Abstract
Interleukin (IL)-8 is a potent neutrophil chemoattractant that drives the inflammatory response in cystic fibrosis (CF). Traditional approaches to the pathophysiology of this inflammation have focused on targeting NF-κB–dependent signaling and therapy with glucocorticoids. We test the hypothesis that an alternative pathway, independent of NF-κB, operates through prostaglandin E2 (PGE-2) receptor EP-2 and stimulates IL-8 chemokine secretion. Using CF bronchial epithelial cells (IB3-1) in vitro, exogenous PGE-2 induces IL-8 release in a dose-dependent manner. These events are associated with elevation in the EP-2 receptors. Inhibition of cyclooxygenase (Cox)-2 with NS-398 was associated with reductions in Cox-2 (2-fold) and IL-6 (1.3-fold) mRNA transcripts, and in IL-8 and PGE-2 chemokine secretion. The inhibition of Cox-2 signaling led to down-regulation of the downstream C/EBP homologous protein (CHOP) transcription factor, resulting in a decrease in IL-8 activation. We confirmed the regulation of IL-8 promoter by CHOP in CF cells using the IL-8 reporter assay. We conclude that PGE-2 stimulates IL-8 production through the CHOP transcription factor in CF cells.
Keywords: PGE-2, chemokine, cAMP, ibuprofen, CFTR
CLINICAL RELEVANCE
IL-8 drives the inflammatory response in cystic fibrosis (CF). We show an alternative, non–NF-κB, signaling pathway for prostaglandin E2–induced IL-8 stimulation in CF. We provide additional data regarding the potential of cyclooxygenase-2 inhibition for CF lung disease.
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-dependent and ATP-gated chloride channel that regulates epithelial surface fluid secretion in respiratory and gastrointestinal tracts (1). Deletion of phenylalanine at position 508 (ΔF508) in CFTR is the most common CF-causing mutation, resulting in a temperature-sensitive folding defect, retention of the protein in the endoplasmic reticulum (ER), and subsequent degradation by the proteasome (2). Patients with CF exhibit a typical phenotype that is characterized by persistent pulmonary infections, leading to pulmonary failure and death. Bronchoalveolar lavage fluid in patients with CF contains increased levels of proinflammatory cytokines and neutrophils. CF cells have increased basal interleukin (IL)-8 levels (a pro-inflammatory C-X-C chemokine) attributed to activated NF-κB (3).
Prostaglandin E2 (PGE-2) is a potent mediator of inflammation produced by cyclooxgenation of arachidonic acid. PGE-2 binds to G protein–coupled receptors, EP-1, EP-2, EP-3, or EP-4 to trigger an inflammatory response. Expression of these receptors is cell specific, and distinct signaling mechanisms contribute to the biological heterogeneity of PGE-2. EP-2 and EP-4 receptors are known to stimulate IL-8 chemokine secretion (4) and intracellular cAMP formation (5). ΔF508 CFTR epithelial cell lines have enhanced expression and activity of cycloxygenase 2 (Cox-2), resulting in PGE-2 hypersecretion. PGE-2 hypersecretion results in elevated IL-8 secretion through unidentified signaling pathway(s). Moreover, elevated PGE-2 levels can be lowered to basal levels by re-trafficking ΔF508 CFTR to plasma membrane (6). It was recently demonstrated in human T lymphocytes that PGE-2 induces C/EBP homologous protein (CHOP) transcription factor that binds to the IL-8 promoter (4). CHOP is a growth arrest– and DNA damage–inducible gene 153 (GADD153) protein. We demonstrate here that PGE-2 mediates the IL-8 inflammatory response in CF cells through the CHOP transcription factor.
MATERIALS AND METHODS
Cell Culture, Transfection, and Metabolic Labeling
The IB3-1 (ΔF508/W1282X; low level expression of ΔF508-CFTR and no W1282X protein) and CFTE (ΔF508-homozygous) airway epithelial cell lines were maintained in LHC-8 media containing 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B, and 10% fetal bovine serum. LHC-8 medium was purchased from Biosource (Camarillo, CA), and other components were purchased from Gibco (Invitrogen, Carlsbad, CA). IL-1β (R&D Systems Inc., Minneapolis, MN), PGE-2 (Sigma, St. Louis, MO), 4-phenylbutyrate (4PBA; Ucyclyd Pharma, Scottsdale, AZ), PS-341 and MLN-273 (Millennium Pharmaceuticals, Cambridge, MA), and NS-398 (Calbiochem, San Diego, CA) were added to IB3-1 cells as indicated. CHOP shRNA construct in pSM2 vector (ΔCHOP) was purchased from Open Biosystems (Huntsville, AL). The IB3-1 cells were seeded on 6-well plates and transfected with CHOP shRNA (4 μg/well), IL-8 firefly reporter (4 μg/well), or renilla luciferase (1 μg/well) constructs using Lipofectamine 2000 (Invitrogen).
Immunoblotting
Cells were washed three times in ice-cold PBS and lysed directly on plates using M-PER (Pierce Biotech, Inc., Rockford, IL) protein lysis buffer containing protease inhibitor cocktail (Pierce). The protein extracts were suspended in Laemmli's sample buffer (30 μl; Invitrogen) containing β-mercaptoethanol (Invitrogen), resolved by 4-10% SDS-PAGE and transferred to a 0.45-μm (β-actin, EP-2 or EP-4) or 0.2-μm (CHOP) pore size nitrocellulose membrane (Invitrogen). The CHOP antibody was purchased from Affinity BioReagents Inc. (Golden, CO), while EP-2, EP-4, and β-actin antibodies were from Santa Cruz Biotech (Santa Cruz, CA). The anti-mouse, -rabbit, or -goat horseradish peroxidase secondary antibodies were from Amersham (Piscataway, NJ).
RNA Extraction, cDNA Synthesis and Quantitative RT-PCR
Real-time reverse transcript PCR was performed using the ABI PRISM 7300HT Sequence Detection System (Applied Biosystems, Foster City, CA). Total RNA (100 ng) was mixed with Superscript Platinum One-Step qRT-PCR kit (Invitrogen) and IL-8, IL-6, or Cox-2 primers (Invitrogen). The real-time RT-PCR reaction was carried out as follows: 95°C for 10 minutes, 40 three-step cycles: 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds with initial 48°C for 10 minutes for reverse transcription. The data were collected and analyzed with Sequence Detection Software 2.0 (Applied Biosystems). The threshold cycle difference Ct = (Ct of IL-8/IL-6/Cox-2 · Ct of GAPDH) · (Ct of IL-8/IL-6/Cox-2 no template control · Ct of GAPDH no template control). The relative expression was calculated for three independent experiments as described before (7).
