Abstract
β2-adrenergic receptors are present throughout the lung, including the alveolar airspace, where they play an important role for regulation of the active Na+ transport needed for clearance of excess fluid out of alveolar airspace. β2-adrenergic receptor signaling is required for up-regulation of alveolar epithelial active ion transport in the setting of excess alveolar edema. The positive, protective effects of β2-adrenergic receptor signaling on alveolar active Na+ transport in normal and injured lungs provide substantial support for the use of β-adrenergic agonists to accelerate alveolar fluid clearance in patients with cardiogenic and noncardiogenic pulmonary edema. In this review, we summarize the role of β2-adrenergic receptors in the alveolar epithelium with emphasis on their role in the regulation of alveolar active Na+ transport in normal and injured lungs.
Keywords: pulmonary edema, acute respiratory distress syndrome, acute lung injury, alveoli, albuterol
CLINICAL RELEVANCE
This review will help clinicians understand the mechanisms by which β2-adrenergic therapy may be useful in the management of acute lung injury.
β2-adrenergic receptors (β2AR) are present throughout the lung. In the alveolar airspace they are important for regulation of the active Na+ transport needed for clearance of excess fluid out of alveolar airspace (1). Both experimental and limited clinical data suggest that β-adrenergic agonists working via the β2AR accelerate clearance of excess fluid from the alveolar airspace, creating the possibility of their use for treatment of pulmonary edema and acute lung injury (ALI).
In this review, we summarize the role of β2-adrenergic receptors in the alveolar epithelium with emphasis on their role in regulation of alveolar active Na+ transport in normal and injured lungs. We also overview data regarding β2-agonist therapy for pulmonary edema and lung injury.
β-ADRENERGIC RECEPTORS
Subtypes and Distribution in the Lung
β-adrenergic receptors are ubiquitous throughout the human body and are classified into three distinct subtypes (β1, β2, and β3) on the basis of their function, agonist-binding patterns, and genetics (Table 1). There is 65 to 70% homology among the β1-, β2-, and β3-receptors. The β3-receptor is found primarily in adipocytes but is also present in pulmonary endothelial cells. The proposed β4-receptor appears to be a conformational state of β1-receptor in myocardial cells (2). Neither the β3 or β4 receptors have been linked with regulation of ion transport in epithelial cells.
TABLE 1.
SUBTYPES OF β-ADRENERGIC RECEPTORS
| Subtype | Location | Physiologic effects | Agonist | Antagonist |
|---|---|---|---|---|
| β1 | Heart | ↑ Myocardial contractility | (−) Ro-363 | Metoprolol |
| Brain | Atenolol | |||
| Kidney | Renin release | CGP 20712 | ||
| Vessels | ||||
| Brain and coronary | Vasodilation | |||
| β2 | Lung | |||
| Alveolar epithelium | ↑ Alveolar fluid clearance | Albuterol | Butoxamine | |
| Bronchial epithelium | Formoterol | ICI 118551 | ||
| Smooth muscle | Bronchodilation | Procaterol | ||
| Skeletal muscle | Salmeterol | |||
| Cerebellum | Terbutaline | |||
| Uterine | S1319 | |||
| Vessels (peripheral) | Vasodilation | |||
| β3 | Adipose tissue | ↑ Lipolysis | L 755,507 | SR 59230 |
| Ureteral muscle | Relaxation of ureter | CL 316,243 | L 748337 | |
| Heart | ↑ Myocardial contractility | LY 368842 | L 747328 | |
| Vasculature | Vasodilation | Ro 40-2148 | ||
| SR 58611 | ||||
| BRL 37344 |
Nonspecific agonists include isoproterenol, norepinephrine, and epinephrine.
Nonselective antagonists include propranol.
In the lung, β2-adrenergic receptor (β2AR) expression increases with each airway generation, with the greatest total amounts in the distal airways and alveoli (3). Greater than 90% of all β-adrenergic receptors in human lung are located in the alveoli (4). Although both β1 and β2 subtypes coexist and are distributed uniformly in the alveolar walls, the β2-subtype predominates (70%) (4). Isolated rat alveolar type II cells possess β2AR and data from autoradiographic and immunohistochemical studies support their presence in the alveolar type 1 cells (4, 5).
β2-Adrenergic Receptor Structure and Function
The β2-adrenergic receptor is a 1,200–base pair, single-copy, intronless gene located on the long arm of human chromosome 5 that encodes a 413–amino acid protein with a molecular mass of approximately 46.5 kD (1). The β2-receptor is a prototypical G protein–coupled receptor (GPCR) with seven-transmembrane domains, an extracellular amino terminus, an intracellular carboxyl terminus, three interconnecting extracellular loops, and three intracellular loops.
β2-adrenergic receptors exist in the plasma membrane in an equilibrium between at least two structural conformations; inactive and active forms that are defined based on their ability to associate with the stimulatory guanosine triphosphate (GTP)-binding protein, Gs. Like many GPCRs, β2-adrenergic receptors spontaneously oscillate between inactive and active conformations. In the absence of agonist, the basal equilibrium favors the inactive conformation (6, 7). Spontaneous receptor activation explains the presence of basal β2AR-driven adenylyl cyclase activity in cells and observations of increased receptor function in the absence of agonist in models of β2AR overexpression (8–10). Engagement of the β2AR by a β2-agonist produces a conformational change shifting the equilibrium between receptor conformations toward the active form causing exchange of guanosine diphosphate (GDP) on Gsα for GTP and dissociation of Gsα from Gsβγ. The inactive β2AR conformation is stabilized by inverse agonists and does not activate Gsα; likewise, replacement of GTP by GDP on Gs uncouples it from the receptor promoting a switch to the inactivate conformation of the β2AR.
The conformational state and hence activity of the β2AR changes with phosphorylation of the receptor. Phosphorylation of ligand occupied receptors by GPCR kinase 2 (GRK2) and protein kinase A (PKA) produces conformational changes that reduce receptor interactions with Gsα and diminish the affinity of receptors for ligand, thereby shifting the β2AR toward an inactive state.
