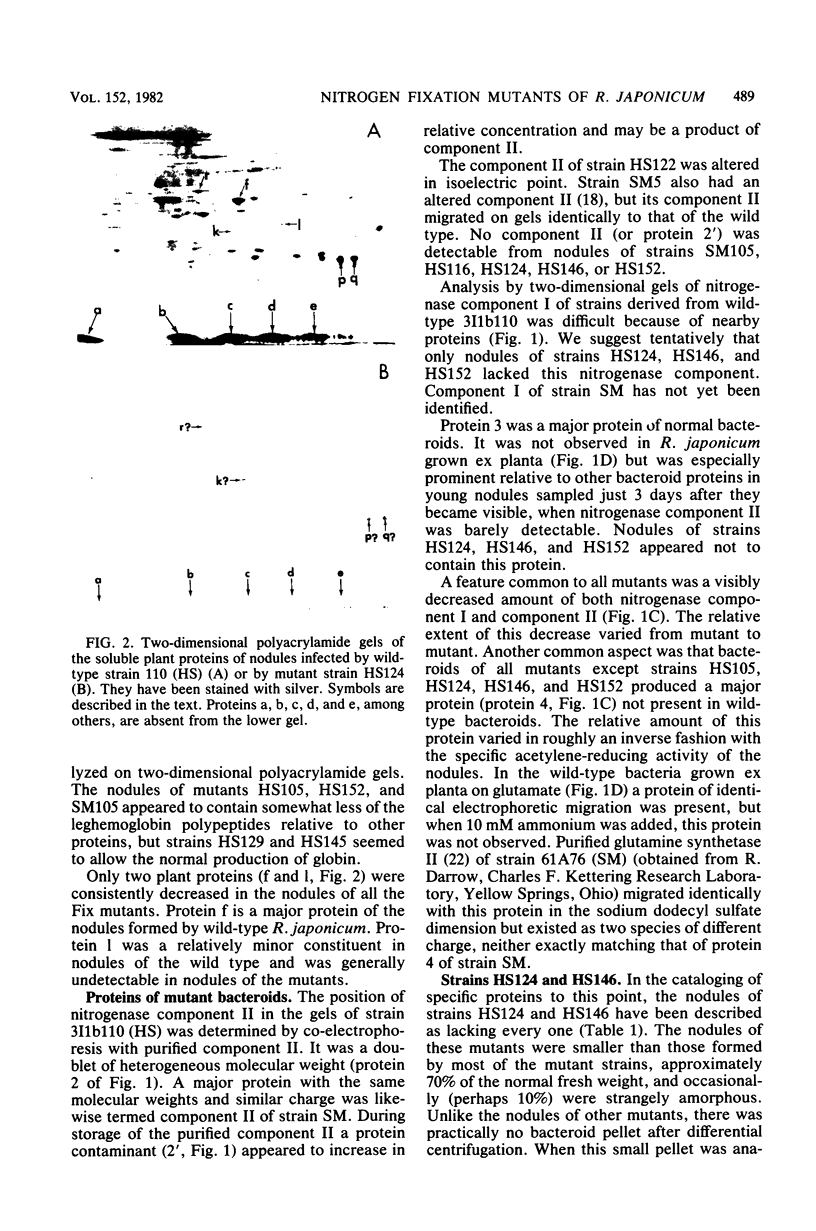
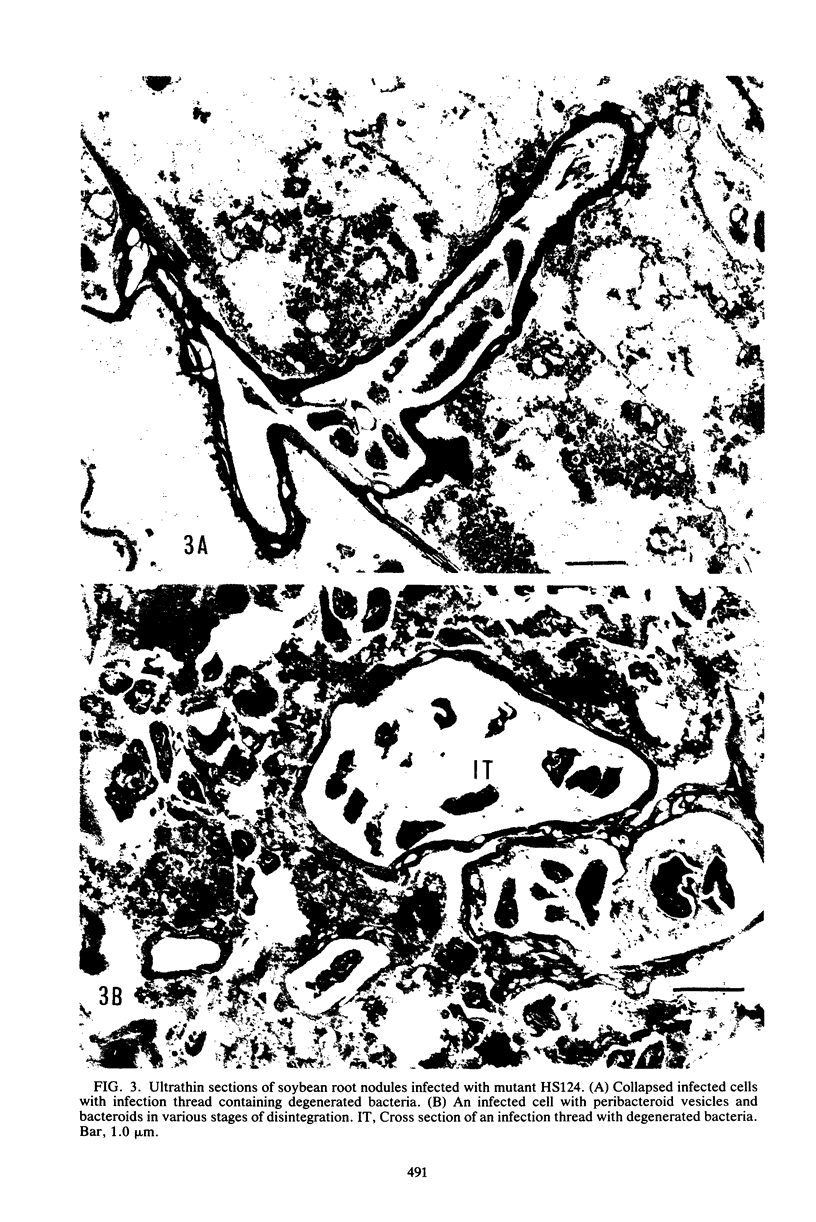
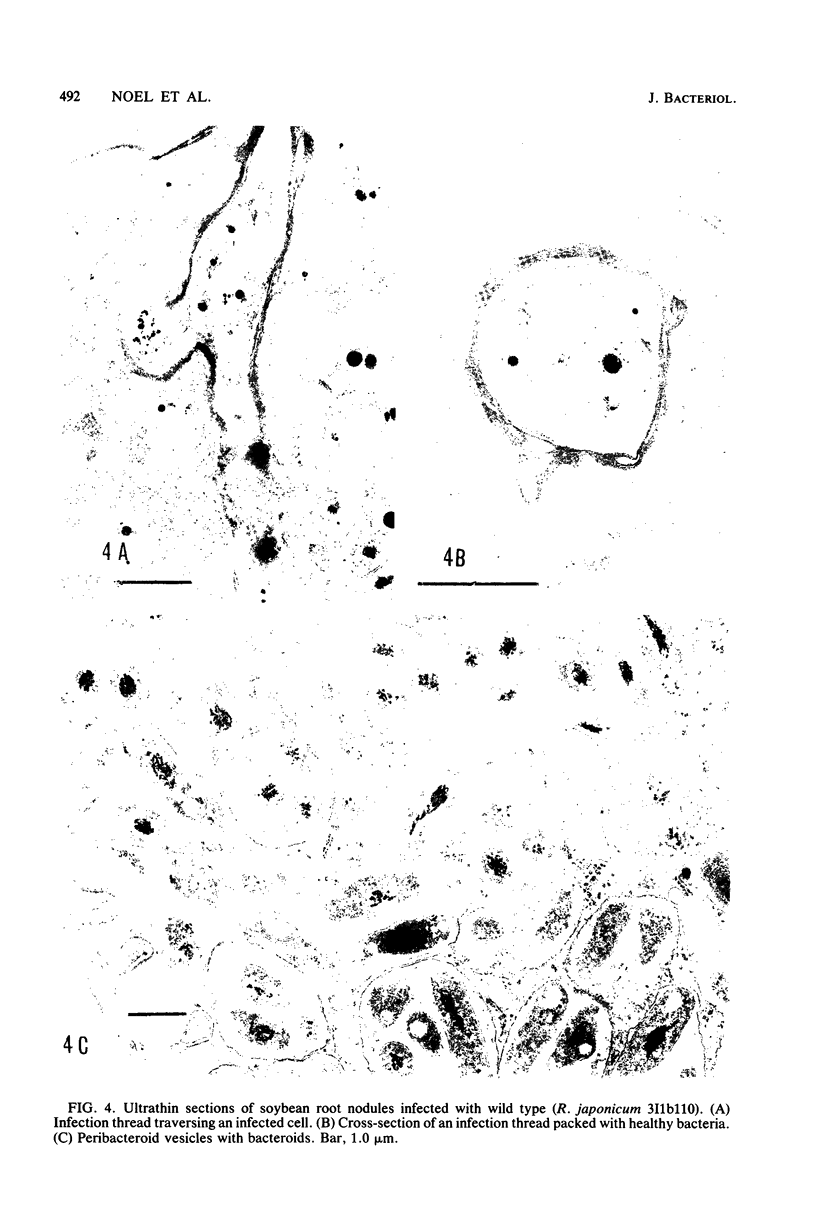
Abstract
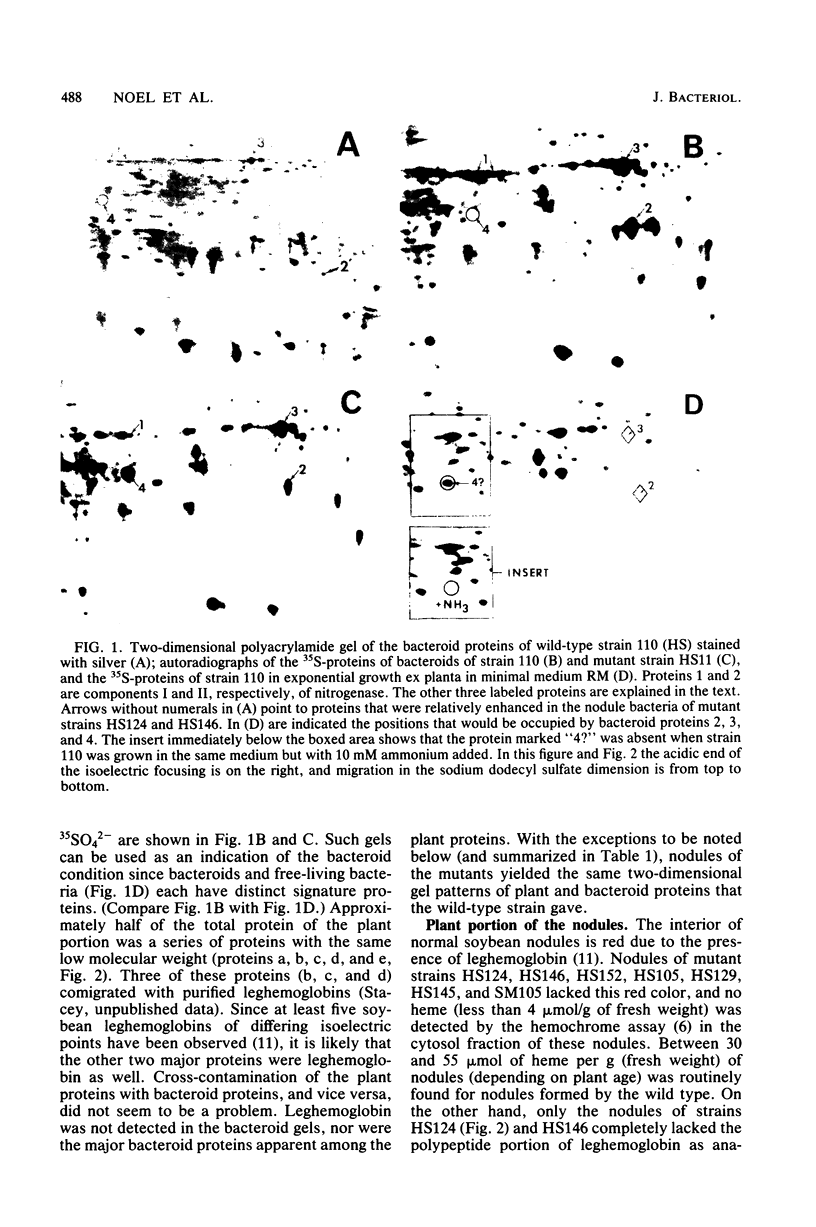
Rhizobium japonicum strains 3I1b110 and 61A76 were mutagenized to obtain 25 independently derived mutants that produced soybean nodules defective in nitrogen fixation, as assayed by acetylene reduction. The proteins of both the bacterial and the plant portions of the nodules were analyzed by two-dimensional polyacrylamide gel electrophoresis. All of the mutants had lower-than-normal levels of the nitrogenase components, and all but four contained a prominent bacteroid protein not observed in wild-type bacteroids. Experiments with bacteria grown ex planta suggested that this protein was derepressed by the absence of ammonia. Nitrogenase component II