IL-8, PGE-2, and cAMP Immunoassay
The IB3-1 cells were transfected with CHOP shRNA (ΔCHOP) or treated with proteasome inhibitor (PS-341 or MLN-273; Millenium Pharmaceuticals), Cox-2 inhibitor (NS-398; Calbiochem, ibuprofen; Sigma, or 5 mM 4PBA; Sigma) or NF-κB inhibitor (Caffeic acid phenyethyl ester; Sigma). IB3-1 cells were induced with 1 ng/ml IL-1β or the indicated concentrations of PGE-2. At the indicated time points, supernatants were collected and IL-8 levels were measured using solid-phase amplified sensitivity immunoassay (EASIA) as specified by the manufacturer (BioSource). Standards, and high and low cytokine controls, were included. The plates were read at 450 nm on 96-well microplate reader (Molecular Devices, Sunnyvale, CA) using SOFT-MAX-Pro software (Molecular Devices). The data represent the mean of three independent experiments ± SD. A PGE-2 high-sensitivity immunoassay kit (R&D Systems) was used to measure the concentration of PGE-2 in IB3-1 cell supernatants. The cAMP levels in IB3-1 cells were determined using cAMP immunoassay kit (R&D Systems). The IB3-1 cells were treated with 1 ng/ml of IL-1β and/or 0.1 or 1 mM ibuprofen (Sigma). The cells were lysed after 20 or 36 hours, and cAMP levels were measured as described by the manufacturer. The standard curve was used to determine the percent cAMP levels.
Chromatin Immunoprecipitation Assay
The ΔF508-homozygous CFTE cells were treated overnight with 10 μM PGE-2 or 1 ng/ml IL-1β. A chromatin immunoprecipitation (ChIP) assay kit (Upstate USA Inc., Charlottesville, VA) was used to determine the binding of CHOP to IL-8. Briefly, histones were crosslinked to DNA by adding formaldehyde to cells and incubating at 37°C for 10 minutes. Cells were washed with ice-cold PBS and incubated with 200 μl SDS cell lysis buffer for 10 minutes on ice. The cell lysate was sonicated by three 10-second pulses on ice followed by centrifugation at 12,000 rpm for 10 minutes at 4°C. Samples were diluted 10-fold in ChIP dilution buffer and pre-cleared by incubating with 75 μl of Salmon Sperm DNA/Protein A agarose beads on a rocking platform for 30 minutes at 4°C. Beads were removed by centrifugation at 1,000 rpm for 10 minutes at 4°C, and supernatants were incubated with 2.5 μg of CHOP antibody at 4°C on rocking platform. After 4 hours, 75 μl of Salmon Sperm DNA/Protein A agarose beads were added to each tube and left overnight. Samples were gently washed with low salt immune complex wash buffer (1×), high salt immune complex wash buffer (1×), LiCl immune complex wash buffer (1×), and TE buffer (2×) (10 min each). Samples were eluted using 500 μl of 1% SDS + 0.1 M NaHCO3 by vortexing and centrifugation at 1,000 rpm for 2 minutes. The supernatants were incubated at 65°C for 4 hours with 20 μl of 5 M NaCl to reverse histone–DNA cross links. DNA was purified by incubating with 10 μl of 0.5 M EDTA, 20 μl 1M Tris-HCl, pH 6.5, and 2 μl of 10 mg/ml Proteinase K for 1 hour at 45°C followed by phenol/chloroform extraction and ethanol precipitation. DNA was dissolved in 10 μl of TE buffer and quantified using a genomic PCR. The 2-μl DNA sample was mixed with 1 μM IL-8 forward primer (CCCTCGAGCATACTCCGTATTTGATAAGGAAC), 1 μM IL-8 reverse primer (GGCTCTTGTCCTAGAAGCTT), and 16 μl PCR supermix (Invitrogen). The PCR reaction was carried out as follows: 95°C for 10 minutes, 28 three-step cycles: 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds. The samples were analyzed by 2% agarose gels containing ethidium bromide.
IL-8 Reporter Assay
IB3-1 cells were transfected with IL-8 luciferase promoter with or without the NF-κB site (pGL-2-firefly luciferase) and renila luciferase (internal control), or CHOP shRNA (ΔCHOP) constructs. Cells were induced with 1 ng/ml IL1-β or 10 μM PGE-2, and luciferase activities were measured after overnight treatment. Dual-Luciferase Reporter (DLRTM) Assay System (Promega, Madison, WI) used to measure IL-8 reporter (firefly luciferase) and renila luciferace activity in IB3-1 cells. Data were normalized with internal renila luciferase control for each sample, and relative change in IL-8 promoter activity with CHOP inhibition or NF-κB mutation was calculated.
Statistical Analysis
All data are represented as the mean ± SD of three experiments. The one-way ANOVA with a Dunnett planned comparison was run for each sample versus control. A P value less than 0.05 was considered to have statistical significance.
RESULTS
PGE-2 Mediates IL-8 Chemokine Induction in CF Cells
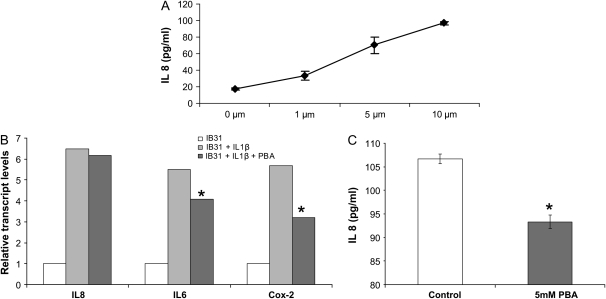
We used two CF airway epithelial cell lines: IB3-1, bronchial ΔF508/W1282X and CFTE, tracheal ΔF508-homozygous epithelial cells to test the hypothesis that higher PGE-2 levels in CF cells (6) result in an elevated IL-8–mediated inflammatory response. In Figure 1, we induced IB3-1 cells with the indicated doses of PGE-2 and challenged with 1 ng/ml IL1-β. IL-8 chemokine levels were measured using the IL-8 enzyme-linked immunosorbent assay. PGE-2 induces IL-8 chemokine levels in a dose-dependent manner (Figure 1A). The ΔF508-CFTR epithelial cell line has enhanced expression and activity of Cox-2, resulting in PGE-2 hypersecretion (6). Since butyrates are known to suppress Cox-2 activation by histone deacetylase (HDAC) inhibition (8), we used 4PBA to suppress relative transcript levels of Cox-2 in CF cells. IB3-1 cells were treated with 4PBA and/or 1 ng/ml IL1-β. IL-8, IL-6, and Cox-2 relative transcript levels were measured by quantitative real-time PCR. 4PBA down-regulated IL-1β–induced relative transcript levels of Cox-2 and IL-6 but not IL-8 (Figure 1B) mRNA. Since 4PBA inhibited the relative transcript levels of Cox-2, we expected that it may have some inhibitory effect on Cox-2–mediated IL-8 chemokine secretion. To explore this further, we used the highly sensitive IL-8 EASIA system and found that 4PBA in fact inhibits IL-1β–induced IL-8 chemokine levels in IB3-1 cells (Figure 1C). This finding suggests that 4PBA may dampen the excessive immune response in CF through the Cox-2 pathway.