Activation of β2AR results in a variety of distinct signaling events besides activation of adenylyl cyclase. GRK2 phosphorylation of β2AR allows for binding of β-arrestins to the receptor, which uncouples the receptor from Gs, and promotes receptor endocytosis via clathrin-coated vesicles (11). Phosphorylation of β2AR also promotes signaling via Giβγ with subsequent down-regulation of adenylyl cyclase activity and activation of mitogen-activated protein kinase (p44/p42). Both of these inhibitory pathways are important for regulation of Na,K-ATPase trafficking and function in alveolar epithelial cells (12–14). Table 2 summarizes protein–protein interactions and signal transduction systems in which the β2AR participates (15–35).
TABLE 2.
PROTEINS/PATHWAYS INVOLVED IN β2-ADRENERGIC RECEPTOR SIGNALING
| Protein Kinases | ||
| Protein kinase A (15, 16) | Receptor phosphorylation | |
| ↓ Interaction with Gs | ||
| ↑ Interaction with Gi → Activation of MAPK (ERK) | ||
| Protein kinase C | Receptor phosphorylation | |
| ↓ Interaction with Gs | ||
| Tyrosine kinases | ||
| Insulin receptor (17, 18) | Receptor phosphorylation (Tyrosine residues) | |
| ↓ (Tyr 141) or ↑ (Tyr 350/354) cAMP production | ||
| ↑ Src binding and GRK2 activation (Tyr 350/354) | ||
| ↑ Internalization | ||
| Insulin-like growth factor-1 | Receptor tyrosine phosphorylation | |
| EGFR (19) | EGFR transactivation | |
| ERK1/2 activation | ||
| G protein–coupled receptor kinases (GRK) | ||
| GRK2 (20–22) | Receptor phosphorylation (agonist occupied) | |
| ↑ Arrestin binding → ↓ Interaction with Gs | ||
| GRK5 (23, 24) | Receptor phosphorylation (agonist occupied) | |
| ↓ Interaction with PDZ-domain-containing proteins | ||
| ↑ Arrestin binding → ↓ Interaction with Gs | ||
| Scaffold Proteins | ||
| A-kinase anchoring proteins (AKAP) (25–27) | ||
| AKAP250/AKAP79/150 | Receptor phosphorylation, and internalization | |
| (Gravin) | Bind receptor to apical cytoskeleton | |
| Bring receptor in proximity to PKA | ||
| Arrestins (28–31) | Bind to GRK-phosphorylated receptor | |
| ↓ Interaction with Gs | ||
| Promote ubiquitination of receptor | ||
| ↑ Internalization (via interaction with Src) | ||
| Facilitate receptor interaction with MAPK | ||
| PDZ-domain-containing proteins (24, 32, 33) | ||
| EBP50 | ↓ Attenuation of activity of Na+/H+-exchanger 3 | |
| ↑ Receptor cycling | ||
| Other Proteins (34, 35) | ||
| N-ethylmaleimide–sensitive factor | ↑ Internalization and receptor cycling | |
| Eukaryotic initiation factor 2B α-subunit | ↓ Agonist-promoted adenylyl cyclase activity | |
Definition of abbreviations: cAMP, cyclic-adenosine monophosphate; EBP50, ezrin-radixin-moesin–binding phoshoprotein 50; EGFR, epidermal growth factor receptor; ERK, extracellular signal–regulated kinase; MAPK, mitogen-activated protein kinase; PKA, protein kinase A.
Adapted from Ref. 1.
Traditionally, β2-agonists have been classified simply as full, near-full, or partial agonists based on their ability to promote cAMP production. A recent study by Swift and coworkers suggested that the possibility of Gs/cAMP-independent signaling by the β2AR creates much pleiotropy among β2AR ligands and as such quantification of agonist activity in terms of cAMP production may no longer be relevant (7).
ROLE OF ALVEOLAR EPITHELIAL β2-ADRENERGIC RECEPTORS
Regulation of Alveolar Active Na+ Transport
β2-adrenergic receptors, via cAMP-dependent and -independent pathways, regulate several of the key proteins needed for alveolar epithelial ion and fluid transport including amiloride-sensitive epithelial Na+ channels, the cystic fibrosis transmembrane conductance regulator (CFTR), and the Na, K-ATPase (36). The initial observation by Goodman and colleagues showing β-agonist–induced active transcellular ion flux in confluent monolayers of isolated rat alveolar epithelial cells (37) was followed by many studies from isolated rat lungs (38) and anesthetized sheep (39) demonstrating that β-adrenergic agonists might be useful for the treatment of pulmonary edema.
Endogenous and exogenous catecholamines stimulate alveolar fluid clearance in newborn and adult animals via activation of β-receptors. Nonspecific βAR (isoproterenol, epinephrine, dobutamine) and β2AR-specific agonists (procaterol, salmeterol, terbutaline) increase alveolar fluid clearance in normal rat (40), dog (41), sheep (42), guinea pig (43), and mouse lungs (44, 45), and in human lung tissue (46). β2-receptors appear to be responsible for the bulk of the β-receptor–sensitive alveolar active Na+ transport (9). Activation of the β1-adrenergic receptor can accelerate alveolar active Na+ transport (47); however, data from studies in β2AR knockout mice suggest that the β2AR is responsible for most of the β-adrenergic–mediated up-regulation of AFC in fluid-filled lungs (9).
