Abstract
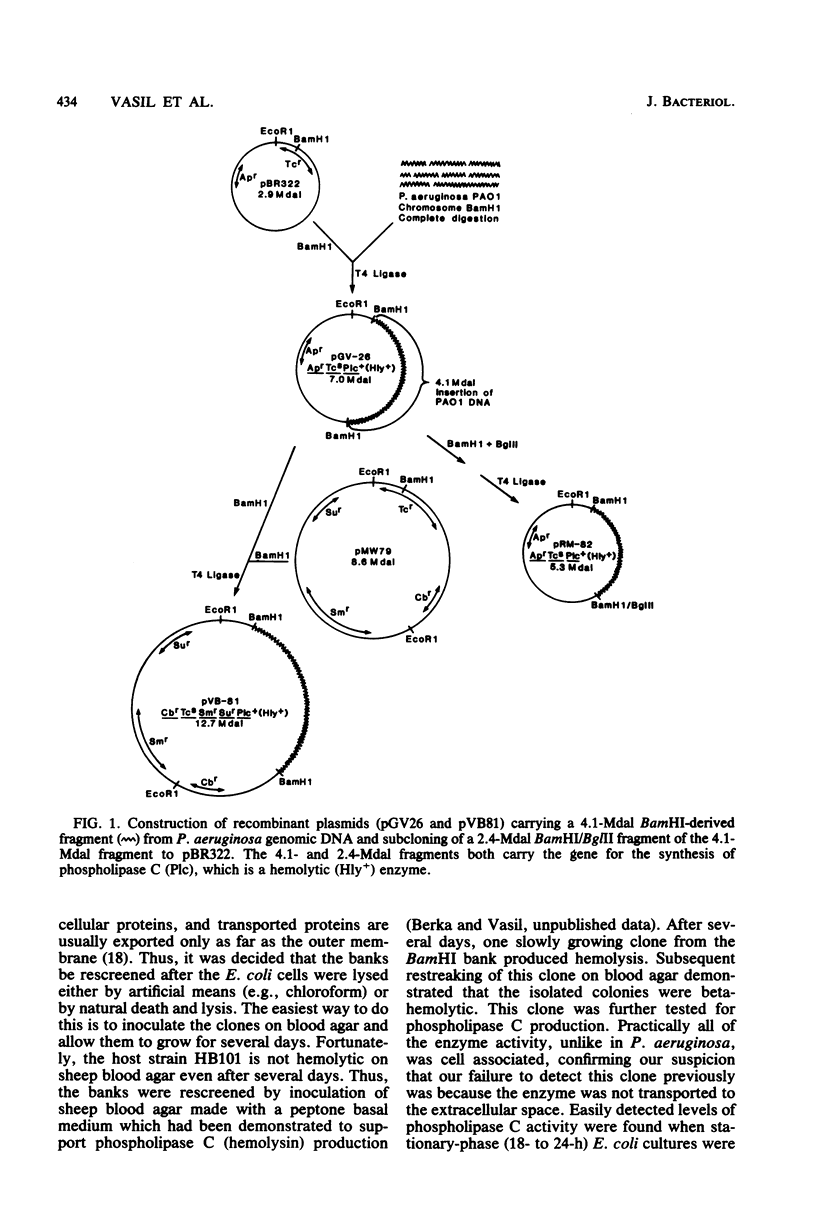
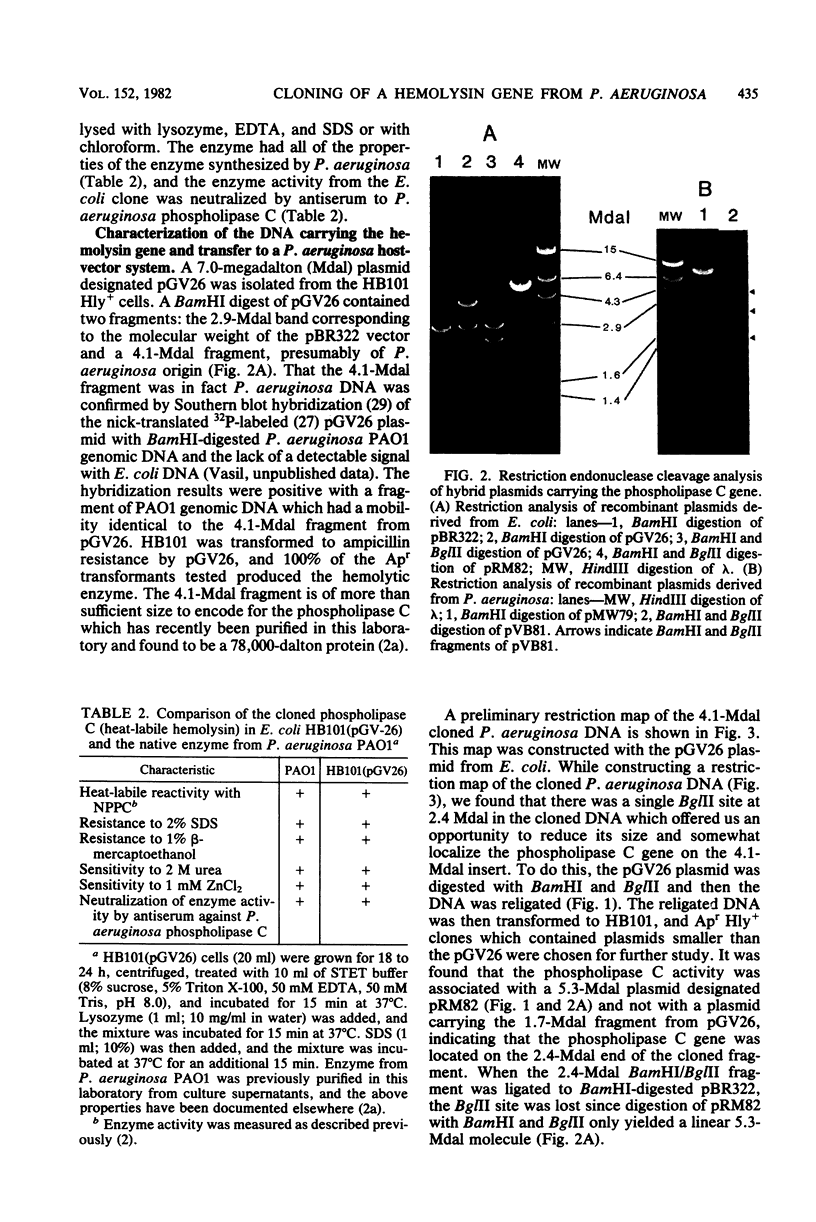
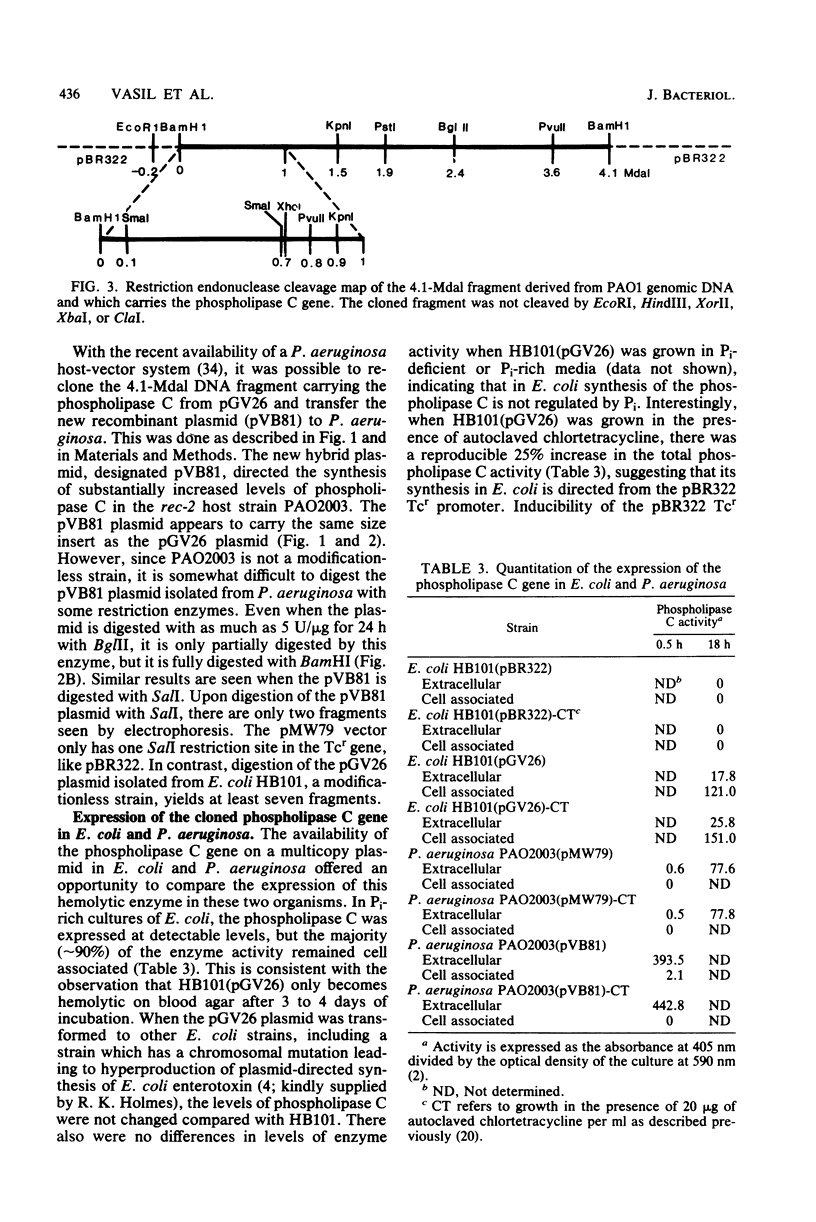
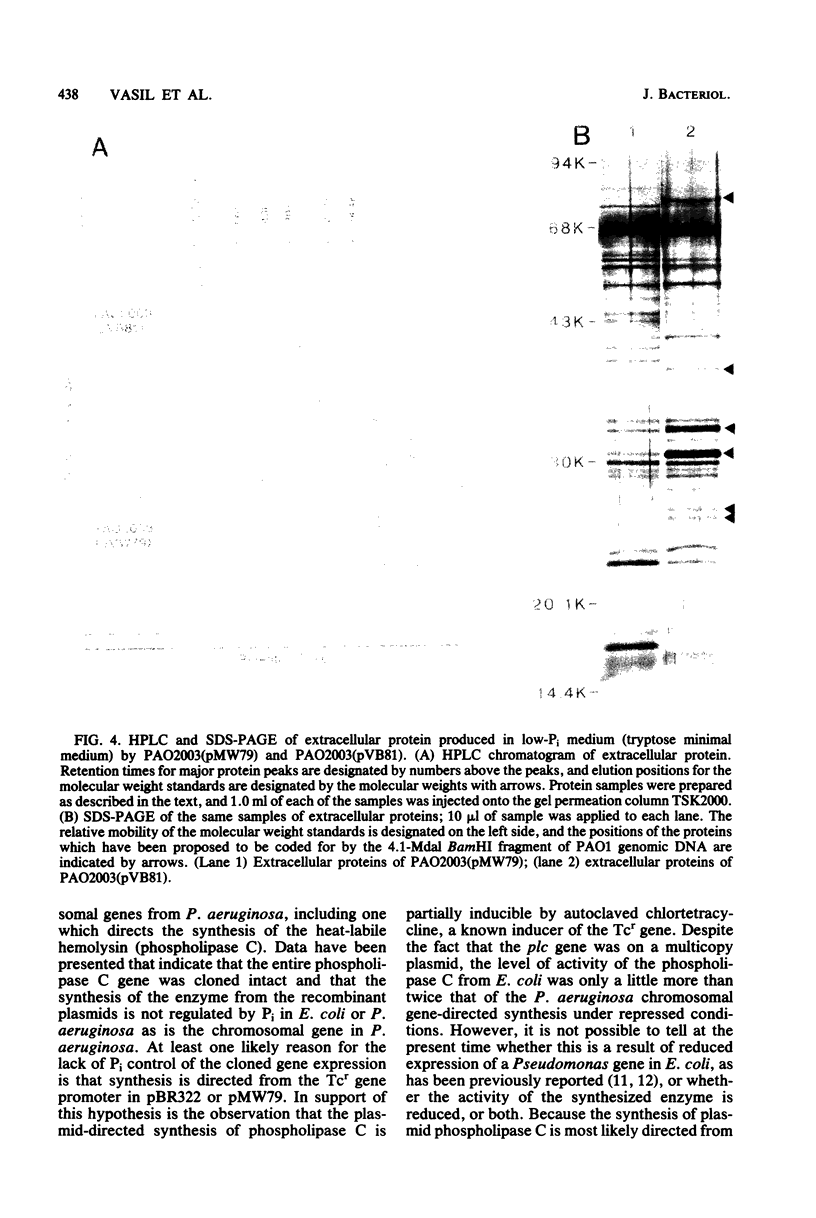
Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa is a phosphate (Pi)-regulated extracellular protein which may be a significant virulence factor of this organism. The gene for this hemolytic enzyme was cloned on a 4.1-megadalton (Mdal) fragment from a BamHI digest of P. aeruginosa PAO1 genomic DNA and was inserted into the BamHI sites of the multicopy Escherichia coli(pBR322) and P. aeruginosa(pMW79) vectors. The E. coli and P. aeruginosa recombinant plasmids were designated pGV26 and pVB81, respectively. A restriction map of the 4.1-Mdal fragment from pGV26 was constructed, using double and single digestions with BamHI and EcoRI and several different restriction enzymes. Based on information from this map, a 2.4-Mdal BamHI/BglII fragment containing the gene for phospholipase C was subcloned to pBR322. The hybrid plasmids pGV26 and pVB81 direct the synthesis of enzymatically active phospholipase C, which is also hemolytic. The plasmid-directed synthesis of phospholipase C in E. coli or P. aeruginosa is not repressible by Pi as is the chromosomally directed synthesis in P. aeruginosa. Data are presented which suggest that the synthesis of phospholipase C from pGV26 and pVB81 is directed from the tetracycline resistance gene promoter. The level of enzyme activity produced by E. coli(pGV26) is slightly higher than the levels produced by P. aeruginosa(pMW79) under repressed conditions. In contrast, the levels produced by P. aeruginosa(pVB81) are