Abstract
Background
Endometrial cancer is the most common female gynecologic cancer in the United States. Excessive and prolonged exposure of the endometrium to estrogens unopposed by progesterone and a high body mass are well-established risk factors for endometrial cancer. Although dietary fiber has been shown to beneficially reduce estrogen concentrations and prevent obesity, its role in endometrial cancer has received relatively little attention.
Objective
The objective was to summarize and quantify the current evidence of a role of dietary fiber consumption in endometrial cancer risk and to identify research gaps in this field.
Design
We conducted a systematic literature review of articles published through February 2007 to summarize the current evidence of a relation between dietary fiber consumption and endometrial cancer risk and to quantify the magnitude of the association by conducting a dose-response meta-analysis.
Results
Ten articles representing 1 case-cohort study and 9 case-control studies that evaluated several aspects of fiber consumption and endometrial cancer risk were identified through searches in various databases. On the basis of 7 case-control studies, the random-effects summary risk estimate was 0.82 (95% CI: 0.75, 0.90) per 5 g/1000 kcal dietary fiber, with no evidence of heterogeneity (I2: 0%, P for heterogeneity: 0.55). The random-effects summary estimate was 0.71 (95% CI: 0.59, 0.85) for the comparison of the highest with the lowest dietary fiber intake in 8 case-control studies, with little evidence of heterogeneity (I2: 20.8%, P for heterogeneity: 0.26). In contrast, the only prospective study that evaluated this association did not find an association.
Conclusions
Although the current evidence, based on data from case-control studies, supports an inverse association between dietary fiber and endometrial cancer, additional population-based studies, particularly cohort studies, are needed before definitive conclusions can be drawn.
Keywords: Endometrial carcinoma, diet, fiber, meta, analysis, systematic literature review
INTRODUCTION
Endometrial cancer is the most common female gynecologic cancer in the United States, ranking fourth among all cancers in women in age-adjusted incidence (1). Excess body mass and excessive and prolonged exposure to estrogens unopposed by progesterone are well-established and strong risk factors for endometrial cancer (2). Dietary fiber has been shown to influence estrogen absorption, metabolism, and bioavailability (3) and weight loss (4). Thus, it is reasonable to expect an inverse association of dietary fiber intake with endometrial cancer. However, surprisingly, the relation between fiber intake and endometrial cancer risk has received relatively little attention.
In support of the Second World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) Report on Food, Nutrition, Physical Activity and the Prevention of Cancer (Internet: www.wcrf.org), and commissioned by the WCRF, we conducted a systematic and comprehensive literature review of diet, nutrition, physical activity, and endometrial cancer (5) to enhance and update the previous 1997 report (6). In the 1997 report, the possible role of fiber on endometrial cancer was not mentioned. In fact, to our knowledge, this is the first systematic literature review and meta-analysis on this topic. The objective of this article was to summarize and quantify the current evidence for a role of dietary fiber consumption on endometrial cancer risk and to identify research gaps in this field.
METHODS
In general, we followed the WCRF Specification Manual, available online at www.wcrf.org. The methods used in this article diverge from the WCRF instructions in that we followed our own criteria for inclusion of studies and used our own methods for data tabulation and analysis and interpretation of the evidence. Although WCRF required the inclusion of all studies regardless of quality, this systematic review and meta-analysis was limited to case-control and cohort studies. Randomized trials would have been included, but none were found. Ecologic and cross-sectional studies were excluded. For the purposes of this study, we conducted additional analyses, such as sensitivity analyses to evaluate the impact of excluding studies that did not meet certain a priori criteria (ie, population-based studies with >200 cases, known hysterectomy status among controls, and adjustment for total energy intake and body mass). Interpretation of the evidence does not necessarily represent the views of WCRF.
Search strategy
Searches were conducted in July 2003, October 2004, and December 2005. The databases searched included Medline, ISI Web, Embase, Biosis, Ingenta, CINAHL, Science Direct, LILACS, Pascal, ExtraMed, and Allied CompMEd. On the basis of results from the 2003 searches, we excluded databases that did not produce any new results in subsequent searches. Bibliographic searches were complemented with manual searches of references in published articles. Translations were provided by WCRF when necessary. For the purposes of this study we also monitored the literature using PubMed Alerts for all new papers on endometrial cancer from January 2006 through February 2007.
Exposure terms for PubMed were provided by the WCRF and can be found in the Specification Manual and in the Appendix of another article (7). General terms included diet[tiab] OR diets-[tiab] OR dietetic[tiab] OR dietary[tiab] OR eating[tiab] OR intake[tiab] OR nutrient*[tiab] OR nutrition[tiab] OR vegetarian*[tiab] OR vegan*[tiab] OR “seventh day adventist-”[tiab] OR macrobiotic[tiab] OR food and beverages[MeSH Terms]. Specifically for dietary fiber, terms included fiber[tiab] OR fiber[tiab] OR polysaccharide*[tiab].
