Abstract
A cannabis withdrawal syndrome has been characterized, but its clinical significance remains uncertain. One method of assessing the significance of cannabis withdrawal is to compare it directly to an established withdrawal syndrome. The present study was a within-subject comparison of cannabis, tobacco, and combined cannabis and tobacco withdrawal among users of both substances. Participants (N=12) completed three 5-day periods of abstinence in a randomized order, separated by 9-day periods of usual substance use. Overall withdrawal severity associated with cannabis alone and tobacco alone was of a similar magnitude. Withdrawal during simultaneous cessation of both substances was more severe than for each substance alone, but these differences were of short duration and substantial individual differences were noted. These results are consistent with other evidence suggesting cannabis withdrawal is clinically important and warrants detailed description in the DSM-V and ICD-11. Additional research is needed to replicate these findings and to further investigate the effects of abstaining from multiple drugs simultaneously.
Keywords: cannabis, marijuana, tobacco, nicotine, withdrawal, dependence
1. Introduction
Cannabis is currently the most widely used illicit drug in the United States and treatment admissions in which cannabis was the primary problem substance have more than doubled since the early 1990’s and are now comparable in number with treatment admissions for cocaine and heroin (SAMHSA, 1998, 2001, 2003). A reliable cannabis withdrawal syndrome that involves increased anger and aggression, anxiety, depressed mood, irritability, restlessness, sleep difficulty and strange dreams, decreased appetite, and weight loss has been clearly demonstrated (Budney et al., 2004). Headaches, physical tension, sweating, stomach pain, and general physical discomfort have also been observed during cannabis withdrawal, but are less common (Budney et al., 2004). Most symptoms onset within the first 24 hours of cessation, peak within the first week, and last approximately 1–2 weeks (Budney et al., 2003; Haney et al., 1999; Kouri and Pope, 2000). Because these withdrawal symptoms are time-limited, occur shortly after cannabis cessation, and are reduced or eliminated following administration of the CB1 receptor agonist delta-9-tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis, it appears that they represent a true withdrawal syndrome (Budney et al., 2007; Haney et al., 2004; Hart, 2005; Lichtman and Martin, 2002).
The clinical significance of cannabis withdrawal, however, has not been clearly established. Cannabis withdrawal is included, but not well defined, as a clinical diagnosis in the ICD-10 (World Health Organization, 1992), and is not included in the DSM-IV because the “clinical significance is uncertain” (American Psychiatric Association, 2000). However, a number of empirical observations are suggestive of its importance. The majority of adults and adolescents seeking outpatient treatment for cannabis dependence have great difficulty achieving initial periods of abstinence (Budney et al., 2000; Budney et al., in press; Copeland et al., 2001; Stephens et al., 2002). Many complain that withdrawal contributes to their inability to quit and report using cannabis or other substances to alleviate withdrawal symptoms (Budney and Hughes, 2006; Budney et al., 2004; Coffey et al., 2002; Copersino et al., 2006). Withdrawal symptoms are also observable to third-party observers, and comments made by these observers suggest that symptoms can be disruptive of daily living (Budney et al., 2001; Budney et al., 2003).
Another method of estimating the clinical significance of cannabis withdrawal is by comparing it with a syndrome of known clinical importance. In an initial archival study, we compared cannabis withdrawal with nicotine (tobacco) withdrawal effects observed in two separate outpatient studies that used similar methodologies (Vandrey et al., 2005). Close similarities in the type, magnitude, and time course of symptoms expressed during cannabis and tobacco withdrawal were observed on 6 participant-rated and 4 observer-rated withdrawal symptoms suggesting that the two syndromes showed comparable severity. Several important methodological limitations of this study (lack of inferential statistics, omission of some common symptoms, robust sample differences) limited confidence in the conclusions that could be drawn.