Figure 1.
Prostaglandin E2 (PGE-2) regulates IL-8 chemokine levels in IB3-1 cells. (A) IB3-1 cells were induced with the indicated doses of PGE-2 for 24 hours. IL-8 chemokine levels were measured using an IL-8 enzyme-linked immunosorbent assay (ELISA). PGE-2 induces IL-8 chemokine levels in a dose-dependent manner. Data are presented as increase in IL-8 concentration (pg/ml) ± SD of three independent experiments. *P < 0.001. (B) IB3-1 cells were treated with 5 mM 4PBA and/or 1 ng/ml IL-1β for 24 hours. IL-8, IL-6, and cyclooxygenase (Cox)-2 mRNA transcripts were measured by real-time PCR. 4PBA down-regulates relative transcript levels of Cox-2 and IL-6 but not IL-8. The data is shown as average (n = 3) of relative expression level in each sample compared with IB3-1 control. *P < 0.01. (C) IB3-1 cells were treated with 5 mM 4PBA (48 h) and 1 ng/ml IL-1β (24 h). IL-8 chemokine levels were measured using the IL-8 ELISA in 4PBA-treated and untreated cells. 4PBA down-regulates IL-8 chemokine levels. Data are the mean ± SD of three independent experiments. *P < 0.05.
Cox-2 Inhibition Down-Regulates PGE-2–Mediated IL-8 Induction in CF Cells
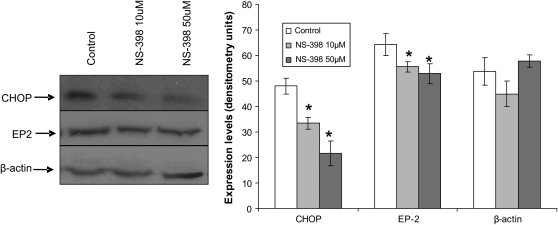
Our results indicate that 4PBA, which (as we showed previously) can rescue ΔF508-mediated chloride secretion in subjects with CF (9), also inhibits Cox-2 in IB3-1 cells. To confirm these results, we compared the effect of Cox-2 inhibition by 4PBA to a specific inhibitor of Cox-2 enzyme, NS-398, by measuring PGE-2 and IL-8 levels. Both 1mM 4PBA and 10μM NS-398 treatment of IB3-1 cells for 24 hours down-regulate PGE-2 levels (Table 1). We observed a similar effect on IL-8 chemokine levels (Table 1). The combination of 4PBA (1 mM) and NS-398 (10 μM) had a synergistic effect on PGE-2 and IL-8 levels, indicating PGE-2–mediated IL-8 induction through Cox-2 in IB3-1 cells (Table 1). The protein extracts from NS-398–treated IB3-1 cells were immunoblotted for downstream prostaglandin E2 receptor (EP-2) and transcription factor CHOP, recently shown to activate IL-8 chemokine levels in T lymphocytes (4). We observed that NS-398 (50 μM) down-regulates protein levels of both CHOP and EP-2 receptor (Figure 2).
TABLE 1.
COX-2 INHIBITION DOWN-REGULATES PGE-2–MEDIATED IL-8 INDUCTION IN IB3-1 CELLS
| Treatment | PGE-2 ± SD (pg/ml) | IL-8 ± SD (pg/ml) |
|---|---|---|
| Control | 19.7 ± 0.4 | 225 ± 32.5 |
| 1 mM 4PBA | 16.8 ± 0.9* | 201 ± 9.8* |
| 10 μM NS-398 | 16.8 ± 0.9* | 204.1 ± 5.8* |
| 1 mM 4PBA + 10 μM NS-398 | 17.8 ± 1.2* | 164.3 ± 5.7* |
Definition of abbreviations: 4PBA, 4-phenylbutyrate; Cox, cyclooxygenase; IL-8, interleukin-8; PGE-2, prostaglandin E2.
IB3-1 cells were treated with 4PBA or NS-398 as described in Materials and Methods, and PGE-2 and IL-8 levels were measured by a PGE-2 and IL-8 immunoassay. 4PBA and NS-398 treatment for 24 hours down-regulates PGE-2 and IL-8 chemokine levels. Data are the mean ± SD of three independent experiments.
P < 0.05.
Figure 2.
Cox-2 inhibition down-regulates EP-2 and CHOP expression in IB3-1 cells. IB3-1 cells were treated with NS-398 for 24 hours. EP-2, C/EBP homologous protein (CHOP), and β-actin levels were measured by immunoblotting. Cox-2 or PGE-2 inhibition by 50 μM NS-398 suppressed CHOP protein levels. EP-2 protein levels were also inhibited by NS-398 treatment. Densitometric quantification of immunoblot is shown in right-hand panel. PGE-2 mediates EP-2 and CHOP expression in IB3-1 cells. *P < 0.05.