Per WCRF instructions, our searches included endometrial hyperplasia because this includes precancerous lesions. However, we only found a few papers evaluating the role of diet and nutrition on endometrial hyperplasia, and none of them evaluated dietary fiber. Outcomes terms included: 1) endometrial neoplasm [MeSH]; 2) malign* [tiab] OR cancer*[tiab] OR carcinoma*[tiab] OR tumor*[tiab] OR tumor*[tiab]; 3) endometr* [tiab] OR corpus uteri [tiab] OR uterine [tiab]; 4): #2 AND #3; and 5): #3 AND hyperplasia [tiab].
Article selection and data extraction
Overall search results and the criteria for selection were described elsewhere (8). In brief, citations identified from these searches were reviewed independently by 2 of us (LHK and EVB) for relevance. For the overall systematic literature review, we included peer-reviewed manuscripts containing original data in any language that evaluated the relation between any aspect of diet, nutrition, physical activity (ie, what we classified as “relevant exposures” for the overall systematic literature review) and endometrial cancer or endometrial hyperplasia published through June 2006. Studies that evaluated the influence of these factors on survival after cancer were excluded. Following WCRF instructions, no article was excluded on the basis of quality, except when the data provided were not useful (eg, only P values were presented for a given relevant factor). For the overall review we identified 522 possible relevant citations. Five articles could not be located but they did not appear to contain primary data. Of the remaining 517 articles, we included 285 and excluded 232. Reasons for excluding manuscripts after a full review included the following: published only as an abstract (n = 5), case series (n = 76), no relevant exposure data (n = 83), no relevant outcome (n = 12), survival data only (n = 1), combined outcomes (eg, hyperplasia and cancer) (n = 3), non-peer reviewed publication (n = 9), reviews (n = 39), and no useful data, such as only P values (n = 4).
Of the 285 included papers, 9 mentioned dietary fiber (9–17) and all were written in English. Through monitoring the endometrial cancer literature through February 2007 using PubMed Alerts for “endometrial cancer,” one additional article was identified (18).
Data on study characteristics and results were extracted by trained research personnel using an Access program developed by Leeds University under WCRF sponsorship. Each entry was reviewed by at least one of us.
Assessment of study quality and sensitivity analyses
We decided not to grade individual articles according to a quality score for 2 reasons: 1) no widely accepted tool for quality assessment of epidemiologic studies is available, and 2) we had few articles in which dietary fiber was evaluated, which limited these types of analyses. Instead, we present evidence from all case-control studies that examined fiber intake and then repeated certain analyses, excluding studies that did not meet certain a priori quality criteria. In the forest plots, studies excluded are marked and the specific reasons for exclusion are indicated in the footnotes. The a priori quality criteria were as follows: 1) population-based studies as the appropriateness of hospital controls in diet and cancer studies is controversial, 2) sample size of ≥200 cases for more optimal statistical power, 3) exclusion of hysterectomies from the control group, and 4) adjustment for important confounders such as total energy and body mass.
Statistical analysis
To conduct dose-response meta-analyses, published results were transformed into a common scale. For example, results related to dietary fiber were reported as units g/d or g · 1000 kcal−1 ·d−1. The average daily energy intake from study to study varied considerably, and much of this variance was probably attributable to dietary assessment methods rather than to true differences in food intake. For example, the median daily energy intake in the study by Potischman et al (11) was 1248 kcal, whereas in the study by McCann et al (13) it was 2102 kcal. Both of these studies were conducted in the United States, which made it unlikely that cultural or lifestyle differences were a major contributor to these differences in reported intake. Thus, for dose-response meta-analyses, we elected to convert reported intakes into nutrient density measures expressed as g/1000 kcal. To convert reported category intakes or medians into nutrient density measures, it was necessary to have a reported value for median (or mean) kcal/d intake for the study population; this was available in all studies, except for one hospital-based study (14).
For studies reporting only categorical analyses, an estimate of mean intake for each category was computed following the methodology developed by Chêne and Thompson (19). The iterative method described in Greenland and Longnecker (20) was used to estimate a single logistic regression parameter per study. This method imputes expected numbers of cases and controls (or cases for a prospective study) and computes the logistic regression slope parameter (which may be interpreted as the log relative risk) and SE. Finally, we estimated fixed-effects and random-effects pooled logistic regression coefficients across studies by study design. We used the random-effects models in forest plots and for interpretation of the evidence, because it uses a combination of within-study variance and between-study variance for computing weights. The Chêne and Thompson (19) and Greenland and Longnecker (20) algorithms described above were implemented in the statistical language R (R: A Language and Environment for Statistical Computing, version 2.4.1, 2006; R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria). Fixed- and random-effects pooled estimates and forest plots were produced by using the “metan” package of STATA/SE version 8.2 (StataCorp LP, College Station, TX). Heterogeneity was assessed by conducting Q tests (ie, testing for the presence or absence of heterogeneity) and quantifying the degree of heterogeneity by estimating the I2 index (21).
RESULTS
Our systematic literature review included 10 articles representing 1 cohort study and 9 case-control studies that evaluated various aspects of fiber intake (9–18). In Table 1, the characteristics of these studies and the variables evaluated are listed. As shown in the table, these studies were conducted in several countries and varied considerably concerning the quality of dietary assessment and in the evaluation and classification of dietary fiber. The case-control study by Salazar-Martinez et al (14) was not included in the dose-response meta-analyses because it did not present an estimate of total energy intake; therefore, transformation into a nutrient density measure was not possible.