β-receptor–mediated increases in alveolar active Na+ transport are likely due to direct and indirect up-regulation of the epithelial Na+ channel, CFTR, and Na,K-ATPase (38, 43, 48–51) (Figure 1). In vitro, β-agonists stimulate both highly selective Na+ channels and amiloride-sensitive, Na+-permeable, nonselective cation channels (52). Yue and coworkers have demonstrated that stimulation of βAR with terbutaline increases the number of epithelial Na+ channels and their open time in alveolar type II cells (51). These effects of β2-agonists are mediated via PKA, which phosphorylates cytoskeleton proteins and promotes trafficking of Na+ channels to the cell membrane (53) and direct phosphorylation of epithelial Na+ channel β and γ subunits. In addition to translocation from intracellular pools to the plasma membrane, β2-receptors increase the expression of epithelial Na+ channel α-subunit mRNA and protein (54). β-agonists and cAMP analogs increase the open probability and open time of amiloride-sensitive Na+ channels in confluent rat alveolar type II cells in vitro (55). Thus, β2AR agonists increase Na+ flux across the apical cell membrane by increasing both membrane-bound channel abundance and Na+ flux through the channels. Data supporting this conclusion come primarily from rat alveolar type II cells; however, the observation that alveolar type I cells have functional ion transporters and β2AR suggests similar regulation of ion transport in alveolar type I cells (5, 56, 57).
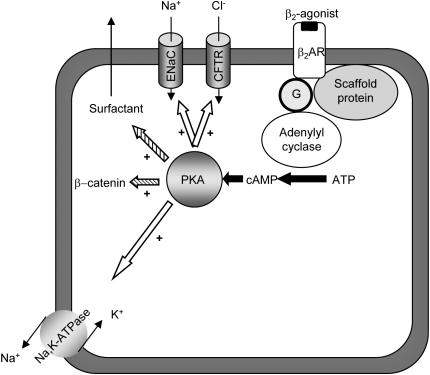
Figure 1.
Effects of β2-adrenergic receptor activation in alveolar epithelial cells. Activation of β2-adrenergic receptor (β2AR) increases alveolar active Na+ transport via upregulation of epithelial Na+ channel (ENaC) and cystic fibrosis transmembrane conductance regulator (CFTR) as well as basolaterally located Na,K-ATPase (open arrows). Activation of the receptor also increases β-catenin and surfactant release, which might be important in the pathogenesis/resolution of acute lung injury. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A.
Activation of β2-adrenergic receptor increases cellular Na,K-ATPase activity in alveolar epithelial cells in vitro and lung tissue (8, 39, 49, 58). β2-adrenergic receptor–mediated short-term regulation of Na+ pumps occurs within minutes of receptor engagement via highly regulated recruitment of assembled Na,K-ATPases from intracellular compartments through phosphorylation of intermediary proteins and RhoA-kinase (49, 59). Long-term regulation is carried out via transcription (54) and translation of α1-subunit of Na,K-ATPase through PKA-induced phosphorylation of cAMP-responsive elements and post-transcriptional regulation (via mitogen-activated protein kinase/extracellular signal–regulated kinase and rapamycin-sensitive pathways) (60). There are no clear data to support a role for β-agonist–mediated up-regulation of the activity of individual Na,K-ATPases. These mechanistic findings suggest that up-regulation of Na,K-ATPase activity by β2-receptor signaling is a complex process that occurs primarily through increased number of Na+ pumps in the cell membrane rather than increased activity of individual pumps.
β2AR-mediated up-regulation of fluid transport also involves Cl− transport via CFTR (61–63). Data from alveolar epithelial cells convincingly indicate that β2-receptor signaling increases Cl− flux through the CFTR (61, 64, 65), similar to that seen in proximal airway epithelial cells (32, 66). Data from CFTR-deficient mice (Δφ508 transgenics) indicate that CFTR is not required for alveolar fluid homeostasis in the uninjured lung but is essential in the presence of excess airspace fluid and β2AR-mediated enhancement of AFC (61, 67). In vitro studies reveal that cAMP produces an initial and rapid increase in Cl− current, which precedes increases in amiloride-sensitive Na+ current offering the possibility that CFTR and/or Cl− flux may influence ENaC function and Na+ flux into the cell (68). Both β-agonists or adenoviral overexpression of β2AR do not increase alveolar fluid clearance in Δφ508 transgenics to the same degree as in wild-type mice (61, 67); likewise, overexpression of CFTR in mice that lack the β2AR does not up-regulate alveolar fluid clearance rate compared to control mice infected with adenovirus that encodes CFTR (61). These data suggest an interdependency between β2AR and CFTR and that both are essential in up-regulation of active Na+ transport and fluid clearance in the alveolus (61). They also support a model in which CFTR may the principal effector of β2AR-mediated up-regulation of alveolar ion transport.
An intriguing question is why engagement of β2-receptors produces highly compartmentalized activation of cAMP-sensitive pathways. Recent data indicate that the β2AR interacts with scaffold and adaptor proteins via its carboxy-terminal end (reviewed in Refs. 69 and 70). These interactions link the β2-receptor directly or indirectly via ezrin-radixin-moesin-binding phosphoprotein 50 to the cytoskeleton at the apical domain of the cell membrane, forming a macromolecular complex composed of protein kinase A, GPCR kinases, ion channels (e.g., CFTR) and phosphodiesterases, which hydrolyze cAMP (66) (Figure 2). This regulatory complex brings the receptor in close proximity to its principal effector molecule (protein kinase A) and to its downstream targets (CFTR) as well as proteins that turn receptor signaling off (GPCR kinase) and prevent diffusion of cAMP (phosphodiesterases). Studies by Sun and colleagues suggested that ezrin-radixin-moesin-binding phosphoprotein 50–mediated interactions between the receptor and CFTR are important for regulation of Cl− transport in airway epithelial cells (Calu-3) (71, 72). Whether similar interactions occur in alveolar epithelial cells is not yet known.