This paper describes a study designed to provide a more rigorous comparison of the cannabis and nicotine withdrawal syndromes. Comparisons were made for all symptoms commonly reported in prior studies of both cannabis and tobacco withdrawal (aggression, anger, depressed mood, irritability, anxiety/nervousness, restlessness, sleep difficulty, strange dreams, and tension). A within-subject design was used to eliminate inter-group variability, and because this required participation of regular users of both substances it also provided the opportunity to assess the effects of abstaining from both substances simultaneously. Examining withdrawal that occurs following cessation of both substances is important because approximately 9% of heavy smokers are daily or near daily cannabis users (Ford et al., 2002) and approximately 50% of heavy cannabis users also use tobacco regularly (Moore and Budney, 2001). Moreover, among cannabis-dependent individuals, those that smoke tobacco have increased psychosocial problems and poor cannabis cessation outcomes compared with non-smokers (Moore and Budney, 2001). Similarly, cannabis use among tobacco smokers is predictive of continued long-term tobacco use and is associated with poor tobacco cessation outcomes (Amos et al., 2004; Ford et al., 2002). Whether simultaneous drug abstinence results in significantly greater withdrawal or difficulty in achieving abstinence compared with abstinence from either drug alone has not been previously studied.
2. Methods
Two laboratories collaborated to conduct the experiment. Site 1 was the Treatment Research Center of the University of Vermont in Burlington, VT (UVM). Site 2 was the Department of Physiology and Pharmacology of the Wake Forest University School of Medicine in Winston-Salem, NC (WAKE).
2.1 Participants
Current users of cannabis and tobacco were recruited through newspaper advertisements and flyers posted on community bulletin boards. Criteria for participation included: age ≥18 years old; heavy use of cannabis (at least 25 days/month) and tobacco (at least 10 cigarettes/day) for the 6 months prior to participating; no intent to quit or change cannabis or tobacco use; not currently dependent on other substances; no use of illicit drugs (other than cannabis) in the prior month; not currently using any psychotropic medication; not meeting DSM-IV criteria for a current episode of an Axis I psychiatric disorder; and not pregnant. Treatment seekers were excluded because the protocol included periods during which participants were required to resume cannabis and tobacco use following brief periods of abstinence (see below). The cannabis and tobacco use criteria are consistent with that used in prior studies in which significant withdrawal effects were observed in a majority of users at these levels (e.g. Budney et al., 2001; Budney et al., 2003; Fernando et al., 2006; Pomerleau et al., 2000). The criteria requiring cannabis use on 25 of 30 days was used to enhance generality because a substantial number of heavy users are occasionally unable to obtain the drug for a day or two due to its illicit status.
At the UVM site, 23 participants were enrolled, and 9 completed the study. Of those who enrolled and did not complete, 8 failed to achieve the required periods of abstinence, 3 discontinued participation voluntarily prior to the first abstinence period, 2 were discontinued from the study due to use of restricted substances (e.g. cocaine), and 1 person was discontinued for testing negative for cannabis during the first baseline condition. At the WAKE site, 19 enrolled, and 3 completed the study. Of those who did not complete, 4 failed to achieve the required periods of abstinence, 6 discontinued participation voluntarily, 4 were discontinued from the study due to use of restricted substances, and 2 were discontinued from the study for using less tobacco during baseline than reported during screening. Voluntary discontinuation usually resulted from participant problems with time commitment or transportation to the laboratory. Detailed demographics of the 12 completers are provided in Table 1.
Table 1.
Participant Demographics
| N | 12 |
| Age* | 28.2 (10.0) |
| % Male | 50 |
| % Caucasian | 100 |
| Education | 13.4 (1.4) |
| Cannabis use per day | 3.8 (2.1) times |
| Years daily cannabis use | 9.8 (9.2) |
| Tobacco use per day | 20.3 (8.7) cigs |
| Years daily tobacco use | 9.9 (8.2) |
| Alcohol drinks per week | 13.0 (11.5) |
Data presented as Mean (SD) where appropriate
2.2 Procedures
A brief telephone interview was conducted followed by an in depth laboratory screening to determine study eligibility. Informed consent was obtained at the beginning of the laboratory assessment, and the Committees on Human Research at the University of Vermont and Wake Forest University approved all study procedures.
An ABACAD within-subjects design was used to compare abstinence effects associated with cessation from cannabis only (cannabis), tobacco only (tobacco), and both cannabis and tobacco simultaneously (dual). In this design, A-conditions represent periods of cannabis and tobacco smoking-as-usual (SAU), and conditions B, C, and D correspond to randomly assigned 5-day periods of cannabis, tobacco, or dual abstinence. Participants attended 30-minute laboratory sessions each weekday to obtain self-reported affective and behavioral measures, physiological measures, and staff-observed urine and breath samples. The study always began on a Monday and each 5-day abstinence condition began following collection of a urine specimen on the prior Sunday and ended after the laboratory session the following Friday. Data collection did not occur on Saturdays or Sundays prior to SAU conditions. Participants were instructed to treat those days as SAU days and maintain use of cannabis and tobacco as they did prior to participation. Therefore, each 5-day abstinence condition was separated by a period of at least 9 days of usual substance use.