TABLE 1.
Characteristics of observational studies that evaluated dietary fiber and endometrial cancer risk1
| Reference | Country | Cases/controls or cohort size | Age | Dietary assessment | Time frame of dietary assessment | Variables evaluated |
|---|---|---|---|---|---|---|
| Case-cohort study | ||||||
| Jain et al, 2000 (9) | Canada | 221/3697 | 40–59 y | FFQ (86 items) | 1 mo prior | Dietary fiber, insoluble fiber, soluble fiber, cereal fiber, fruit fiber, vegetable fiber |
| Population-based case-control studies | ||||||
| Shu et al, 1993 (17) | China | 268/268 | 18–74 y | FFQ (63 items) | 10 y | Crude fiber |
| Potischman et al, 1993 (11) | United States | 399/296 | 20–74 y | FFQ (Block, 60 items) | Past few years | Fiber |
| Goodman et al, 1997 (12) | United States | 332/511 | 18–84 y | Dietary history (250 items) | 1 y | Crude fiber, nonstarch polysaccharides, cellulose, noncellulosic polysaccharides, dietary fiber, cereal fiber, vegetable fiber, fruit fiber |
| Jain et al, 2000 (15) | Canada | 552/562 | 30–79 y | Dietary history (unknown items) | 1 y | Dietary fiber, insoluble fiber, cereal fiber, fruit fiber, vegetable fiber |
| McCann et al, 2000 (13) | United States | 232/639 | 40–85 y | FFQ (172 items) | 2 y | Dietary fiber |
| Littman et al, 2001 (16) | United States | 679/944 | 45–74 y | FFQ (modified Block, 98 items) | 5 y | Total fiber |
| Xu et al, 2007 (18) | China | 1204/1212 | 30–69 y | FFQ (71 foods) | 5 y | Dietary fiber |
| Hospital-based case-control studies | ||||||
| Barbone et al, 1993 (10) | United States | 103/236 | FFQ (Willett, 116 items) | 1 y | Dietary fiber | |
| Salazar-Martinez et al, 2005 (14) | Mexico | 85/629 | 18–81 y | FFQ (116 items) | 1 y | Dietary fiber |
FFQ, food-frequency questionnaire.
As shown in Table 2, 7 (10–13, 15, 16, 18) of the 8 case-control studies that evaluated dietary fiber and endometrial cancer risk suggested an inverse relation, with odds ratios ranging from 0.5 to 0.71 for comparisons of the highest with the lowest category of intake. In contrast, a small hospital-based case-control study in Mexico (14) and a case-cohort study in Canada (9) reported risk estimates >1, but the CIs included 1.
TABLE 2.
Studies that evaluated fiber intake and endometrial cancer risk1
| Covariates considered2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Age | Cases/controls or total cohort |
Exposure evaluated |
Contrast | RR/OR | 95% CI | P for trend | A | B | E | S | H | R |
| Case-cohort study | ||||||||||||||
| Jain et al, 2000 (9) | Canada | 40–59 y | 221/3697 | Dietary fiber | >23.2 vs <15.1 g/d | 1.24 | (0.82, 1.87) | NS | 1 | 1 | 1 | 1 | 1 | 2 |
| Population-based case-control studies | ||||||||||||||
| Potischman et al, 1993 (11) | United States | 20–74 y | 399/296 | Dietary fiber | >13.6 vs <7.7 g/d | 0.7 | (0.4, 1.3) | 1 | 1 | –3 | 1 | 1 | 1 | |
| Goodman et al, 1997 (12) | United States | 18–84 y | 332/511 | Dietary fiber | >23.9 vs <12.3 g/d | 0.47 | (0.25, 0.86) | 0.02 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al, 2000 (15) | Canada | 30–79 y | 552/562 | Dietary fiber | >27.5 vs <17.2 g/d | 0.71 | (0.49, 1.03) | 0.14 | 1 | 1 | 1 | 1 | 1 | 2 |
| Per 10 g | 0.89 | (0.75, 1.05) | 1 | 1 | 1 | 1 | 1 | 2 | ||||||
| McCann et al, 2000 (13) | United States | 40–85 y | 232/639 | Dietary fiber | >32 vs <20 g/d | 0.5 | (0.3, 1) | 0.002 | 1 | 1 | 1 | 1 | 1 | 3 |
| Littman et al, 2001 (16) | United States | 45–74 y | 679/944 | Total fiber | >10.7 vs <5.6 g · 1000 kcal−1 · d−1 | 0.68 | (0.47, 0.99) | 0.16 | 1 | 1 | 1 | 1 | 1 | |
| Xu et al, 2007 (18) | China | 30–69 y | 1204/1212 | Dietary fiber | 8.1 vs 4.8 g · 1000 kcal−1 ·d−1 | 0.8 | (0.6, 1.0) | 1 | 1 | 1 | 1 | |||
| Hospital-based case-control studies | ||||||||||||||
| Barbone et al, 1993 (10) | United States | 103/236 | Dietary fiber | >21.5 vs <16.6 g/d | 0.6 | (0.3, 1.1) | 0.43 | 1 | 1 | 1 | 1 | 1 | 3 | |
| Salazar-Martinez et al, 2005 (14) | Mexico | 18–81 y | 85/629 | Dietary fiber | >24 vs <13 g/d | 1.46 | (0.76, 2.79) | 0.33 | 1 | 1 | 1 | 1 | ||
OR, odds ratio; RR, relative risk; NS, P > 0.05.