CHOP Inhibition Suppresses IL-8 Chemokine Levels in CF Cells
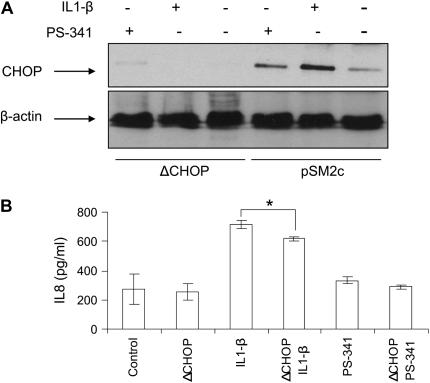
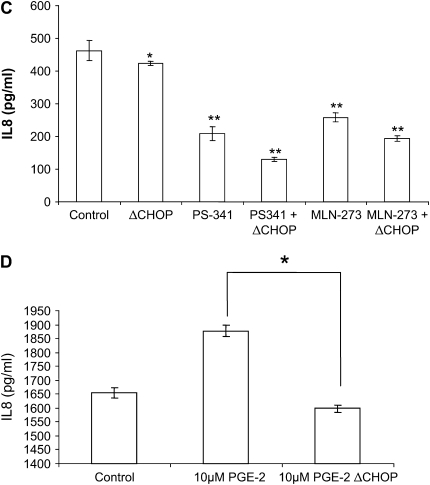
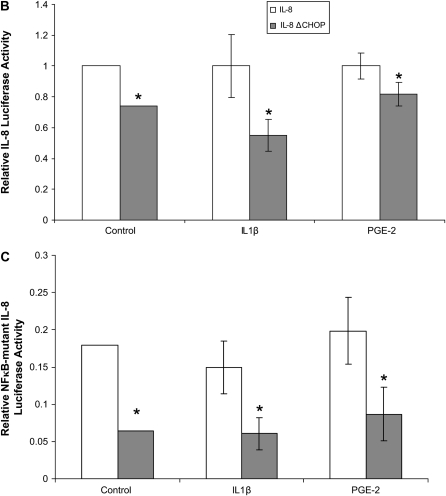
To further confirm the role of CHOP-mediated IL-8 secretion in CF, we treated IL-1β–induced IB3-1 cells with a proteasome inhibitor, PS-341, to block the NF-κB–mediated IL-8 induction or transfected IB3-1 cells with CHOP shRNA (ΔCHOP) to inhibit CHOP-mediated IL-8 induction. We found both IL1-β and PS-341 induce, while ΔCHOP suppresses, CHOP protein levels (Figure 3A). Moreover, ΔCHOP down-regulates IL-1β–induced IL-8 chemokine levels (Figure 3B). To investigate the effect of CHOP inhibition on IL-1β–induced IL-8 levels, we used two boronic acid dipeptide analogues, PS-341 and MLN-273 to block IκB–NF-κB activity (10). ΔCHOP has a synergistic effect on inhibition of IL-1β–induced IL-8 levels by PS-341 or MLN-273 (Figure 3C). We previously reported that PS-341–mediated proteasome inhibition results in rescue of functional ΔF508-CFTR on IB3-1 cell surfaces (10), and others have reported that elevated PGE-2 levels can be lowered by re-trafficking ΔF508-CFTR to cell surface (6). On the contrary our results indicate that ΔCHOP-mediated IL-8 inhibition is independent of proteasome inhibitor–mediated CFTR or IκB–NF-κB rescue.
Figure 3.
CHOP inhibition suppress IL-8 chemokine levels. IB3-1 cells were transfected with ΔCHOP or treated with IL-1β (1 ng/ml), PGE-2 (10 μM), PS-341, or MLN-273 (10 μM). CHOP and β-actin levels were determined by immunoblotting, and IL-8 chemokine levels were measured by ELISA. (A) IL-1β and PS-341 induce, while ΔCHOP down-regulates, CHOP protein levels. (B) ΔCHOP down-regulates IL-1β–induced IL-8 chemokine levels. In the absence of IL-1β–mediated proinflammatory response, we do not observe the inhibitory effect of ΔCHOP and/or PS-341 on IL-8 chemokine levels. Data are the mean ± SD of three independent experiments. (C) ΔCHOP or proteasome inhibition using PS-341 or MLN-273 down-regulate IL-1β–induced IL-8 chemokine levels. Moreover, proteasome inhibition by PS-341 or MLN-273 and ΔCHOP has a synergistic effect on inhibition of IL-1β–induced IL-8 chemokine levels. Data are the mean ± SD of three independent experiments. (D) ΔCHOP suppress the PGE-2–mediated IL-8 induction. Data are the mean ± SD of three independent experiments. *P < 0.05 for all panels, and **P < 0.01 for panel C.
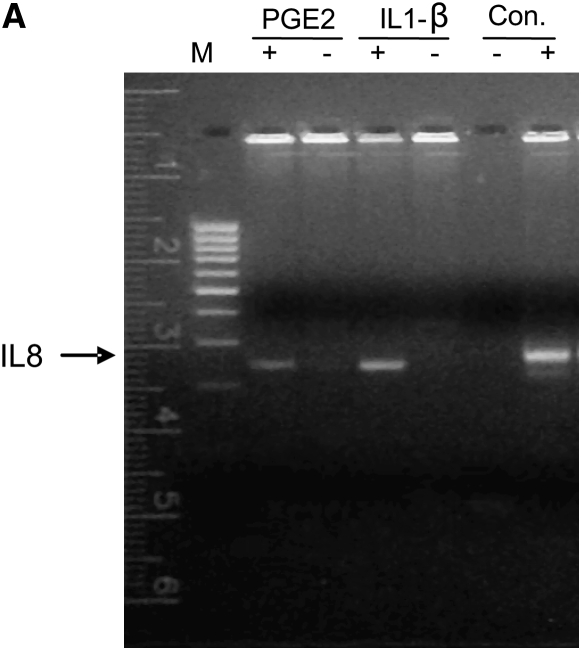
In addition to inhibition of IL-1β–induced IL-8 levels, ΔCHOP inhibited the PGE-2–mediated IL-8 induction (Figure 3D). To elucidate the mechanism of CHOP-mediated IL-8 induction in CF cells, we used the Chromatin Immunoprecipitation (ChIP) assay to detect the binding of CHOP transcription factor to the IL-8 promoter. Since CHOP was recently shown to regulate IL-8 transcriptional activity in T cells (4), it is possible that CHOP binds to the IL-8 promoter in response to pro-inflammatory stimuli in CF cells. This assay was designed to detect CHOP-mediated mRNA transcription of IL-8. Indeed, we found that both PGE-2 and IL-1β induce interaction of CHOP with the IL-8 promoter in ΔF508-CFTR homozygous CFTE cells (Figure 4A) as well as IB3-1 cells (data not shown). Moreover, we further confirmed regulation of the IL-8 promoter by CHOP in IB3-1 cells using IL-8 promoter reporter assays. We observed CHOP inhibition could down-regulate both basal and induced (IL-1β or PGE-2) IL-8 promoter activity (Figure 4B). To rule out the role of NF-κB in this regulation, we used the NF-κB mutant IL-8 promoter and again observed the down-regulation of both basal and induced (IL-1β or PGE-2) promoter activity (Figure 4C). In the absence of the NF-κB site in IL-8 promoter, CHOP inhibition had a more significant inhibitory effect on basal and PGE-2–induced IL-8 promoter activity, supporting our hypothesis that CHOP is downstream or independent of NF-κB.