During the study, participants were asked to maintain their usual patterns of alcohol and caffeine use and abstain from using illegal drugs (other than cannabis) and psychoactive medications. To eliminate the influence of acute intoxication on subjective reporting, participants were instructed not to smoke cannabis or drink alcohol for at least 2 hours prior to each laboratory visit. Though there was no objective way to verify compliance with the requirement to not smoke cannabis during this time, compliance with the alcohol restriction was confirmed via negative breathalyzer readings obtained prior to all laboratory data collection. During periods of tobacco abstinence, participants were explicitly instructed not to use alternative tobacco or tobacco cessation products. Participants were also asked to not make significant changes to their diet or exercise patterns during the study.
For ethical reasons, on the last day of each abstinence period participants were asked if they intended to resume their usual use of cannabis and/or tobacco and would like to continue in the study, or whether they would instead like to remain abstinent and be referred for help with abstinence. During the study, all participants indicated they planned to resume use. At the end of the study, cannabis and tobacco treatment referral lists were offered and provided to those who would accept them.
2.3 Measures
Prior to enrollment, a drug history questionnaire assessed past and present patterns (e.g. age of first use and progression to daily use) and consequences (e.g. prior treatment episodes or experience of withdrawal) of substance use and abuse. The Time-Line Follow-Back method (Sobell and Sobell, 1992) was used to obtain the amount and frequency of substance use during the previous 6 months. The DSM Checklist (Hudziak et al., 1993) was used to diagnose current (past 6 months) Axis I psychiatric disorders.
During each weekday laboratory visit, participants completed a battery of self-report questionnaires. The Withdrawal Symptom Checklist (WSC), an adaptation of the Marijuana Withdrawal Checklist (Budney et al., 2001; Budney et al., 2003), was used to assess withdrawal symptoms prospectively over the course of the study. The WSC included common symptoms of both cannabis and tobacco withdrawal and several filler items rated on a 0–3 scale (0 = not at all, 1= mild, 2 = moderate, 3 = severe) based on their experience over the prior 24 hours. The primary outcome variable for this measure was a composite withdrawal discomfort score (WDS), a sum of 10 items previously reported as common symptoms of withdrawal for both cannabis and tobacco (aggression, anger, appetite change (increased for tobacco, decreased for cannabis), depressed mood, irritability, anxiety/nervousness, restlessness, sleep difficulty, and strange dreams).
The Profile of Mood States (POMS) (McNair et al., 1971) is a 65-item measure that provided composite scores of tension (range 0–36), depression (range 0–60), anger (range 0–48), and confusion (range 0–28) based on participants’ ratings of the previous 24 hours. Craving for cannabis and tobacco was measured by the short (12-item) versions of the Marijuana Craving Questionnaire (MCQ) (Heishman et al., 2001) and Tobacco Craving Questionnaire (TCQ) (Heishman et al., 2003) respectively. The MCQ and TCQ each yield 4 composite scores: purposefulness, expectancy, emotionality, and compulsivity. Participants kept a daily diary detailing their use of tobacco, cannabis, alcohol, and both prescription and OTC medications. Research staff obtained measures of resting heart rate, supine blood pressure, body weight, breath CO, and breath alcohol during each laboratory session. At the end of the final session, participants completed an End of Study Questionnaire. Participants rated the overall level of discomfort they experienced during each abstinence condition on a 0–3 scale, responded to an unstructured question asking what aspects of each abstinence period were most difficult for them, and then they rank-ordered the difficulty of completing each abstinence condition.
Observed urine samples were collected daily and qualitatively tested on-site using an enzyme immunoassay technique for evidence of cannabis, benzodiazepine, opiate, cocaine, methamphetamine, and amphetamine use. Specimens were then shipped overnight to Dominion Diagnostics (Kingstown, RI) for quantitative analysis (gas-chromatography-mass spectroscopy for cannabis, gas-chromatography for cotinine) of the primary metabolites of THC and nicotine (11-nor-9-carboxy-delta9-tetrahydrocannabinol (THCCOOH) and cotinine respectively) and creatinine. Results were typically received within 72 hours of specimen collection. During abstinence periods, participants were judged abstinent from cannabis using the previously validated criteria of a ratio of THCCOOH to creatinine that did not increase by 50% on consecutive days and decreased by at least 50% during the 5-day abstinence period (Huestis and Cone, 1998). Participants were judged abstinent from tobacco if cotinine levels decreased by at least 40% on consecutive days until specimens tested at or below a cutoff of 80ng/mL (Benowitz et al., 2002).