The numbers in each column refer to the number of covariates adjusted for under that grouping. A, age; B, BMI/weight; E, total energy; S, smoking; H, hormone replacement therapy or estrogen replacement therapy; R, reproductive factors; (1), matched for age.
Adjusted for noncarbohydrate calories.
Other fiber-related exposures examined in case-control studies of endometrial cancer included crude fiber (12, 17), soluble and insoluble fiber (9), cellulose and noncellulosic polysaccharides (12), and fiber categorized by food group: cereal or grain fiber, fruit fiber, and vegetable fiber (9, 12, 15). Jain et al (9) examined the role of soluble and insoluble fiber in a case-cohort study and found limited evidence of an association. Insoluble fiber was also unrelated to endometrial cancer risk in a population-based case-control study conducted by the same group (15). Goodman et al (12) conducted the most thorough evaluation of fiber subtypes and found similar results for cellulose (adjusted OR: 0.59; 95% CI: 0.32, 1.07) and noncellulosic polysaccharides (OR: 0.62; 95% CI: 0.34, 1.15). Other results for fiber subtypes are shown in Table 3. Two studies evaluated crude fiber, one study found a suggestion of an inverse association (12), whereas no evidence of an association was found in the other study (17). Fiber intake by food source was evaluated in 4 studies, with inconsistent results. Goodman et al (12). found a stronger relation for fruit and cereal fiber intakes than for vegetable fiber. In contrast, Jain et al (15) found a relation only for vegetable fiber (Table 3). Littman et al (16) found an association only from fiber from fruit and vegetables combined (OR: 0.67; 95% CI: 0.47, 0.96), whereas there was no association with fiber from grains or fiber from beans (data not shown). As shown in Table 3, the case-cohort study (9) failed to find an association for any of these fiber subtypes.
TABLE 3.
Studies that evaluated fiber subtypes and endometrial cancer risk1
| Covariates considered2 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Age | Cases/controls or total cohort | Type of study | Exposure | Contrast | RR/OR (95% CI) | P for trend | A | B | E | S | H | R |
| Crude fiber | ||||||||||||||
| Shu et al, 1993 (17) | China | 18–74 y | 268/268 | Population-based CC | Crude fiber | >4.68 vs <2.65 g/d | 1.1 | 0.7 | 1 | 1 | 1 | 1 | ||
| Goodman et al, 1997 (12) | United States | 18–84 y | 332/511 | Population-based CC | Crude fiber | >6.04 vs <3.01 g/d | 0.6 (0.33, 1.09) | 0.31 | (1) | 1 | 1 | 1 | 1 | |
| Cereal fiber | ||||||||||||||
| Jain et al, 2000 (9) | Canada | 40–59 y | 221/3697 | Case cohort | Cereal fiber | >4.8 vs <2.7 g/d | 1.07 (0.73, 1.58) | 1 | 1 | 1 | 1 | 1 | 2 | |
| Goodman et al, 1997 (12) | United States | 18–84 y | 332/511 | Population-based CC | Cereal fiber | >2.51 vs <0.01 g/d | 0.55 (0.33, 0.92) | 0.001 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al, 2000 (15) | Canada | 30–79 y | 552/562 | Population based CC | Cereal fiber | >10.5 vs <4.8 g/d | 1.03 (0.72, 1.47) | 0.83 | 1 | 1 | 1 | 1 | 1 | 2 |
| Vegetable fiber | ||||||||||||||
| Jain et al, 2000 (9) | Canada | 40–59 y | 221/3697 | Case cohort | Vegetable fiber | >9.5 vs <5.5 g/d | 0.9 (0.61, 1.34) | 1 | 1 | 1 | 1 | 1 | 2 | |
| Goodman et al, 1997 (12) | United States | 18–84 y | 332/511 | Population-based CC | Vegetable fiber | >6.25 vs <2.94 g/d | 0.71 (0.4, 1.23) | 0.22 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al, 2000 (15) | Canada | 30–79 y | 552/562 | Population based CC | Vegetable fiber | >12.8 vs <6.6 g/d | 0.64 (0.44, 0.91) | 0.02 | 1 | 1 | 1 | 1 | 1 | 2 |
| Per 6.2 g | 0.87 (0.75, 1.01) | 1 | 1 | 1 | 1 | 1 | 2 | |||||||
| Fruit fiber | ||||||||||||||
| Jain et al, 2000 (9) | Canada | 40–59 y | 221/3697 | Case cohort | Fruit fiber | >5.7 vs <2.2 g/d | 1.08 (0.73, 1.61) | 1 | 1 | 1 | 1 | 1 | 2 | |
| Goodman et al, 1997 (12) | United States | 18–84 y | 332/511 | Population-based CC | Fruit fiber | >6.27 vs <2.21 g/d | 0.54 (0.32, 0.92) | 0.22 | (1) | 1 | 1 | 1 | 1 | |
| Jain et al, 2000 (15) | Canada | 30–79 y | 552/562 | Population based CC | Fruit fiber | >8.9 vs <3.4 g/d | 1.34 (0.92, 1.95) | 0.31 | 1 | 1 | 1 | 1 | 1 | 2 |
| Per 5.5 g | 1.0 (0.86, 1.18) | 1 | 1 | 1 | 1 | 1 | 2 | |||||||
OR, odds ratio; RR, relative risk; CC, case-control.