Figure 4.
CHOP binds to the IL-8 promoter region. (A) CFTE cells were treated with 10 μM PGE-2 or 1 ng/ml IL-1β. The interaction of CHOP with IL-8 DNA sequences was evaluated using a Chromatin Immunoprecipitation (ChIP) assay. Both PGE-2 and IL-1β–induced binding of CHOP to the IL-8 promoter region as detected through PCR of the IL-8 promoter from the immunoprecipitate. IL-8 could not be amplified from the no-antibody IP negative control, and the CFTE DNA extract is the IL-8–positive sample. (B) IB3-1 cells were transfected with the IL-8 promoter construct tagged to reporter (IL-8 firefly luciferase) and renilla luciferase (internal control), or CHOP shRNA (ΔCHOP) constructs. Cells were induced with 1 ng/ml IL-1β or 10 μM PGE-2, and luciferase activities were measured after overnight treatment. Data are shown as relative change in IL-8 promoter activity with CHOP inhibition and is normalized with renilla luciferase internal control for each sample. The CHOP inhibition suppresses the IL-8 promoter activity. (C) IB3-1 cells were transfected with IL-8 promoter construct with deletion of NF-κB site (NF-κB mutant IL-8 firefly luciferase) and renilla luciferase (internal control), or CHOP shRNA (ΔCHOP) constructs. Cells were induced with 1 ng/ml IL-1β or 10 μM PGE-2, and luciferase activities were measured after overnight treatment. Data are shown as relative change in IL-8 promoter activity with NF-κB mutation and CHOP inhibition, and are normalized with renilla luciferase internal control for each sample. The CHOP inhibition suppresses basal NF-κB mutant IL-8 promoter activity as well as IL-1β– or PGE-2–mediated promoter activity. *P < 0.05.
PGE-2 Mediates IL-8 Induction through an NF-κB–Independent Pathway
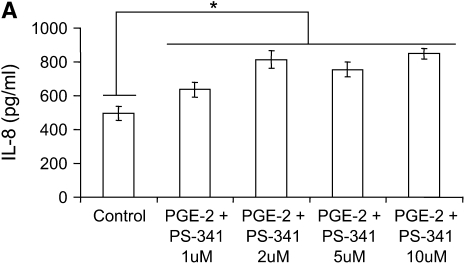
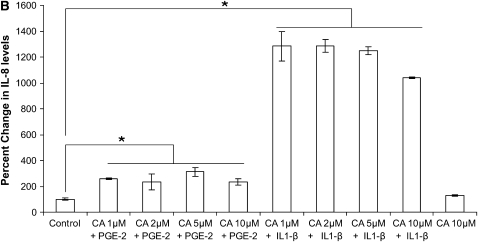
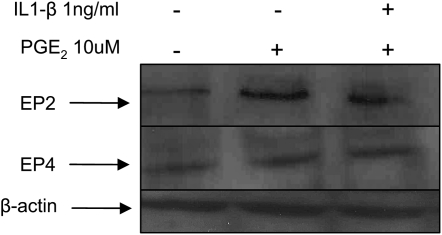
We used two CF airway epithelial cell lines, IB3-1 (ΔF508/W1282X bronchial) and CFTE (homozygous ΔF508 tracheal) to test the hypothesis that PGE-2–mediated IL-8 induction is independent of NF-κB. We treated IB3-1 cells with increasing concentrations of proteasome inhibitor to block NF-κB–mediated IL-8 induction followed by treatment with equimolar concentrations of PGE-2. Although PGE-2 did not have any effect on IL-8 chemokine levels at 16 hours (data not shown), by 36 hours PGE-2 induced IL-8 chemokine levels (Figure 5A). The inhibition of proteasome-mediated IκB degradation by increasing concentrations of PS-341 does not suppress the IL-8 levels in the presence of PGE-2, indicating that PGE-2 can rescue IL-8 suppression. Our results also demonstrate that PGE-2–mediated IL-8 signaling is either independent or downstream of IκB–NF-κB. We confirmed these results by using a highly specific inhibitor of NF-κB, caffeic acid (CA) phenyethyl ester (11), and observed similar levels of PGE-2–mediated IL-8 induction in the presence of increasing doses of CA (Figure 5B). We observed significant down-regulation of IL-8 chemokine levels with CA in the presence of IL-1β but not with PGE-2, confirming our hypothesis that PGE-2–mediated IL-8 regulation is independent or downstream of NF-κB. We also observed that proteasome inhibitor (10 μM PS-341) induces the expression of the CHOP protein levels (Figure 3A), thus providing an explanation for the minimal IL-8 induction by proteasome inhibitor in the presence of PGE-2 (Figure 5A). The EP-2 and EP-4 receptors stimulate IL-8 chemokine secretion (4) and intracellular cAMP levels (5). We detected the induction of EP-2 receptor by PGE-2 but did not observe any change in EP-4 receptor (Figure 6). IL-1β treatment had no additional effect on EP-2 or EP-4 protein levels (Figure 6).
Figure 5.
PGE-2–mediated IL-8 induction is NF-κB independent. IB3-1 cells were treated with 10 μM PGE-2 for 24 hours followed by 12 hours of treatment with increasing concentrations of PS-341. IL-8 chemokine levels were measured by standard ELISA. (A) PGE-2 induces IL-8 chemokine levels by 36 hours of induction. Inhibition of proteasome-mediated IκB degradation by PS-341 does not suppress IL-8 levels, indicating that PGE-2–mediated IL-8 induction is independent of IκB–NF-κB. Data are the mean ± SD of three independent experiments. (B) PGE-2 (10 μM) or IL-1β (1 ng/ml) induces IL-8 chemokine levels by overnight exposures. NF-κB inhibition by increasing caffeic acid (CA) concentration suppress IL-1β–induced IL-8 secretion but does not suppress PGE-2–mediated IL-8 levels, indicating that PGE-2–mediated IL-8 induction is independent of NF-κB. Data are the mean ± SD of three independent experiments. *P < 0.05.
Figure 6.
PGE-2 signaling cascade in CF cells. The total protein extracts (40 μg) from IB3-1 were immunoblotted for EP-2, EP-4, and β-actin. EP-2 protein levels were induced by 10 μM PGE-2 treatment for 24 hours, while IL-1β treatment had no effect on EP-2 or EP-4 protein levels. β-actin signals are equivalent, confirming equal protein loading in each lane.