Participants were compensated up to $750 for study participation. During SAU periods compensation was $15 per laboratory visit, and during abstinence periods compensation was $25 per day, $12.50 of which was earned for completing study measures, and $12.50 of which was contingent on verified abstinence from the target substance(s). The payment for abstinence was delayed until verification was obtained from the off-site laboratory (usually 2–3 days). A $50 completion bonus was provided for completion of each of the three phases of the study.
2.4 Data Analysis
2.4.1 Preliminary Analyses
Quantitative urine results for each participant were evaluated based on the criteria described above to verify abstinence. A two-way repeated measures analysis of variance (ANOVA) was conducted to compare the mean variable scores obtained during each of the 3 SAU periods. Repeated measures ANOVA’s using mean scores collapsed across the SAU and abstinence study conditions were also conducted to assess effects due to abstinence condition order, and to assess the stability of self-reported cannabis, tobacco and alcohol use on days other than when abstinence was required.
2.4.2 Primary Analyses
Two-way repeated measures ANOVA’s (3 × 6; condition × day) were performed to examine effects of condition (cannabis, tobacco, and dual abstinence), day (Mean SAU collapsed across days and Abstinence Days 1–5), and condition by day interactions for each dependent variable. When significant main effects or interactions were detected, post-hoc analyses were conducted to test for abstinence manipulation effects and condition differences within each day using the corresponding ANOVA for testing simple effects. Bonferonni adjustments for multiple comparisons were used for post-hoc comparisons and statistical difference was determined based on α = .05 for all analyses.
3. Results
3.1 Results of Preliminary Analyses
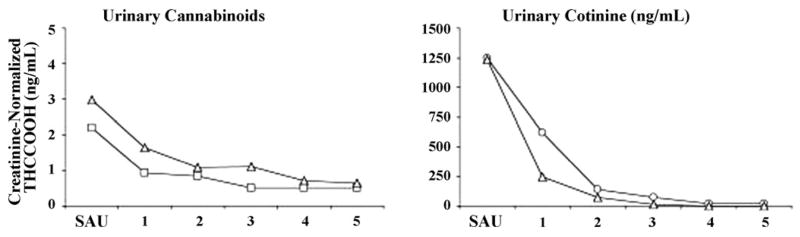
Quantitative levels of urinary THCCOOH (adjusted for creatinine) and cotinine decreased as expected during each abstinence phase (Figure 1). No differences of SAU condition were found for any outcome variables indicating stable responding across the 3 baseline periods. Similarly, no effects of abstinence condition order were observed. No effects of study condition were detected in participants’ self-reported use of cannabis, tobacco, and alcohol (excluding abstinence condition days) were detected indicating that participants followed the instruction to not significantly alter their use of these drugs during the study except when instructed to abstain.
Figure 1.
Mean creatinine-normalized tetrahydrocannabinol (THCCOOH) and cotinine levels. SAU values reflect the average of specimens collected on Days 1, 3, and 5 of the SAU condition preceding each abstinence condition.
3.2 Results of Primary Analyses
3.2.1 Withdrawal Symptom Checklist (WSC)
Main effects of study day were found for WSC items anxiety/nervousness (F = 4.99, p ≤ .05), decreased appetite (F = 3.03, p ≤ .05), depressed mood (F = 2.35, p ≤ .05), difficulty concentrating (F = 2.22, p ≤ .05), feverish (F = 2.62, p ≤ .05), increased anger (F = 2.55, p ≤ .05), irritability (F = 5.53, p ≤ .05), physical discomfort (F = 3.07, p ≤ .05), restlessness (F = 5.75, p ≤ .05), shakiness (F = 2.69, p ≤ .05), sleep difficulty (F = 5.50, p ≤ .05), stomach pain (F = 2.47, p ≤ .05), strange dreams (F = 2.49, p ≤ .05), sweating (F = 2.71, p ≤ .05), tension (F = 2.41, p ≤ .05), and the composite WDS (F = 8.76, p ≤ .05). Post-hoc tests comparing ratings by study day indicate that these differences reflect an abstinence manipulation or withdrawal effect (i.e. ratings during abstinence were significantly greater than SAU). Significant withdrawal effects were observed for anxiety/nervousness, decreased appetite, difficulty concentrating, irritability, sleep difficulty, strange dreams, and WDS in the cannabis abstinence condition. Significant withdrawal effects were observed for anxiety/nervousness, increased anger, irritability, physical discomfort, restlessness, shakiness, sleep difficulty, tension, and WDS in the tobacco abstinence condition.