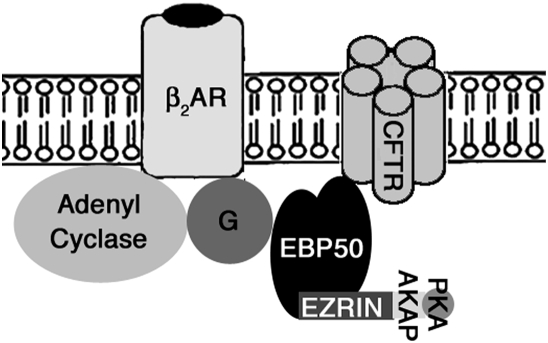
Figure 2.
Proposed interaction of β2-adrenergic receptor with CFTR and other proteins. In the cell membrane, the β2AR is in close proximity to transport molecules such as CFTR and possibly ENaC. This macromolecular complex is maintained through interactions of the β2AR with scaffold and adaptor proteins including EBP50 and ezrin, which also link the receptor with submembrane cytoskeletal elements. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; EBP50, ezrin-radixin-moesin-binding phosphoprotein 50; PKA, protein kinase A. (Adapted from Reference 32.)
The concentration of cAMP in the cell is determined by the activities of adenylyl cyclases and phophodiesterases, which inactivate cAMP (73). There are nine types of adenylyl cyclases, which are all transmembrane proteins, synthesizing cAMP at the plasma membrane (73, 74). This results in a gradient in which higher concentrations of cAMP are found at the membrane and lower concentrations in the cytosol (73, 75–78). This gradient may allow localization of the physiologic effect of βAR and resultant production of cAMP to microenvironments, which in turn activate specific target proteins.
Maintenance of Alveolar Fluid Homeostasis
Recent data provide some clues regarding whether β2AR are required for maintenance of alveolar fluid balance in the normal lung (39, 41, 44–46, 79, 80). While initial studies of adrenalectomized animals (8, 45) or desensitization of β-receptors (81, 82) note no net effect on lung water content or basal alveolar fluid clearance, a more recent study showed reduced basal alveolar fluid clearance in adrenelectomized mice (83). The differences between these studies may be due to inability to fully desensitize alveolar β2-receptors or completely eliminate serum catecholamines and methods used to quantify alveolar fluid clearance (in vivo live model versus ex vivo). Data from mice with no functional β2-receptor (β2-knockout) or β1-receptor and β2-receptor (β1β2-knockout) (9) similarly reveal normal water content when uninjured but decreased ability to clear excess water from the airspace and more pulmonary edema and decreased survival from acute lung injury (hyperoxia). These findings suggest that the β2AR is functionally required in the presence of excess airspace fluid, but may not be required to maintain lung fluid balance in the uninjured lung or when fluid shifts are modest. Importantly, these data indicate that other regulatory pathways are not sufficient to accelerate alveolar active Na+ transport mechanisms in the injured lung.
Other Functions
The effects of β-adrenergic receptor in the alveolar epithelium are not limited to up-regulation of active Na+ transport. β2-adrenergic receptor may also increase levels of the Wnt pathway member β-catenin, which regulates cell-to-cell adhesions via cadherin (84, 85). Phosphorylation of β-catenin by PKA stabilizes β-catenin post-translationally through inhibition of its ubiquitination (86). Experimental data also suggest that β-agonists can improve endothelial barrier function (87–89) by inhibition of endothelial cell contraction and reduced intracellular gap formation (90, 91). β2AR also regulate surfactant secretion by alveolar type II cells (92, 93). These additional effects may be responsible for some of the protective effects of β2-agonists in the acutely injured lung.
In contrast to the beneficial effects of β2-agonists on the alveolar epithelium, activation of β2AR (94) has an inhibitory effect on the function of alveolar macrophages where they diminish motility (95) via increased levels of cAMP, which has also been reported to result in diminished fluid phase endocytosis (96) and phagocytosis function (97).
β2-AGONIST THERAPY FOR TREATMENT OF PULMONARY EDEMA
Experimental Studies
There is a great body of experimental data indicating that specific and nonspecific β-agonists enhance AFC in experimental models of cardiogenic and noncardiogenic pulmonary edema (1, 9, 61). Recent data suggest that preservation of β-adrenergic receptor signaling attenuates loss of endothelial cell barrier function in mice with severe bacterial pneumonia (98). Stimulation of β-adrenergic receptors is also capable of preventing hypoxia-induced reduction in alveolar active Na+ transport and fluid clearance in rats (99). Physiologic concentrations of β-agonists do not alter neutrophil chemotaxis, death/apoptosis in vitro, or affect alveolar recruitment and activation of neutrophils in vivo (100); thus, the beneficial effects of β-agonists in these models are unlikely to be a reflection of their putative anti-inflammatory effects.
McAuley and colleagues investigated the effect of clinically relevant doses of β2-agonists (in the alveolar epithelial lining fluid) on alveolar fluid clearance in an acid aspiration model of acute lung injury in rats (89). Racemic albuterol (10−5 M), salmeterol (10−6 M), and isoproterenol (10−6 M) each stimulated basal alveolar fluid clearance to levels comparable to maximal cAMP-dependent alveolar fluid clearance using a stable analog of cAMP (dibutyryl cAMP 10−3 M) (89). This improvement in alveolar fluid clearance correlated with attenuation of acid aspiration lung injury.
Both transgenic and adenoviral-mediated overexpression of β2-receptor in the alveolar epithelium increase alveolar active Na+ transport (8, 9, 101), probably by increasing the number of receptors in active conformation. Transgenic overexpression of β2-receptor in alveolar type II cells increases alveolar fluid clearance in mice by approximately 40% (101). Adenoviral-mediated transfer of a human β2-receptor to the alveolar epithelium increases alveolar fluid clearance in normal rats and mice by up-regulating the expression and/or function of amiloride-sensitive epithelial Na+ channels and Na,K-ATPases in the distal lung (8, 9). These effects were attributed, in part, to improved responsiveness to endogenous catecholamines. Importantly, overexpression of the β2-receptor in mouse lungs markedly improved survival of mice exposed to 100% oxygen.