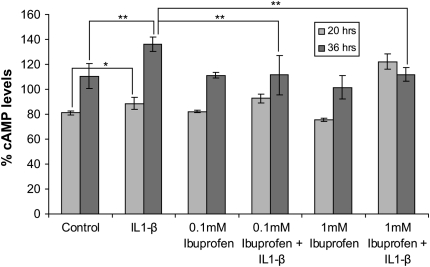
Ibuprofen Inhibits IL-1β–Induced cAMP Levels in IB3-1 Cells
We next hypothesized that use of Cox-2 inhibitors to suppress inflammation in CF will also reduce cAMP levels and hence CFTR activation that may not only adversely affect the disease but also the efficacy of therapeutic strategies designed to increase CFTR expression and/or function. To test this hypothesis, we analyzed the effect of the commonly used broad-spectrum Cox inhibitor, ibuprofen, on IL-1β–induced cAMP levels in CF cells. IL-1β (1 ng/ml) significantly induces cAMP levels in IB3-1 cells, and ibuprofen (0.1 or 1mM) treatment for 36 hours suppresses these elevated cAMP levels (Figure 7). Importantly, treatment with 1 mM ibuprofen for 20 hours stimulated IL-1β–mediated cAMP induction, indicating time- and dose-dependent effects of ibuprofen on cAMP levels in CF cells. We observed that the broad-spectrum Cox-inhibitor ibuprofen suppresses the IL-1β–induced cAMP levels in CF cells. This may reduce the efficacy of other therapeutic strategies used to increase CFTR expression and function in a subset of patients with CF.
Figure 7.
Ibuprofen inhibits IL-1β–induced cAMP levels in IB3-1 cells. IB3-1 cells were induced with 1ng/ml IL1-β and treated with 0.1 or 1 mM dose of ibuprofen. Cells were lysed after 20 or 36 hours and cAMP levels were measured using cAMP immunoassay kit (R&D Systems). IL-1β significantly induces cAMP levels in IB3-1 cells, and both 0.1 and 1 mM ibuprofen inhibits these elevated cAMP levels in IB3-1 cells by 36 hours of treatment. Data are shown as percent cAMP levels ± SD. *P < 0.06; **P < 0.05.
DISCUSSION
IL-8, the C-X-C chemokine, is a potent chemoattractant for neutrophils (12) that has been implicated in a number of inflammatory diseases, such as CF (13), adult respiratory distress syndrome (14), chronic obstructive pulmonary disease, and asthma (15). The airway epithelium is one of several sources of IL-8 in the airway (16). The airway epithelium serves as a barrier against invading microorganisms. Airway epithelial release of IL-8 contributes to host defense by promoting neutrophil chemotaxis and airway inflammation. The exaggerated inflammatory responses in chronic diseases such as CF contribute to neutrophil-driven lung destruction (17–19). Several cytokines, such as IL-1β, TNF-α, IFN-γ, and bacterial products, induce IL-8 release from airway epithelial cells (20, 21), thus exacerbating the baseline inflammatory milieu in CF.
In CF, infection followed by chronic inflammation is the major factor leading to respiratory failure and death (1, 22). Nonsteroidal anti-inflammatory drugs (NSAIDs) attenuate the acute inflammatory reaction and impair proinflammatory events dependent on neutrophils (23). The main mechanism of action of NSAIDs is inhibition of Cox and thus PG biosynthesis. It has been reported that a ΔF508-CFTR epithelial cell line has enhanced expression and activity of Cox-2 resulting in PGE-2 hypersecretion. Moreover, it was also shown that elevated PGE-2 levels can be rescued by re-trafficking ΔF508-CFTR to plasma membrane (6). The effects of PGE-2 on the airways are wide ranging, from inflammation to relaxation and inhibition of contractile responses in airway smooth muscle (24). Much of the PGE-2 in airways is likely to be derived from the epithelium (25), and the stimulation of chloride secretion in airway epithelial cells by proinflammatory mediators such as bradykinin (BK) occurs through the induced release of PGE-2 (26). Moreover, BK induces IL-8 secretion in non-CF and CF human airway epithelia via Cox-2–derived prostanoids like PGE-2 (27). In the present study we tested the hypothesis that CF airway epithelia express a hyper-inflammatory phenotype resulting from increased IL-8 release in response to PGE-2.
We found that PGE-2 mediates IL-8 chemokine induction in CF epithelial cells. Since butyrates are known to both rescue ΔF508-CFTR from degradation (28) and suppress Cox-2 activation by HDAC inhibition (8), we used 4PBA to suppress relative transcript levels of Cox-2 in CF cells. We observed that 4PBA inhibited the relative transcript levels of Cox-2 and IL-6, and IL-8 chemokine protein secretion. We further confirmed that Cox-2 mediates IL-8 secretion in CF cells by using the specific Cox-2 inhibitor NS-398, and found that Cox-2 inhibition down-regulates IL-8 chemokine levels. It has been reported that physiologic as well as pathologic concentrations up to 100 μM PGE-2 up-regulate endogenous IL-8 expression in human intestinal epithelial cells (29, 30) and enhance IL-8 production in human synovial fibroblasts stimulated with IL-1β (31). We show here that PGE-2 induces IL-8 secretion in CF airway epithelial cells, both in the absence and presence of IL-1β. Although PGE-2 is well known as a mediator of immune and phlogistic responses, the role of PGE-2 in IL-8 induction in airway epithelial cells has not been well documented. We observed that PGE-2 significantly increased IL-8 secretion by a novel signaling mechanism mediated by CHOP transcription factor. We demonstrate that the signaling pathway triggered by the EP-2 receptor is involved in this process. We postulate that PGE-2 may enhance phlogistic responses by inducing the release of IL-8 in CF.