of one mutant was altered in isoelectric point. The soluble plant fraction of the nodules of seven mutants had very low levels of heme, yet the nodules of five of these seven mutants contained the polypeptide of leghemoglobin. Thus, the synthesis of the globin may not be coupled to the content of available heme in soybean nodules. The nodules of the other two of these seven mutants lacked not only leghemoglobin but most of the other normal plant and bacteroid proteins. Ultrastructural examination of nodules formed by these two mutants indicated normal ramification of infection threads but suggested a problem in subsequent survival of the bacteria and their release from the infection threads.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., van den Bos R. C., van Kammen A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim Biophys Acta. 1978 Feb 13;539(1):1–11. doi: 10.1016/0304-4165(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S. Isolation of bacteria, transforming bacteria, and bacteroids from soybean nodules. Plant Physiol. 1977 Nov;60(5):771–774. doi: 10.1104/pp.60.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A. Regulation of Rhizobium nitrogen fixation by the unadenylylated glutamine synthetase I system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5817–5821. doi: 10.1073/pnas.77.10.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rao V. R., Darrow R. A., Keister D. L. Effect of oxygen tension on nitrogenase and on glutamine synthetases I and II in Rhizobium jaonicum 61A76. Biochem Biophys Res Commun. 1978 Mar 15;81(1):224–231. doi: 10.1016/0006-291x(78)91653-4. [DOI] [PubMed] [Google Scholar]