Clinical Studies
Pulmonary edema clearance is impaired in animal models of hydrostatic and noncardiogenic pulmonary edema (102, 103). Loss of the ability to increase clearance of pulmonary edema is associated with increased risk of pulmonary edema and mortality from acute lung injury in humans (104–106) and animals (9). In a study of 79 patients with ALI, greater than half had impaired pulmonary edema clearance and only 13% had maximal expected clearance rate (106). Hospital mortality was 20% in patients with maximal clearance, compared with 62% in patients with impaired or submaximal clearance. These data raise the possibility that reduced alveolar fluid clearance may contribute to mortality in acute lung injury. Recent human studies of fluid management in ALI/ARDS (107) do not clearly link total body fluid balance with clinical outcome; thus, it is not yet possible to implicate reduced alveolar fluid clearance as a contributor to, or cause of, respiratory failure.
Limited clinical data regarding the use of β-agonists for pulmonary edema has expanded in the last few years. Salmeterol, a long-acting β2-agonist, has been shown to reduce the incidence of high-altitude pulmonary edema in mountain climbers when used as preventive therapy (104). Aerosol delivery of albuterol at clinically approved doses to mechanically ventilated patients with respiratory failure yields clinically significant levels of this β-agonist in lung edema fluid (108). Furthermore, β-agonist use correlates with improved outcome in patients with acute lung injury (109). In a single-center, double-blind, randomized controlled trial (BALTI, The β-Agonist Lung Injury Trial), treatment with intravenous salbutamol (15 μg/kg/h) in patients with ARDS significantly lowered extravascular lung water content measured by thermodilution at Day 7 compared with placebo (110). Patients who received salbutamol had improved respiratory system compliance and a trend toward lower lung injury scores at Day 7. In contrast to experimental data (46, 89), the effect of β-agonist therapy on lung water content was not evident until 48 hours after initiation of therapy. The mechanism responsible for this delay in β-agonist response might be linked to alveolar epithelial damage during early ARDS (110).
Limitations
The question of whether the alveolar epithelium may be critically injured and even denuded, interfering with and offsetting the beneficial effects of β-agonists on the alveolar epithelium, remains unanswered (36). As such, it is unclear whether β2AR-mediated up-regulation of active Na+ transport is possible during severe lung injury. Some experimental ALI models (i.e., prolonged hemorrhagic shock, hyperoxia, ischemia reperfusion after lung transplantation, and ventilator-induced lung injury) have been linked with diminished β-receptor function (111–116). Recently, Davis and coworkers have reported decreased sensitivity to β-agonists in a murine model of respiratory syncytial virus infection (83). The inhibitory effect of viral infection was attributed to impaired β2-AR signaling as a consequence of GRK-2–mediated uncoupling of the receptor from adenylyl cyclase (83). Restoration of β-agonist–sensitive active Na+ transport with inhibition of inducible nitric oxide synthase (111) and N-acetylcysteine (114) in some of these models implicates oxidation-dependent impairment of β2-adrenergic receptor signaling. NF-κB–dependent activation of inducible nitric oxide synthase impairs the function of membrane proteins (i.e., adenylyl cyclase) involved in the β2-receptor signaling pathway (111). These effects may be due to alterations in β2AR signaling but could also be attributed to diminished alveolar barrier function, loss of epithelial cells, or down-regulation of transport protein function.
Theoretically, a potential limitation of β2-agonist therapy for treatment of pulmonary edema is receptor desensitization (a regulated process that leads to attenuation of the biologic effect of receptor during prolonged agonist exposure) and down-regulation (a form of desensitization during which density/number of receptors decreases), both of which will diminish the efficacy of β-agonist therapy (82, 117, 118).
Regulation of β2-receptors occurs primarily through phosphorylation-dependent loss of sensitivity to agonist. These processes have been extensively studied in cardiac cells and airway smooth muscle cells. Proclivity for desensitization varies among tissues; for example, cardiac myocytes are readily desensitized, whereas airway smooth muscle cells may not have the necessary GPCR kinases to affect receptor phosphorylation.
Continuous stimulation with isoproterenol causes impairment in the ability of β-agonists to increase alveolar fluid clearance only when nonspecific β-agonists (isoproterenol) or high doses of a β2-agonist are used (119, 120). Prolonged stimulation with a β-agonist impairs its ability to continue to up-regulate alveolar fluid clearance, likely due to reduction in receptor density (120) and impaired of post-receptor signaling (82). A broad base of data supports β-agonist–induced attenuation of β2AR-mediated airway relaxation. Whether similar agonist-dependent (homologous) or -independent (heterologous) desensitization occurs in alveolar epithelial cells in humans is not known, and thus the implications of prolonged β2AR engagement on the protective effects of β-agonists are not known.
Another potentially limiting factor for use of β2-agonists is the β2-adrenergic receptor polymorphism, which might influence the response to the agonists and β2AR regulation (121, 122). While the effect of β2-adrenergic receptor polymorphism in asthma has been studied and has been shown to affect clinical response, it has not been evaluated in the alveolar epithelium of humans (123–124).
Finally, it is important to recognize the detrimental effects of β2-agonist therapy such as induction of tachycardia and increased oxygen consumption, which may cause adverse effects particularly in patients with underlying cardiovascular disease. Another concern is the worsening of ventilation–perfusion mismatch that results from β-agonist–mediated vasodilation, which precedes bronchodilation and thereby causes deterioration of oxygenation.
CONCLUSIONS
β2-adrenergic receptor signaling is required for up-regulation of alveolar epithelial active ion transport in the setting of excess alveolar edema fluid. The positive, protective effects of β2AR signaling on alveolar active Na+ transport in normal and injured lungs provide substantial support for the use of β-adrenergic agonists to accelerate alveolar fluid clearance in patients with cardiogenic and noncardiogenic pulmonary edema.