We observed that IL-1β and proteasome inhibition by PS-341 (Velcade) minimally induced CHOP protein levels, resulting in a modest increase in basal IL-8 chemokine levels. We were able to inhibit CHOP protein levels by CHOP shRNA (ΔCHOP), and observed significant down-regulation of both IL-1β– and PGE-2–induced IL-8 chemokine levels by CHOP inhibition. Proteasome inhibition by PS-341 or MLN-273 also down-regulates IL-1β–induced IL-8 chemokine levels. It has been previously shown that CHOP deletion protects cells from endoplasmic reticulum (ER) stress by decreasing the ER client protein load and changing the redox conditions within the organelle (32). The misfolded client protein, proteasome inhibition, or hyper-inflammatory response imposes a stress on the ER. The cells under ER stress induce pro-apoptotic signals mediated by CHOP (33). CHOP deletion may protect against lethal consequences due to ER stress. Previously, we found that a dipeptide boronic acid analogue (PS-341) can rescue ΔF508-CFTR from ER-associated degradation (ERAD) and also rescue CFTR-mediated chloride efflux (10). We predicted that deleting CHOP will not only inhibit IL-8 induction in CF cells, but also rescue CF cells from the unfolded protein response (UPR) or proteasome inhibition–mediated ER stress. We observed that CHOP inhibition not only suppresses the PGE-2–mediated IL-8 induction but also has a synergistic effect on proteasome inhibitor–mediated IL-8 suppression. We and others observed that proteasome inhibition rescues IκB from degradation and NF-κB–mediated IL-8 induction (34, 35). We were able to overcome the proteasome inhibitor–mediated IL-8 suppression by adding PGE-2, indicating that the PGE-2–mediated IL-8 induction pathway is downstream of NF-κB. Moreover, we could not completely suppress the IL-8 induction by NF-κB inhibition, indicating the presence of alternate pathways for IL-8 induction or the induction of downstream components of the PGE-2–signaling pathway by some other pathway. We also confirmed these results with a highly specific NF-κB inhibitor, CA phenylethyl ester.
We further confirmed that PGE-2 mediates IL-8 induction via the EP-2-CHOP pathway, and found that CHOP binds to the IL-8 promoter in the presence of PGE-2 or IL-1β in CF cells. A variety of transcription factors such as NF-κB, NF–IL-6, activator protein (AP)-1, and octamer-1 have been shown to regulate the IL-8 gene transcription (36–39). To our knowledge, there is only one report on PGE-2–induced IL-8 gene transcription in human T lymphocytes via CHOP transcription factor (4). PGE-2 has profoundly different effects on the production of IL-8 that are dependent on cell type and environment. We confirmed CHOP as a regulator of PGE-2 or IL-1β–mediated IL-8 induction in CF cells using an IL-8-promoter reporter assay. Moreover, we used the NF-κB mutant IL-8 promoter construct to demonstrate that CHOP-mediated IL-8 regulation is independent or downstream of NF-κB. Our data describe for the first time that CHOP functions as a transcription factor that regulates IL-8 chemokine levels in CF cells. The NF-κB–mediated IL-8 induction in CF is well known (40), and we show here for the first time the presence of downstream PGE-2–mediated IL-8 signaling via CHOP transcription factor.
The NSAID ibuprofen is known to inhibit 5-lipooxygenase and hence leukotreine formation (41), suggesting that it may be useful in treatment of CF. This led to clinical trials in which it was demonstrated that ibuprofen slows the rate of loss in FEV1 in patients with CF with mild lung disease (42, 43), but did not address the mechanism by which ibuprofen improves lung function. However, there is evidence that use of a low dose of ibuprofen is associated with worsening FEV1, and the best evidence for a therapeutic benefit was in children, not adults, with CF. We anticipated that use of ibuprofen in CF can exacerbate the disease in individuals with partially functional CFTR by suppressing the IL-1β– or PGE-2–mediated cAMP induction that is requisite for CFTR activation. We observed that ibuprofen can indeed inhibit IL-1β–induced cAMP levels in IB3-1 cells at both higher (1 mM) and lower (0.1 mM) doses (Figure 7). Nasal potential difference (NPD) monitoring of individuals with CF with pancreatic sufficiency (PS) alleles, on and off ibuprofen, might help us estimate this risk. Moreover, it was recently reported that ibuprofen inhibits cAMP-mediated chloride secretion in human colonic and airway epithelia by direct inhibition of CFTR chloride channels as well as basolateral membrane K+ channels (44). This may not only further accelerate the disease, but also affect the efficacy of therapeutic strategies designed to increase CFTR expression and/or function in patients with cell surface conductance mutations. Our results on Cox-2/PGE-2 inhibition by 4PBA also similarly explain the differences in efficacy of 4PBA treatment in a subset of patients with CF, with submaximal low chloride/isoproterenol response in NPD measurements (9), due to lack of optimal cAMP-mediated CFTR activation.
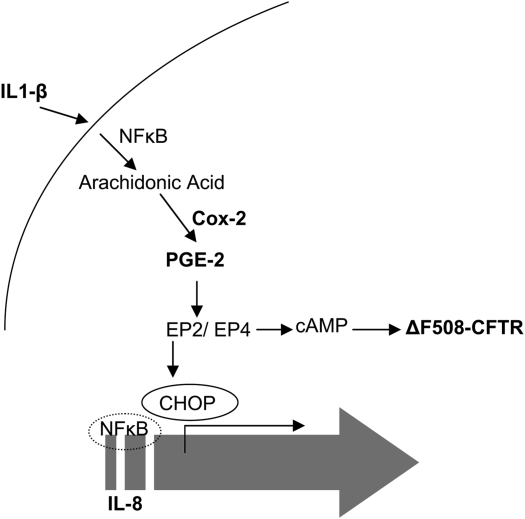
Overall, our study unravels the complexity of IL-8 regulation in CF cells (Figure 8). This will not only help us in understanding the mechanism of CF pathophysiology but also help in targeting more specific drugs for treatment of CF lung disease. We anticipate that the use of Cox-2 or NF-κB inhibitors in CF for controlling inflammation may augment the progression of the lung disease in a subset of patients with CF with mild alleles by inhibiting other downstream pathways like EP-2/EP-4–mediated cAMP levels (4, 44) or by switching off the proinflammatory response altogether. They may also reduce the efficacy of other therapeutic strategies used to increase CFTR expression and function in these patients. We propose that sequential deciphering of IL-8 regulation in CF may lead to identification of better and more specific therapeutic target(s) to improve the overall CF pathophysiology and lung function.
Figure 8.
Schematic of the proposed IL-8 signal transduction cascade in CF cells. Proinflammatory stimuli (IL-1β) increase the levels of PGE-2 via NF-κB–mediated Cox-2 induction, which results in induction of IL-8 through the CHOP transcription factor. CHOP is activated via the EP-2 receptor in the PGE-2–signaling cascade. The broad-spectrum Cox inhibitor, ibuprofen, suppresses the IL-1β–mediated IL-8 and cAMP induction. The Cox inhibition control IL-8 mediated inflammation but it may further deteriorate the CF pathophysiology due to inefficient cAMP-mediated CFTR activation.
Acknowledgments
The authors thank Dr. H. R. Wong, Cincinnati Children's Hospital, for providing IL-8 promoter constructs. IB3-1 cells are under a licensing agreement between Pfizer Inc., Japan Tobacco, Inc., and Johns Hopkins University (JHU). MLN-273 was kindly provided by Millennium Pharmaceuticals under a material transfer agreement.