at least 600-fold higher than the levels produced by P. aeruginosa(pMW79) under repressed conditions and approximately 20-fold higher than those produced by P. aeruginosa(pMW79) under derepressed conditions. The majority (85%) of the enzyme produced by E. coli(pGV26) remained cell associated, whereas >95% of the enzyme produced by P. aeruginosa(pVB81) was extracellular. Analysis of extracellular proteins from cultures of P. aeruginosa(pMW79) and P. aeruginosa(pVB81) by high-performance liquid chromotography and sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed that the phospholipase C gene was cloned intact, and it is likely that several additional genes were cloned on the 4.1-Mdal fragment of DNA. It was also found that some of these genes encode proteins which are the same molecular weight as some previously described Pi-repressible proteins of P. aeruginosa. The existence of a Pi regulon of P. aeruginosa is proposed. It is likely that one of these genes also regulates the level of pyocyanin production by P. aeruginosa and that one or more play a role in transport or binding of Pi. The availability of the hybrid plasmids described herein will be useful in further studies on the role of this hemolysin in the virulence of P. aeruginosa and in the study of the genetics and physiology of Pi-regulated proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Vasil M. L. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol. 1982 Oct;152(1):239–245. doi: 10.1128/jb.152.1.239-245.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bramucci M. G., Twiddy E. M., Baine W. B., Holmes R. K. Isolation and characterization of hypertoxinogenic (htx) mutants of Escherichia coli KL320(pCG86). Infect Immun. 