This work was supported by the American Lung Association, American Lung Association Metropolitan Chicago, the American Heart Association, by NIEHS ES015024 (G.M.M.), and by NHLBI HL-66211 and HL-71042 (P.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0198TR on August 20, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta 2-adrenergic receptors: their role in regulation of alveolar active sodium transport. Am J Respir Crit Care Med 2004;170:1270–1275. [DOI] [PubMed] [Google Scholar]
- 2.Granneman JG. The putative beta4-adrenergic receptor is a novel state of the beta1-adrenergic receptor. Am J Physiol Endocrinol Metab 2001;280:E199–E202. [DOI] [PubMed] [Google Scholar]
- 3.Spina D, Rigby PJ, Paterson JW, Goldie RG. Autoradiographic localization of beta-adrenoceptors in asthmatic human lung. Am Rev Respir Dis 1989;140:1410–1415. [DOI] [PubMed] [Google Scholar]
- 4.Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis 1985;132:541–547. [DOI] [PubMed] [Google Scholar]
- 5.Liebler JM, Borok Z, Li X, Zhou B, Sandoval AJ, Kim KJ, Crandall ED. Alveolar epithelial type I cells express beta2-adrenergic receptors and G-protein receptor kinase 2. J Histochem Cytochem 2004;52:759–767. [DOI] [PubMed] [Google Scholar]
- 6.Liggett SB. Update on current concepts of the molecular basis of beta2-adrenergic receptor signaling. J Allergy Clin Immunol 2002;110:S223–S227. [DOI] [PubMed] [Google Scholar]
- 7.Swift SM, Schwarb MR, Mihlbachler KA, Liggett SB. Pleiotropic β-agonist-promoted receptor conformations and signals independent of intrinsic activity. Am J Respir Cell Mol Biol 2007;2:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. beta(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res 2001;89:907–914. [DOI] [PubMed] [Google Scholar]
- 9.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius D, Mohemahmadi N, Thakuria G, Hardiman K, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on b2-adrenergic receptor signaling. Circ Res 2004;94:1091–1100. [DOI] [PubMed] [Google Scholar]
- 10.Akhter SA, Skaer CA, Kypson AP, McDonald PH, Peppel KC, Glower DD, Lefkowitz RJ, Koch WJ. Restoration of beta-adrenergic signaling in failing cardiac ventricular myocytes via adenoviral-mediated gene transfer. Proc Natl Acad Sci USA 1997;94:12100–12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science 2005;308:512–517. [DOI] [PubMed] [Google Scholar]
- 12.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997;390:88–91. [DOI] [PubMed] [Google Scholar]
- 13.Tepe NM, Liggett SB. Functional receptor coupling to Gi is a mechanism of agonist-promoted desensitization of the beta2-adrenergic receptor. J Recept Signal Transduct Res 2000;20:75–85. [DOI] [PubMed] [Google Scholar]
- 14.Pesce L, Guerrero C, Comellas A, Ridge KM, Sznajder JI. Beta-agonists regulate Na,K-ATPase via novel MAPK/ERK and rapamycin-sensitive pathways. FEBS Lett 2000;486:310–314. [DOI] [PubMed] [Google Scholar]
- 15.Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, Caron MG, Lefkowitz RJ. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase: regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem 1985;260:7094–7101. [PubMed] [Google Scholar]
- 16.Okamoto T, Murayama Y, Hayashi Y, Inagaki M, Ogata E, Nishimoto I. Identification of a Gs activator region of the beta 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell 1991;67:723–730. [DOI] [PubMed] [Google Scholar]
- 17.Hadcock JR, Port JD, Gelman MS, Malbon CC. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of beta 2-adrenergic receptors in response to insulin. J Biol Chem 1992;267:26017–26022. [PubMed] [Google Scholar]
- 18.Karoor V, Baltensperger K, Paul H, Czech MP, Malbon CC. Phosphorylation of tyrosyl residues 350/354 of the beta-adrenergic receptor is obligatory for counterregulatory effects of insulin. J Biol Chem 1995;270:25305–25308. [DOI] [PubMed] [Google Scholar]
- 19.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 2000;275:9572–9580. [DOI] [PubMed] [Google Scholar]
- 20.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J Biol Chem 2001;276:15192–15199. [DOI] [PubMed] [Google Scholar]
- 21.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA 1986;83:2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benovic JL, DeBlasi A, Stone WC, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science 1989;246:235–240. [DOI] [PubMed] [Google Scholar]
- 23.Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J Biol Chem 1996;271:13796–13803. [DOI] [PubMed] [Google Scholar]
- 24.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 1999;401:286–290. [DOI] [PubMed] [Google Scholar]
- 25.Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD. Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol 2000;10:409–412. [DOI] [PubMed] [Google Scholar]
- 26.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem 1999;274:1588–1595. [DOI] [PubMed] [Google Scholar]
- 27.Lin F, Wang H, Malbon CC. Gravin-mediated formation of signaling complexes in beta 2-adrenergic receptor desensitization and resensitization. J Biol Chem 2000;275:19025–19034. [DOI] [PubMed] [Google Scholar]
- 28.Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol 2001;13:139–145. [DOI] [PubMed] [Google Scholar]
- 29.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001;294:1307–1313. [DOI] [PubMed] [Google Scholar]
- 30.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999;283:655–661. [DOI] [PubMed] [Google Scholar]
- 31.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 2000;148:1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, et al. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA 2003;100:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall RA, Premont RT, Chow CW, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 1998;392:626–630. [DOI] [PubMed] [Google Scholar]
- 34.Cong M, Perry SJ, Hu LA, Hanson PI, Claing A, Lefkowitz RJ. Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem 2001;276:45145–45152. [DOI] [PubMed] [Google Scholar]