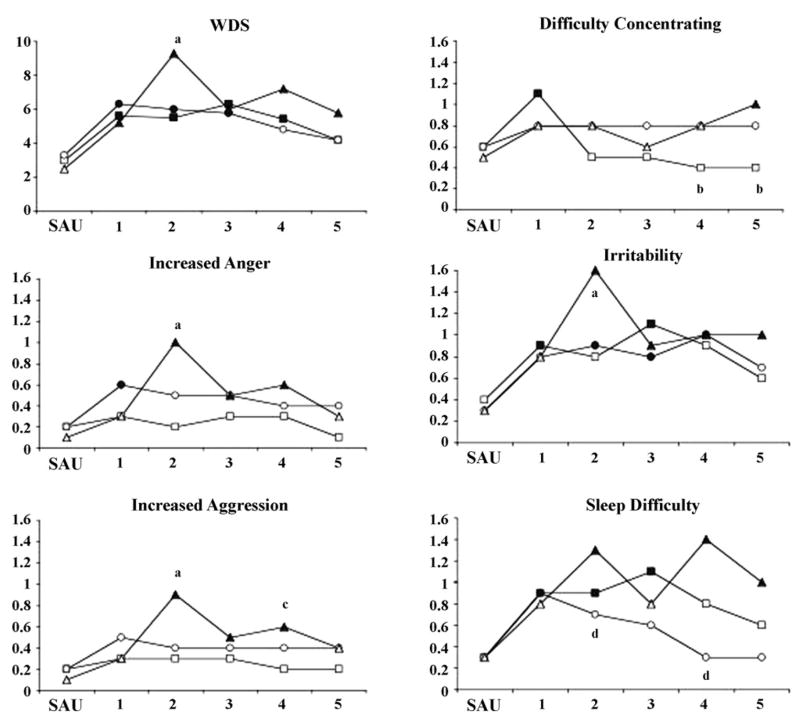
Significant withdrawal effects were observed for each of these symptoms except decreased appetite in the dual abstinence condition. Significant condition by day interactions were observed for ratings of difficulty concentrating (F = 2.22, p ≤ .05), increased aggression (F = 2.37, p ≤ .05), increased anger (F = 2.80, p ≤ .05), irritability (F = 1.91, p ≤ .05), sleep difficulty (F = 2.33, p ≤ .05), and the composite WDS score (F = 2.72, p ≤ .05). Illustrations of these data are provided in Figure 2. Post-hoc analyses indicated that ratings of difficulty concentrating were greater on Days 4 and 5 in the tobacco and dual abstinence conditions compared with the cannabis abstinence condition. Increased aggression, increased anger, irritability, and WDS were greater in the dual abstinence condition compared with the cannabis and tobacco abstinence conditions on Day 2. Increased aggression was also greater in the dual abstinence condition compared with the cannabis abstinence condition on Day 4. Sleep difficulty was greater in the dual abstinence condition compared with the tobacco abstinence condition on Days 2 and 4.
Figure 2.
Mean ratings for WSC items for which significant condition by day interactions were observed. Filled symbols indicate values significantly different from SAU. Subscripts designate differences by condition on a given study day (a = dual > cannabis and tobacco; b = dual and tobacco > cannabis; c = dual > cannabis; d = dual > tobacco).