The numbers in each column refer to the number of covariates adjusted for under that grouping. A, age; B, BMI/weight; E, total energy; S, smoking; H, hormone replacement therapy or estrogen replacement therapy; R, reproductive factors; (1), matched for age.
Meta-analysis
The data only allowed for a meta-analysis of total dietary fiber, because there were only a few studies that evaluated fiber subtypes, and the results tended to be inconsistent. As shown in Figure 1, 7 case-control studies were included in the dose-response meta-analyses (10–13, 15, 16, 18). A continuous relative risk for the same increment was also computed for the case-cohort study for comparison purposes. The study by Salazar-Martinez et al (14) was not included in the dose-response meta-analyses because it did not present an estimate of total energy intake and, therefore, transformation into a nutrient density measure was not possible. There was no evidence of heterogeneity among case-control studies (I2: 0.0%, P for heterogeneity: 0.55). On the basis of data from case-control studies, we estimated an 18% reduction in endometrial cancer risk per 5 g/1000 kcal total fiber intake (random- and fixed-effects pooled OR: 0.82; 95% CI: 0.75, 0.90). In contrast, the case-cohort study showed no association, with a derived RR of 1.15 (95% CI: 0.89, 1.49) per 5 g/1000 kcal fiber intake. Exclusion of the one case-control study that did not meet our quality criteria (10) essentially did not change results. A forest plot showing results of the comparison between the highest and lowest categories of fiber intake is shown in Figure 2. There was little evidence of heterogeneity among case-control studies, and, overall, we estimated a reduction of ≈30% in endometrial cancer risk associated with high fiber intake. However, as mentioned earlier, the only prospective study that evaluated this association failed to find an association.
FIGURE 1.
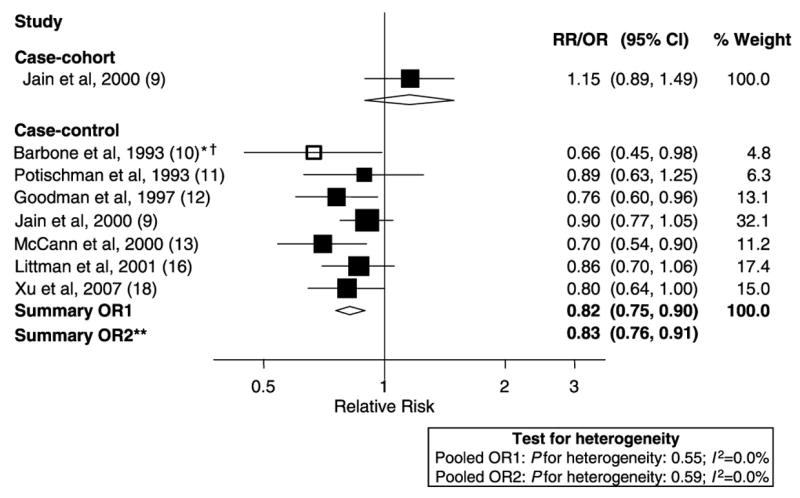
Random-effects meta-analysis of studies that evaluated dietary fiber and endometrial cancer risk (per 5 g/1000 kcal). All studies excluded hysterectomies from the control group and adjusted for BMI (weight) and total energy intake [Potischman et al (11), adjusted for noncarbohydrate calories]. **Excluded studies for the following reasons: *hospital based, †<200 cases. OR, odds ratio; RR, relative risk.
FIGURE 2.