1981 Jun;32(3):1034–1044. doi: 10.1128/iai.32.3.1034-1044.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr, Bass J. A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Höhne C., Noble M. A., Haldane E. V., Lior H., Young L. S. Hemolysin and K antigens in relation to serotype and hemagglutination type of Escherichia coli isolated from extraintestinal infections. J Clin Microbiol. 1981 Jan;13(1):171–178. doi: 10.1128/jcm.13.1.171-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. A Pseudomonas aeruginosa mutant non-derepressible for orthophosphate-regulated proteins. J Bacteriol. 1981 Aug;147(2):675–678. doi: 10.1128/jb.147.2.675-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. Phospholipase C regulatory mutation of Pseudomonas aeruginosa that results in constitutive synthesis of several phosphate-repressible proteins. J Bacteriol. 1982 Jun;150(3):1221–1226. doi: 10.1128/jb.150.3.1221-1226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Vasil M. L. Isolation and genetic characterization of toxin-deficient mutants of Pseudomonas aeruginosa PAO. J Bacteriol. 1981 Aug;147(2):275–281. doi: 10.1128/jb.147.2.275-281.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E., Crawford I. P. Wide ranging plasmid bearing the Pseudomonas aeruginosa tryptophan synthase genes. Nature. 1977 May 19;267(5608):283–284. doi: 10.1038/267283a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ingledew W. M., Campbell J. J. A new resuspension medium for pyocyanine production. Can J Microbiol. 