3.2.2 POMS, MCQ, and TCQ
A main effect of study day was observed for the anger sub-scale of the POMS (F = 2.50, p ≤ .05). Post-hoc tests indicated a withdrawal effect during the tobacco and dual abstinence conditions. Significant condition by day interactions were observed for the anger (F = 2.28, p ≤ .05) and confusion (F = 2.05, p ≤ .05) sub-scales of the POMS. Post-hoc tests indicated that, on abstinence Day 2, scores on the anger sub-scale were greater in the dual abstinence condition compared to the cannabis and tobacco conditions, and that scores in the tobacco condition were greater compared with the cannabis condition. Scores on the confusion sub-scale were greater in the dual abstinence condition compared with the cannabis abstinence condition on Day 2, and in the tobacco condition compared with the cannabis condition on Days 4 and 5.
A main effect of study day was observed for the compulsivity (F = 5.03, p ≤ .05) and purposefulness (F = 3.64, p ≤ .05) sub-scales of the MCQ. Post-hoc tests indicated a withdrawal effect during the cannabis abstinence condition for both sub-scales.
Main effects of study day were observed for the compulsivity (F = 2.95, p ≤ .05), emotionality (F = 4.99, p ≤ .05), expectancy (F = 2.81, p ≤ .05), and purposefulness (F = 2.77, p ≤ .05) sub-scales of the TCQ. Post-hoc tests indicated a withdrawal effect during the tobacco and dual abstinence conditions for all 4 sub-scales. A significant interaction was also observed for the compulsivity sub-scale of the TCQ (F = 2.48, p ≤ .05), and post-hoc tests indicated greater ratings during the tobacco abstinence condition compared with the dual abstinence condition on Day 3.
3.2.3 Physiological Measures
Main effects for study day were observed for heart rate (F = 5.49, p ≤ .05) and systolic blood pressure (F = 2.43, p ≤ .05). Post-hoc tests indicated a withdrawal effect for heart rate during all three abstinence conditions and for systolic blood pressure during tobacco abstinence. Heart rate increased during cannabis abstinence and decreased during the tobacco and dual abstinence conditions. Systolic blood pressure decreased during tobacco abstinence.
Main effects of study condition were observed for systolic blood pressure (F = 9.26, p ≤ .05), diastolic blood pressure (F = 11.96, p ≤ .05), and body weight (F = 3.62, p ≤ .05). Post-hoc tests indicated that systolic and diastolic blood pressure was greater during cannabis abstinence compared with tobacco abstinence, while the opposite was true for body weight. Blood pressure and body weight during the dual abstinence condition was intermediate and did not differ from the cannabis or tobacco conditions.
A condition by day interaction was observed for heart rate (F = 3.27, p ≤ .05). Post-hoc tests indicated that heart rate was greater during the cannabis abstinence condition compared with the tobacco abstinence condition on all 5 abstinence days and compared with the dual abstinence condition on Days 1, 3, 4, and 5 of abstinence.
3.2.4 End of Study Questionnaire
No differences were detected for ratings of overall level of discomfort on the End of Study Questionnaire. Interestingly, notable individual variability was observed on the rank-order assessment of abstinence phase difficulty (Table 2). No single abstinence phase was consistently ranked as being more difficult than the others. Five participants rated the dual abstinence condition as the most difficult, 4 rated the cannabis condition as the most difficult, and 3 rated tobacco abstinence as the most difficult. Similarly, 5 participants rated the dual abstinence condition as least difficult, 4 rated the cannabis condition least difficult, and 3 rated tobacco abstinence as the least difficult.
Table 2.