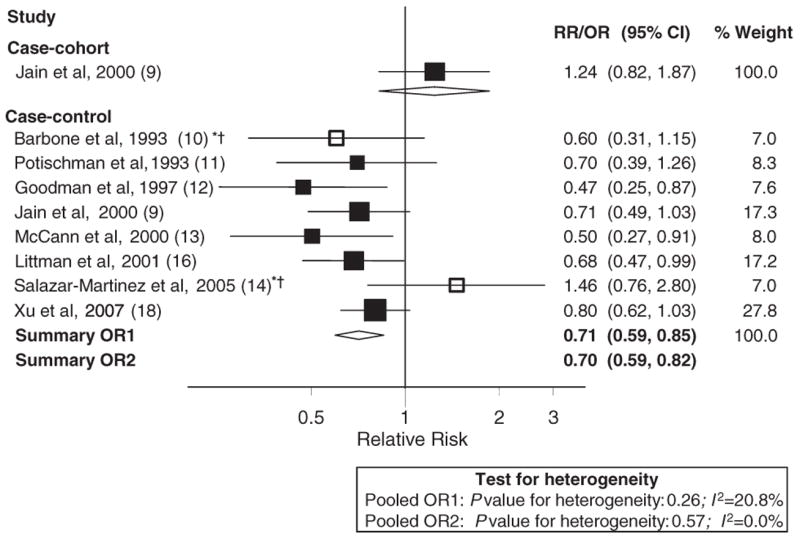
Random-effects meta-analysis of studies that evaluated dietary fiber and endometrial cancer risk: highest compared with lowest category. All studies excluded hysterectomies from the control group and adjusted for BMI (weight) and total energy intake [Potischman et al (11), adjusted for noncarbohydrate calories]. **Excluded studies for the following reasons: *hospital based, †<200 cases.
DISCUSSION
This meta-analysis of 8 case-control studies (10–13, 15, 16, 18) suggested an inverse association between dietary fiber intake and endometrial cancer risk, with little evidence of heterogeneity among studies. We estimated a reduction in risk of endometrial cancer of ≈30% for women with the highest fiber consumption compared with the lowest consumption and a reduction in risk of ≈20% per 5 g/1000 kcal fiber intake. However, the only prospective study that evaluated dietary fiber and endometrial cancer suggested, if anything, a positive association (9). Because there were only a few studies that evaluated this exposure, we had limited power to assess publication bias through funnel plots or to conduct sensitivity analyses. We repeated some analyses excluding studies that did not meet our quality criteria, with essentially no change in these findings. Results for fiber subtypes were limited and inconsistent, thus precluding meta-analysis and any conclusions based on the current evidence.
It is generally thought in epidemiology that cohort studies provide stronger evidence regarding an association than case-control studies because they are less prone to differential recall of dietary habits or selection bias. However, when considering the available data for the relation between dietary fiber and endometrial cancer one must question whether the whole body of evidence from case-control studies should be discarded based on the results of one single case-cohort study, particularly considering that there are reasonable biological mechanisms to suggest that dietary fiber may decrease endometrial cancer risk.
Dietary fiber in broad terms is defined as the endogenous components of plant foods that are resistant to digestion in the intestinal tract (22). However, its classification and definition are not standardized (23). Although there is a relatively large body of literature evaluating the role of dietary fiber with other cancers, particularly with colorectal cancer, the overall evidence for a role of fiber on cancer is weak (24). The chemical complexity of fiber, inconsistencies in its definition, and the limitations of currently available dietary assessment tools have been postulated as possible explanations for the inconsistent findings stemming from studies of fiber and cancer (22).
Several lines of evidence support a role of dietary fiber in the reduction of endometrial cancer risk. Dietary fiber intake may result in reduced exposure to endogenous estrogens. Through mechanical effects on the gut, it decreases transit time and therefore may result in less reabsorption of bile acid, metabolites of cholesterol, which is itself a precursor of endogenous synthesis of estrogens (25). Dietary fiber also can bind bile acids and may prevent the deconjugation of sterols that are excreted into the gut, thereby preventing their reabsorption (26). Diets high in dietary fiber have a relatively lower glycemic load, and these characteristics are associated with favorable effects on glucose and insulin metabolism and insulin resistance (4), which have been implicated in endometrial cancer etiology (2). Epidemiologic studies have also shown an inverse association between fiber intake and weight (4). Lignans, a class of compounds that have weak estrogenic effects, are found in foods that are high in dietary fiber, so a diet high in fiber may also provide high phytoestrogen exposure (27). High dietary fiber intake is also characteristic of diets high in whole grains, which contain other compounds, including phenolic compounds and antioxidants, which may also lower the risk of cancers in general (28). Dietary fiber consumption has been shown to result in lower blood pressure levels (29) and diabetes risk (30, 31). Both hypertension and diabetes are risk factors for endometrial cancer (32). High dietary fiber intake may also be a marker for a generally “healthier” dietary pattern and lifestyle, and various associated factors, such as increased vegetable consumption, lower fat intake, increased physical activity, and lower adiposity, which are associated with lower endometrial cancer risk (5). Thus, there is strong indirect evidence to support an inverse association of dietary fiber intake on endometrial cancer risk, based on its effects on weight maintenance, estrogen concentrations, glucose and insulin metabolism, diabetes, and hypertension.