1969 Jun;15(6):595–598. doi: 10.1139/m69-101. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immun. 1980 Sep;29(3):1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol. 1967 Feb;93(2):670–674. doi: 10.1128/jb.93.2.670-674.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni J. C., Portalier R. C. Genetic and biochemical characterization of periplasmic-leaky mutants of Escherichia coli K-12. J Bacteriol. 1981 Mar;145(3):1351–1358. doi: 10.1128/jb.145.3.1351-1358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaroni J. C., Portalier R. C. Genetic and biochemical characterization of periplasmic-leaky mutants of Escherichia coli K-12. J Bacteriol. 1981 Mar;145(3):1351–1358. doi: 10.1128/jb.145.3.1351-1358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Jorgensen J., Counts G. W., Falkow S. Association of hemolysin production, hemagglutination of human erythrocytes, and virulence for chicken embryos of extraintestinal Escherichia coli isolates. Infect Immun. 1978 Apr;20(1):50–54. doi: 10.1128/iai.20.1.50-54.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Obara Y., Nikkawa T., Yamai S., Kato T., Yamada Y., Ohashi M. Simplified purification and biophysicochemical characteristics of Kanagawa phenomenon-associated hemolysin of Vibrio parahaemolyticus. Infect Immun. 1980 May;28(2):567–576. doi: 10.1128/iai.28.2.567-576.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Petit J. C., Sicard D., Bayo S., Daguet G. L. Effect of active and passive immunization on the development of experimental Pseudomonas aeruginosa pyelonephritis in mice. Can J Microbiol. 1981 Jan;27(1):93–97. doi: 10.1139/m81-015. [DOI] [PubMed] [Google Scholar]