This work was supported by grant R01 HL59410 (to P.Z.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0197OC on August 20, 2007
Conflict of Interest Statement: 4-phenylbutyrate (4-PBA) is under a licensing agreement between JHU, Ucyclyd Pharma, Inc., and P.L.Z. The terms of these arrangements are being managed by the JHU in accordance with its conflict of interest policies. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001. [DOI] [PubMed] [Google Scholar]
- 2.Thibodeau PH, Brautigam CA, Machius M, Thomas PJ. Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol 2005;12:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, Kelly C, O'Sullivan BP, Freedman SD. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol 2004;11:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caristi S, Piraino G, Cucinotta M, Valenti A, Loddo S, Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J Biol Chem 2005;280:14433–14442. [DOI] [PubMed] [Google Scholar]
- 5.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci 2003;74:143–153. [DOI] [PubMed] [Google Scholar]
- 6.Medjane S, Raymond B, Wu Y, Touqui L. Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L816–L824. [DOI] [PubMed] [Google Scholar]
- 7.Vij N, Zeitlin PL. Regulation of the ClC-2 lung epithelial chloride channel by glycosylation of SP1. Am J Respir Cell Mol Biol 2006;34:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun 2004;317:463–471. [DOI] [PubMed] [Google Scholar]
- 9.Zeitlin PL, Diener-West M, Rubenstein RC, Boyle MP, Lee CK, Brass-Ernst L. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol Ther 2002;6:119–126. [DOI] [PubMed] [Google Scholar]
- 10.Vij N, Fang S, Zeitlin PL. Selective inhibition of endoplasmic reticulum-associated degradation rescues {delta}F508-cystic fibrosis transmembrane regulator and suppresses interleukin-8 levels: therapeutic implications. J Biol Chem 2006;281:17369–17378. [DOI] [PubMed] [Google Scholar]
- 11.Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA 1996;93:9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA 1987;84:9233–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest 1992;89:1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 1993;341:643–647. [DOI] [PubMed] [Google Scholar]
- 15.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol 1992;89:1001–1009. [DOI] [PubMed] [Google Scholar]
- 16.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP III, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990;86:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995;310:1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birrer P, McElvaney NG, Rudeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal RG. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med 1994;150:207–213. [DOI] [PubMed] [Google Scholar]
- 19.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 1994;150:448–454. [DOI] [PubMed] [Google Scholar]
- 20.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 1995;96:2204–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massion PP, Inoue H, Richman-Eisenstat J, Grunberger D, Jorens PG, Housset B, Pittet JF, Wiener-Kronish JP, Nadel JA. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Invest 1994;93:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratjen F, Doring G. Cystic fibrosis. Lancet 2003;361:681–689. [DOI] [PubMed] [Google Scholar]
- 23.Abramson SB, Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Arthritis Rheum 1989;32:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Smith AP, Cuthbert MF, Dunlop LS. Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clin Sci Mol Med 1975;48:421–430. [DOI] [PubMed] [Google Scholar]
- 25.Barnett K, Jacoby DB, Nadel JA, Lazarus SC. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am Rev Respir Dis 1988;138:780–783. [DOI] [PubMed] [Google Scholar]
- 26.Leikauf GD, Ueki IF, Widdicombe JH, Nadel JA. Alteration of chloride secretion across canine tracheal epithelium by lipoxygenase products of arachidonic acid. Am J Physiol 1986;250:F47–F53. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers HC, Pang L, Holland E, Corbett L, Range S, Knox AJ. Bradykinin increases IL-8 generation in airway epithelial cells via COX-2-derived prostanoids. Am J Physiol Lung Cell Mol Physiol 2002;283:L612–L618. [DOI] [PubMed] [Google Scholar]
- 28.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol 2000;278:C259–C267. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Chadee K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J Immunol 1998;161:3746–3752. [PubMed] [Google Scholar]
- 30.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem 2001;276:38658–38664. [DOI] [PubMed] [Google Scholar]
- 31.Agro A, Langdon C, Smith F, Richards CD. Prostaglandin E2 enhances interleukin 8 (IL-8) and IL-6 but inhibits GMCSF production by IL-1 stimulated human synovial fibroblasts in vitro. J Rheumatol 1996;23:862–868. [PubMed] [Google Scholar]
- 32.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004;18:3066–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, del Rio G, Gibson BW, Ellerby HM, Bredesen DE. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem 2004;279:177–187. [DOI] [PubMed] [Google Scholar]
- 34.Dai RM, Chen E, Longo DL, Gorbea CM, Li CC. Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IkappaBalpha. J Biol Chem 1998;273:3562–3573. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava M, Eidelman O, Zhang J, Paweletz C, Caohuy H, Yang Q, Jacobson KA, Heldman E, Huang W, Jozwik C, et al. Digitoxin mimics gene therapy with CFTR and suppresses hypersecretion of IL-8 from cystic fibrosis lung epithelial cells. Proc Natl Acad Sci USA 2004;101:7693–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira IC, Mukaida N, Matsushima K, Vilcek J. Transcriptional inhibition of the interleukin-8 gene by interferon is mediated by the NF-kappa B site. Mol Cell Biol 1994;14:5300–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol 1994;56:554–558. [PubMed] [Google Scholar]
- 38.Mastronarde JG, He B, Monick MM, Mukaida N, Matsushima K, Hunninghake GW. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-kappa B and NF-IL-6. J Infect Dis 1996;174:262–267. [DOI] [PubMed] [Google Scholar]
- 39.Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter: the role of Oct-1 as a transcriptional repressor. J Biol Chem 1997;272:2396–2403. [PubMed] [Google Scholar]
- 40.Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros 2005;4:15–23. [DOI] [PubMed] [Google Scholar]
- 41.Vanderhoek JY, Bailey JM. Activation of a 15-lipoxygenase/leukotriene pathway in human polymorphonuclear leukocytes by the anti-inflammatory agent ibuprofen. J Biol Chem 1984;259:6752–6756. [PubMed] [Google Scholar]
- 42.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995;332:848–854. [DOI] [PubMed] [Google Scholar]
- 43.Konstan MW, Hoppel CL, Chai BL, Davis PB. Ibuprofen in children with cystic fibrosis: pharmacokinetics and adverse effects. J Pediatr 1991;118:956–964. [DOI] [PubMed] [Google Scholar]
- 44.Devor DC, Schultz BD. Ibuprofen inhibits cystic fibrosis transmembrane conductance regulator-mediated Cl- secretion. J Clin Invest 1998;102:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]