- 35.Klein U, Ramirez MT, Kobilka BK, von Zastrow M. A novel interaction between adrenergic receptors and the alpha-subunit of eukaryotic initiation factor 2B. J Biol Chem 1997;272:19099–19102. [DOI] [PubMed] [Google Scholar]
- 36.Mutlu GM, Sznajder JI. beta(2)-Agonists for treatment of pulmonary edema: ready for clinical studies? Crit Care Med 2004;32:1607–1608. [DOI] [PubMed] [Google Scholar]
- 37.Goodman BE, Brown SE, Crandall ED. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol 1984;57:703–710. [DOI] [PubMed] [Google Scholar]
- 38.Goodman BE, Kim K, Crandall ED. Evidence for active sodium transport across alveolar epithelium of isolated rat lung. J Appl Physiol 1987;62:2460–2466. [DOI] [PubMed] [Google Scholar]
- 39.Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest 1987;79:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol 1994;76:2636–2642. [DOI] [PubMed] [Google Scholar]
- 41.Berthiaume Y, Broaddus VC, Gropper MA, Tanita T, Matthay MA. Alveolar liquid and protein clearance from normal dog lungs. J Appl Physiol 1988;65:585–593. [DOI] [PubMed] [Google Scholar]
- 42.Berthiaume Y. Effect of exogenous cAMP and aminophylline on alveolar and lung liquid clearance in anesthetized sheep. J Appl Physiol 1991;70:2490–2497. [DOI] [PubMed] [Google Scholar]
- 43.Norlin A, Finley N, Abedinpour P, Folkesson HG. Alveolar liquid clearance in the anesthetized ventilated guinea pig. Am J Physiol 1998;274:L235–L243. [DOI] [PubMed] [Google Scholar]
- 44.Icard P, Saumon G. Alveolar sodium and liquid transport in mice. Am J Physiol 1999;277:L1232–L1238. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda N, Folkesson HG, Matthay MA. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated vs. in situ studies. J Appl Physiol 2000;89:672–679. [DOI] [PubMed] [Google Scholar]
- 46.Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med 1997;155:506–512. [DOI] [PubMed] [Google Scholar]
- 47.Sakuma T, Tuchihara C, Ishigaki M, Osanai K, Nambu Y, Toga H, Takahashi K, Ohya N, Kurihara T, Matthay MA. Denopamine, a beta(1)-adrenergic agonist, increases alveolar fluid clearance in ex vivo rat and guinea pig lungs. J Appl Physiol 2001;90:10–16. [DOI] [PubMed] [Google Scholar]
- 48.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 1999;61:627–661. [DOI] [PubMed] [Google Scholar]
- 49.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol 1999;276:L20–L27. [DOI] [PubMed] [Google Scholar]
- 50.Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest 1994;94:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yue G, Shoemaker R, Matalon S. Regulation of low-amiloride-affinity sodium channels in alveolar type II cells. Am J Physiol 1994;267:L94–L100. [DOI] [PubMed] [Google Scholar]
- 52.Chen XJ, Eaton DC, Jain L. Beta-adrenergic regulation of amiloride-sensitive lung sodium channels. Am J Physiol Lung Cell Mol Physiol 2002;282:L609–L620. [DOI] [PubMed] [Google Scholar]
- 53.Berdiev BK, Prat AG, Cantiello HF, Ausiello DA, Fuller CM, Jovov B, Benos DJ, Ismailov IL. Regulation of epithelial sodium channels by short actin filaments. J Biol Chem 1996;271:17704–17710. [DOI] [PubMed] [Google Scholar]
- 54.Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of beta-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol 1998;275:L414–L422. [DOI] [PubMed] [Google Scholar]
- 55.Lazrak A, Nielsen VG, Matalon S. Mechanisms of increased Na(+) transport in ATII cells by cAMP: we agree to disagree and do more experiments. Am J Physiol Lung Cell Mol Physiol 2000;278:L233–L238. [DOI] [PubMed] [Google Scholar]
- 56.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 2002;99:1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 2006;103:4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saldias FJ, Lecuona E, Comellas AP, Ridge KM, Rutschman DH, Sznajder JI. β-adrenergic stimulation restores rat lung ability to clear edema in ventilator-associated lung injury. Am J Respir Crit Care Med 2000;162:282–287. [DOI] [PubMed] [Google Scholar]
- 59.Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell 2003;14:3888–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pesce L, Comellas A, Sznajder JI. Beta-adrenergic agonists regulate Na-K-ATPase via p70S6k. Am J Physiol Lung Cell Mol Physiol 2003;285:L802–L807. [DOI] [PubMed] [Google Scholar]
- 61.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, et al. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 2005;96:999–1005. [DOI] [PubMed] [Google Scholar]
- 62.Jiang X, Ingbar DH, O'Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl- channels. Am J Physiol 1998;275:C1610–C1620. [DOI] [PubMed] [Google Scholar]
- 63.Nielsen VG, Duvall MD, Baird MS, Matalon S. cAMP activation of chloride and fluid secretion across the rabbit alveolar epithelium. Am J Physiol 1998;275:L1127–L1133. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, Ingbar DH, O'Grady SM. Adrenergic regulation of ion transport across adult alveolar epithelial cells: effects on Cl- channel activation and transport function in cultures with an apical air interface. J Membr Biol 2001;181:195–204. [DOI] [PubMed] [Google Scholar]
- 65.O'Grady SM, Jiang X, Ingbar DH. Cl-channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L239–L244. [DOI] [PubMed] [Google Scholar]
- 66.Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA 2001;98:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 2002;119:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chalfant ML, Coupaye-Gerard B, Kleyman TR. Distinct regulation of Na+ reabsorption and Cl- secretion by arginine vasopressin in the amphibian cell line A6. Am J Physiol Cell Physiol 1993;264:C1480–C1488. [DOI] [PubMed] [Google Scholar]
- 69.Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 2002;91:672–680. [DOI] [PubMed] [Google Scholar]
- 70.Malbon CC, Tao J, Wang HY. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem J 2004;379:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem 2000;275:29539–29546. [DOI] [PubMed] [Google Scholar]