The question remains as to whether the evidence from case-control studies should be outweighed by the null findings from a single prospective study (9). However, several issues should be taken into account when interpreting the findings from this prospective study. First, the number of cases included in the analyses was relatively small; therefore, the study may not have had sufficient statistical power to detect an association. Virtually all CIs reported in the article for dietary variables include the null value. Second, women with a history of hysterectomy were excluded from baseline, but women undergoing hysterectomies during the follow-up period were not censored and, as the authors acknowledged, this may have led to an overestimation of person-years at risk. Although analyses were controlled for all relevant confounders, energy intake was adjusted for by simply adding it as continuous variable to multivariate analyses, rather than using other, more robust methods, such as the residual method (33). An additional possible limitation is that the study population consisted of Canadian women undergoing mammographic screening, which may have resulted in a relatively homogeneous population with a narrower range and less variability in nutrient intake compared with other populations. The demographic characteristics of this cohort were not reported in the article. However, quartile cutoffs confirm that the range of intake for the Jain population was narrower than that in most of the other studies (Table 4), including the case-control study by the same authors (15). This case-control study (15) was also conducted using a different, more detailed dietary assessment method than used in the cohort study. This narrower range of intake may have affected the magnitude of the association that could be detected.
TABLE 4.
Range of dietary fiber intake (g/1000 kcal) and magnitude of relative risk (RR) estimate in studies that investigated associations with endometrial cancer risk1
| Study | Fiber intake categories | Lower cutoff2 | Upper cutoff2 | Difference (estimate of range of intake) | High vs low RR estimate | Ln(RR), absolute value3 |
|---|---|---|---|---|---|---|
| g · 1000 kcal−1 · d−1 | g · 1000 kcal−1 · d−1 | |||||
| Jain et al, 2000, case-cohort study | 4 | 7.649 | 11.753 | 4.103 | 1.24 | 0.215 |
| Barbone et al, 1993 (10) | 3 | 10.024 | 12.983 | 2.959 | 0.6 | 0.511 |
| Xu et al, 2007 (18) | 5 | 4.8 | 8.1 | 3.3 | 0.8 | 0.223 |
| Potischman et al, 1993 (11) | 4 | 6.170 | 10.897 | 4.728 | 0.7 | 0.357 |
| Littman et al, 2001 (16) | 5 | 5.6 | 10.7 | 5.1 | 0.68 | 0.386 |
| Jain et al, 2000, case-control study (15) | 4 | 9.529 | 15.235 | 5.706 | 0.71 | 0.342 |
| McCann et al, 2000 (13) | 4 | 9.515 | 15.224 | 5.709 | 0.5 | 0.693 |
| Goodman et al, 1997 (12) | 4 | 6.856 | 13.322 | 6.466 | 0.47 | 0.755 |
The correlation between range of intake and absolute value of ln(RR) is 0.58, including Jain et al (9) and 0.55 excluding Jain et al (9).
Values were estimated from reported g/d and total energy intake, except for Littman et al (16) and Xu et al (18).
Provides a common measure of departure of odds ratio/RR from the null value, regardless of direction of effect.
The range of intakes in the 8 studies that examined the association of dietary fiber with endometrial cancer and for which the range of fiber intake in g/1000 kcal was available or could be estimated is shown in Table 4. The narrowest range of intake was in the study by Barbone et al (10), but that study used tertiles rather than quartiles (or quintiles) of intake. Otherwise, with the exception of the study by Xu et al (18), the range of intake was narrower in the case-cohort study (9) than in any of the other studies. The last column in Table 4, which presents the absolute value of the natural log of the relative risk estimate, provides a measure of the magnitude of the departure from the null on a common scale, regardless of the direction of the association. As illustrated in Table 4, studies with the largest range of intake tended to report the associations of larger magnitude. Overall, the correlation between the range of intake and the magnitude of the association was 0.58, including the Jain et al (case-cohort) study and 0.55 excluding that study.
To our knowledge this is the first systematic literature review and meta-analysis of the role of dietary fiber intake on endometrial cancer risk. The 1997 WCRF/AICR Report, based on a narrative (and not comprehensive) review of the association of diet and endometrial cancer, did not mention a possible role of dietary fiber intake, even though 3 studies (10, 11, 17) that evaluated this relation had been published before 1997.
In conclusion, our meta-analysis of case-control studies suggests an inverse association between dietary fiber intake and endometrial cancer risk. This finding is strongly supported by several indirect lines of evidence. Because only one prospective cohort study examined this association, additional cohort studies are needed before definitive conclusions can be drawn regarding the role of dietary fiber on endometrial cancer prevention. Future studies should address the effects of different fiber characteristics, such as fiber solubility and food source, and should take into account the role of several important confounders, such as body mass index, total energy intake, and physical activity. Furthermore, the effects by body mass, exogenous estrogen use, and menopausal status should be evaluated. Overall, the consistent findings from case-control studies are intriguing and deserve further study.
Acknowledgments
We thank James Thomas for his valuable help with the data extraction program Access.
Footnotes
Supported by World Cancer Research Fund International and the National Cancer Institute (NIH-K07 CA095666 to EVB). However, interpretation of the evidence may not represent the views of WCRF or the NCI, and our conclusions may differ from those in the 2007 WCRF report summarizing evidence related to food, nutrition, physical activity, and cancer risk.
The authors’ responsibilities were as follows—EVB and LHK: study design and implementation; EVB: study management; DFM, LHK, and EVB: data analysis; DMG, EVB, and MLM: bibliographic searches; EVB and DMG: data extraction and tabulation; EVB, LHK, and MLM: interpretation of the evidence; and LHK, DFM, DMG, and MLM: critical revision of the article. None of the authors declared any conflicts of interest.