Mean WDS, Rank-Order, and Substance Use by Participant
| ID | Cannabis WDS | Tobacco WDS | Dual WDS | Rank-Order | Cannabis Times/Day | Tobacco Cigs/Day |
|---|---|---|---|---|---|---|
| 1 | −1.4 | −0.8 | 5.0 | D > T > C | 4 | 15 |
| 2 | 3.2 | 9.0 | 7.0 | T > D > C | 6 | 35 |
| 3 | 0.0 | 0.4 | 1.0 | D > T > C | 2 | 20 |
| 4 | 1.8 | 7.4 | 8.2 | D > T > C | 2 | 40 |
| 5 | 3.2 | 2.2 | 5.8 | T > C > D | 8 | 20 |
| 6 | 5.4 | 1.8 | 1.8 | C > T > D | 6 | 15 |
| 7 | 6.2 | −1.2 | 4.0 | D > C > T | 10 | 17 |
| 8 | −0.4 | 2.8 | 6.8 | D > C > T | 2 | 20 |
| 9 | 2.2 | 6.0 | 3.2 | T > C > D | 3 | 16 |
| 10 | 2.2 | 1.4 | 3.4 | C > T > D | 4 | 15 |
| 11 | 5.8 | −3.2 | 1.2 | C > D > T | 4 | 20 |
| 12 | 0.2 | 0.4 | 4.8 | C > T > D | 1 | 10 |
WDS scores reflect mean change from cannabis and tobacco smoking as usual (SAU)
Rank-Order refers to the order of abstinence condition difficulty on the End of Study Questionnaire
C = cannabis, T = tobacco, D = dual abstinence conditions
4. Discussion
Overall withdrawal discomfort (WDS) and individual symptom severity during cannabis abstinence was similar to that observed during tobacco abstinence in the present study. The differences observed between the cannabis and tobacco abstinence conditions were mostly for symptoms expected to differ based on previous studies (difficulty concentrating/confusion, heart rate, body weight). Exceptions to this were that ratings of anger and craving appeared to be higher during tobacco abstinence compared with cannabis abstinence. We believe the conclusion that the cannabis and tobacco withdrawal syndromes are of comparable severity is valid because the withdrawal effects observed in this study were similar in magnitude to that observed in prior studies using similar measures (Budney et al., 2001; Budney et al., 2003; Hughes, 1992; Hughes and Hatsukami, 1986). Moreover, these findings are consistent with our prior archival comparison (Vandrey et al., 2005).
This experiment provided the first examination of abstinence effects that occur following simultaneous cessation of cannabis and tobacco. The WDS and individual symptoms such as aggression, anger, and irritability appeared to be more severe during simultaneous abstinence compared with abstinence from either drug alone. However, this effect was not very robust (limited to Day 2 for most variables), and the participants’ general rating of discomfort across the three abstinence conditions indicated substantial individual differences. Although it is logical to expect greater withdrawal with dual abstinence, for a subset of participants, the dual abstinence condition was associated with less withdrawal and rated as less difficult. One possible reason for the lower withdrawal in the dual abstinence period for some participants may be that both substances are smoked and thus share smoking-related cues. Thus, the absence of smoking cues during dual abstinence might decrease withdrawal. Exposure to drug cues has been associated with increases in ratings of withdrawal and more rapid relapse in prior studies, but it is not known whether these cues generalize across substances (Childress et al., 1986; Droungas et al., 1995; Juliano et al., 2006).
Though significant methodological improvements over our previous cross-study comparison were made, important limitations remain with regards to the current experiment. First, the sample size was relatively small, which is potentially problematic with regard to the generality of the findings and when trying to demonstrate similarities (the null hypothesis). Replication with a larger sample size would be desirable, however, the feasibility of a larger scale study is uncertain given the high drop out rate and difficulty recruiting eligible participants encountered at both sites in the current study. The high drop out rate may also have resulted in the exclusion of people who experience more severe withdrawal. No clear pattern emerged with regard to which study condition participants were in when they quit the study early, but we cannot rule out the possibility that withdrawal effects observed in one of our abstinence conditions is a more conservative representation of withdrawal severity relative to the others.
Abstinence periods of 5 days duration might not have been sufficient to accurately assess the magnitude and importance of all abstinence effects. Prior research suggests that peak withdrawal effects for cannabis and tobacco occur within the first week of abstinence (Budney et al., 2004; Hughes, 1990; Kouri and Pope, 2000). However, these studies also suggest that some withdrawal symptoms (ex. anger and restlessness for cannabis; sleep difficulty and increased appetite/weight gain for tobacco) have a later onset of peak effects compared with other symptoms and may have been underestimated in the current study. It is also important to note that this study was not placebo controlled and was based on participants who are heavy daily users of cannabis and tobacco. Therefore, we cannot rule out the influence of expectancy effects across conditions, and these results may not generalize to less frequent cannabis and tobacco users.
Though we included measures of withdrawal based on prior cannabis and tobacco withdrawal research, the fact that mean WDS scores were not associated with greater rankings of abstinence difficulty in half the sample suggests our WDS did not capture some important facets of cannabis or tobacco withdrawal. Further research should examine what abstinence effects beyond the withdrawal symptoms included in the WDS may contribute to abstinence difficulty. Two such possibilities are negative consequences that may result from the absence of substance-related social activities or the disengagement of habitual substance use routines (e.g., no longer able to use substance after meals, after work, while driving, or before going to sleep) that accompany an abstinence attempt. Several participants described these types of consequences as being very difficult to endure on the End of Study Questionnaire.
