROMES ALPHA AND ALPHA-3. Biochem Biophys Res Commun. 1965 Apr 9;19:182–186. doi: 10.1016/0006-291x(65)90501-2. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]