References
- 1.Khan M, Mori M, Sakauchi F, et al. Risk of endometrial cancer mortality by ever-use of sex hormones and other factors in Japan. Asian Pac J Cancer Prev. 2006;7:260–6. [PubMed] [Google Scholar]
- 2.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 3.Sowers MR, Crawford S, McConnell DS, et al. Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr. 2006;136:1588–95. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 4.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411–8. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. The association between food, nutrition, and physical activity and the risk of endometrial cancer and underlying mechanisms. [accessed 1 November 2007];Second Report on Food, Nutrition, Physical Activity and the Prevention of Cancer: World Cancer Research Fund International/American Institute for Cancer Research. 2007 Internet: www.wcrf.org.
- 6.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Consumption of animal foods and endometrial cancer risk: a systematic literature review and meta-analysis. Cancer Causes Control. 2007;18:967–88. doi: 10.1007/s10552-007-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandera EV, Kushi LH, Moore DF, Gifkins DM, McCullough ML. Fruits and vegetables and endometrial cancer risk: a systematic literature review and meta-analysis. Nutr Cancer. 2007;58:6–21. doi: 10.1080/01635580701307929. [DOI] [PubMed] [Google Scholar]
- 9.Jain MG, Rohan TE, Howe GR, Miller AB. A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol. 2000;16:899–905. doi: 10.1023/a:1011012621990. [DOI] [PubMed] [Google Scholar]
- 10.Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. 1993;137:393–403. doi: 10.1093/oxfordjournals.aje.a116687. [DOI] [PubMed] [Google Scholar]
- 11.Potischman N, Swanson CA, Brinton LA, et al. Dietary associations in a case-control study of endometrial cancer. Cancer Causes Control. 1993;4:239–50. doi: 10.1007/BF00051319. [DOI] [PubMed] [Google Scholar]
- 12.Goodman MT, Wilkens LR, Hankin JH, Lyu LC, Wu AH, Kolonel LN. Association of soy and fiber consumption with the risk of endometrial cancer. Am J Epidemiol. 1997;146:294–306. doi: 10.1093/oxfordjournals.aje.a009270. [DOI] [PubMed] [Google Scholar]
- 13.McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States) Cancer Causes Control. 2000;11:965–74. doi: 10.1023/a:1026551309873. [DOI] [PubMed] [Google Scholar]
- 14.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero de los Rios P, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–45. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 15.Jain MG, Howe GR, Rohan TE. Nutritional factors and endometrial cancer in Ontario, Canada. Cancer Control. 2000;7:288–96. doi: 10.1177/107327480000700312. [DOI] [PubMed] [Google Scholar]
- 16.Littman AJ, Beresford SA, White E. The association of dietary fat and plant foods with endometrial cancer (United States) Cancer Causes Control. 2001;12:691–702. doi: 10.1023/a:1011292003586. [DOI] [PubMed] [Google Scholar]
- 17.Shu XO, Zheng W, Potischman N, et al. A population-based case-control study of dietary factors and endometrial cancer in Shanghai, People’s Republic of China. Am J Epidemiol. 1993;137:155–65. doi: 10.1093/oxfordjournals.aje.a116655. [DOI] [PubMed] [Google Scholar]
- 18.Xu WH, Dai Q, Xiang YB, et al. Nutritional factors in relation to endometrial cancer: a report from a population-based case-control study in Shanghai, China. Int J Cancer. 2007;120:1776–81. doi: 10.1002/ijc.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–21. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 21.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis. Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Martinez ME, Jacobs ET. Dietary fiber and carbohydrates. In: Heber D, Blackburn GL, Go VLW, Milner J, editors. Nutritional oncology. 2. San Diego, CA: Academic Press; 2006. pp. 521–530. [Google Scholar]
- 23.Lupton JR, Turner ND. Dietary fiber. In: Stipanuk MH, editor. Biochemical, physiological, and molecular aspects of human nutrition. 2. St Louis, MO: Elsevier; 2006. pp. 240–1. [Google Scholar]
- 24.McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004;23:6349–64. doi: 10.1038/sj.onc.1207716. [DOI] [PubMed] [Google Scholar]
- 25.Weisburger JH, Wynder EL. Dietary fat intake and cancer. Hematol Oncol Clin North Am. 1991;5:7–23. [PubMed] [Google Scholar]
- 26.Gorbach SL, Goldin BR. Diet and the excretion and enterohepatic cycling of estrogens. Prev Med. 1987;16:525–31. doi: 10.1016/0091-7435(87)90067-3. [DOI] [PubMed] [Google Scholar]
- 27.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133(suppl 3):956S–64S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 28.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(suppl):300S–7S. doi: 10.1080/07315724.2000.10718964. [DOI] [PubMed] [Google Scholar]
- 29.Streppel MT, Arends LR, van ’t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–6. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 30.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 31.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–30. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 32.Persson I, Adami H-O. Endometrial cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of cancer epidemiology. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 33.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(suppl):1220S–8S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]