These limitations acknowledged, the results do suggest that the severity of the cannabis withdrawal syndrome is of comparable magnitude to that of the tobacco withdrawal syndrome. Tobacco withdrawal has been extensively researched, and by its inclusion in DSM-IV, is judged to be clinically significant (substance withdrawal disorders must produce “clinically significant distress” to be included in DSM-IV). This observed similarity with tobacco withdrawal, combined with several lines of evidence that cannabis withdrawal is clinically significant as described in the introduction, suggest that cannabis withdrawal is clinically significant in a subset of heavy cannabis users and should be characterized in detail in the next iterations of the DSM and ICD manuals.
Several suggestions for future research can be made to extend this area of study. Prospective investigation of the relationships between cannabis withdrawal and relapse or failed quit attempts would provide additional information regarding its clinical significance. The implications of the dual abstinence effect findings are unclear, but also are of clinical importance. Interestingly, we could not locate another prospective study comparing withdrawal severity during cessation from one versus more than one drug simultaneously. Our data that drug withdrawal was not consistently greater across participants and that for some participants withdrawal was less severe with dual abstinence, clearly suggest further studies of single versus dual abstinence are warranted.
Acknowledgments
This research was supported by grants R01-DA12471, T32-DA07242 from the National Institute on Drug Abuse. The authors would also like to thank the research support staff at the University of Vermont and Wake Forest University for their invaluable contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-TR ed) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can’t go without a fag ... you need it for your hash’ - a qualitative exploration of smoking, cannabis, and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P, Ahijevych K, Hall SM, Jarvis MJ, LeHouezec J, Hansson A, Lichenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine and Tob Res. 2002;4:149–159. [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping-skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The Cannabis Withdrawal Syndrome. Curr Opin Psychiatry. 2006;19:233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey RG. A review of the validity and significance of the cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical Trial of Abstinence-Based Vouchers and Cognitive-Behavioral Therapy for Cannabis Dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Bahrenburg B. Dronabinol (oral THC) dose-dependently suppresses cannabis abstinence effects. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addiction. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: an Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. J Subst Abuse Treat. 2001;20:45–52. doi: 10.1016/s0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Fernando WWSA, Wellman RJ, DiFranza JR. The relationship between level of cigarette consumption and latency to the onset of retrospectively reported withdrawal symptoms. Psychopharmacology. 2006;188:335–342. doi: 10.1007/s00213-006-0497-x. [DOI] [PubMed] [Google Scholar]
- Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67:243–248. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: Effects of oral THC or Divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;14:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Hart CL. Increasing treatment options for cannabis dependence: A review of potential pharmacotherapies. Drug Alcohol Depend. 2005;80:147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: Development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco Craving Questionnaire: reliability and validity of a new multi-factorial instrument. Nicotine Tob Res. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Hudziak J, Helzer JE, Wetzel MW, Kessel KB, McBee B, Janca A, Przybeck P. The use of the DSM-III-R Checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. doi: 10.1080/14622200701188919. In Press. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D, editors. Effects of abstinence from tobacco: A critical review. Vol. 10. New York: Plenum Press; 1990. [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. J Abnorm Psychol. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Pope HG. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol. 2002;42:20S–27S. doi: 10.1002/j.1552-4604.2002.tb05999.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Moore BA, Budney AJ. Tobacco smoking in marijuana dependent outpatients. Journal of Substance Abuse. 2001;13:585–598. doi: 10.1016/s0899-3289(01)00093-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine Tob Res. 2000;2:275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National admissions to substance abuse treatment services - The Treatment Episode Data Set (TEDS) 1992–1996. Rockville, MD: USDHHS; 1998. [Google Scholar]
- SAMHSA. Treatment episode data set (TEDS) 1996 –1999: National admissions to substance abuse treatment services. Rockville, MD: DHHS; 2001. [Google Scholar]
- SAMHSA. Summary of findings from the 2002 National Household Survey on Drug Abuse. Rockville, MD: USDHHS; 2003. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Human Press; 1992. pp. 41–72. [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M The Marijuana Treatment Project Research Group. The marijuana treatment project: Rationale, design, and participant characteristics. Addiction. 2002;97(S1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005;14:54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]
- World Health Organization. ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: Author; 1992. [Google Scholar]