- Riddle L. M., Graham T. E., Amborski R. L. Medium for the accumulation of extracellular hemolysin and protease by Aeromonas hydrophila. Infect Immun. 1981 Sep;33(3):728–733. doi: 10.1128/iai.33.3.728-733.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. M., Jr, Mays B. B., Pierce A. K., Sanford J. P. Pulmonary clearance of Pseudomonas aeruginosa. J Lab Clin Med. 1970 Oct;76(4):548–559. [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Latterell P. Mutants affected in alkaline phosphatase, expression: evidence for multiple positive regulators of the phosphate regulon in Escherichia coli. Genetics. 1980 Oct;96(2):353–366. doi: 10.1093/genetics/96.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Wood D. O., Hollinger M. F., Tindol M. B. Versatile cloning vector for Pseudomonas aeruginosa. J Bacteriol. 1981 Mar;145(3):1448–1451. doi: 10.1128/jb.145.3.1448-1451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretlind B., Möllby R., Wadström T. Separation of two hemolysins from Aeromonas hydrophila by isoelectric focusing. Infect Immun. 1971 Oct;4(4):503–505. doi: 10.1128/iai.4.4.503-505.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. The role of exotoxins in the pathogenesis of Pseudomonas aeruginosa infections. J Infect Dis. 1980 Oct;142(4):626–630. doi: 10.1093/infdis/142.4.626. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H. Pseudomonas aeruginosa vasculitis and bacteremia following conjunctivitis: a simple model of fatal pseudomonas infection in neutropenia. J Infect Dis. 1979 Mar;139(3):288–296. doi: 10.1093/infdis/139.3.288. [DOI] [PubMed] [Google Scholar]