- 72.Sun F, Hug MJ, Bradbury NA, Frizzell RA. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J Biol Chem 2000;275:14360–14366. [DOI] [PubMed] [Google Scholar]
- 73.Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 2006;98:675–681. [DOI] [PubMed] [Google Scholar]
- 74.Ludwig MG, Seuwen K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct Res 2002;22:79–110. [DOI] [PubMed] [Google Scholar]
- 75.Jurevicius J, Skeberdis VA, Fischmeister R. Role of cyclic nucleotide phosphodiesterase isoforms in cAMP compartmentation following beta2-adrenergic stimulation of ICa,L in frog ventricular myocytes. J Physiol 2003;551:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci USA 2001;98:13049–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brunton LL. PDE4: arrested at the border. Sci STKE 2003;2003:PE44. [DOI] [PubMed] [Google Scholar]
- 78.Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 1993;260:222–226. [DOI] [PubMed] [Google Scholar]
- 79.Lane SM, Maender KC, Awender NE, Maron MB. Adrenal epinephrine increases alveolar liquid clearance in a canine model of neurogenic pulmonary edema. Am J Respir Crit Care Med 1998;158:760–768. [DOI] [PubMed] [Google Scholar]
- 80.Norlin A, Baines DL, Folkesson HG. Role of endogenous cortisol in basal liquid clearance from distal air spaces in adult guinea-pigs. J Physiol 1999;519:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sartori C, Fang X, McGraw DW, Koch P, Snider ME, Folkesson HG, Matthay MA. Selected contribution: long-term effects of beta(2)-adrenergic receptor stimulation on alveolar fluid clearance in mice. J Appl Physiol 2002;93:1875–1880. [DOI] [PubMed] [Google Scholar]
- 82.Morgan EE, Stader SM, Hodnichak CM, Mavrich KE, Folkesson HG, Maron MB. Postreceptor defects in alveolar epithelial {beta}-adrenergic signaling after prolonged isoproterenol infusion. Am J Physiol Lung Cell Mol Physiol 2003;285:L578–L583. [DOI] [PubMed] [Google Scholar]
- 83.Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to beta-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 2007;293:L281–L289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004;303:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science 2001;292:1718–1722. [DOI] [PubMed] [Google Scholar]
- 86.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol 2005;25:9063–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minnear FL, DeMichele MA, Moon DG, Rieder CL, Fenton JW II. Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am J Physiol 1989;257:H1613–H1623. [DOI] [PubMed] [Google Scholar]
- 88.Khimenko PL, Barnard JW, Moore TM, Wilson PS, Ballard ST, Taylor AE. Vascular permeability and epithelial transport effects on lung edema formation in ischemia and reperfusion. J Appl Physiol 1994;77:1116–1121. [DOI] [PubMed] [Google Scholar]
- 89.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cAMP-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 2004;32:1470–1476. [DOI] [PubMed] [Google Scholar]
- 90.Liu F, Verin AD, Borbiev T, Garcia JG. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am J Physiol Lung Cell Mol Physiol 2001;280:L1309–L1317. [DOI] [PubMed] [Google Scholar]
- 91.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 2000;7:287–308. [DOI] [PubMed] [Google Scholar]
- 92.Chen Q, Bates SR, Fisher AB. Secretagogues increase the expression of surfactant protein A receptors on lung type II cells. J Biol Chem 1996;271:25277–25283. [DOI] [PubMed] [Google Scholar]
- 93.Kumar VH, Christian C, Kresch MJ. Effects of salmeterol on secretion of phosphatidylcholine by alveolar type II cells. Life Sci 2000;66:1639–1646. [DOI] [PubMed] [Google Scholar]
- 94.Liggett SB. Identification and characterization of a homogeneous population of beta 2-adrenergic receptors on human alveolar macrophages. Am Rev Respir Dis 1989;139:552–555. [DOI] [PubMed] [Google Scholar]
- 95.Fukushima T, Sekizawa K, Jin Y, Yamaya M, Sasaki H, Takishima T. Effects of beta-adrenergic receptor activation on alveolar macrophage cytoplasmic motility. Am J Physiol 1993;265:L67–L72. [DOI] [PubMed] [Google Scholar]
- 96.Pataki G, Czopf L, Jilling T, Marczin N, Catravas J, Matalon S. Regulation of fluid-phase endocytosis in alveolar macrophages. Am J Physiol 1995;269:L520–L526. [DOI] [PubMed] [Google Scholar]
- 97.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 2004;173:559–565. [DOI] [PubMed] [Google Scholar]
- 98.Su X, Robriquet L, Folkesson HG, Matthay MA. Protective effect of endogenous beta-adrenergic tone on lung fluid balance in acute bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L769–L776. [DOI] [PubMed] [Google Scholar]
- 99.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem 2006;281:19892–19898. [DOI] [PubMed] [Google Scholar]
- 100.Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax 2006;62:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGraw DW, Fukuda N, James PF, Forbes SL, Woo AL, Lingrel JB, Witte DP, Matthay MA, Liggett SB. Targeted transgenic expression of beta(2)-adrenergic receptors to type II cells increases alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 2001;281:L895–L903. [DOI] [PubMed] [Google Scholar]
- 102.Azzam ZS, Dumasius V, Saldias FJ, Adir Y, Sznajder JI, Factor P. Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation 2002;105:497–501. [DOI] [PubMed] [Google Scholar]
- 103.Olivera W, Ridge K, Wood LD, Sznajder JI. Active sodium transport and alveolar epithelial Na-K-ATPase increase during subacute hyperoxia in rats. Am J Physiol 1994;266:L577–L584. [DOI] [PubMed] [Google Scholar]
- 104.Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, Hutter D, Turini P, Hugli O, Cook S, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 2002;346:1631–1636. [DOI] [PubMed] [Google Scholar]
- 105.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol 1999;87:1301–1312. [DOI] [PubMed] [Google Scholar]
- 106.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1376–1383. [DOI] [PubMed] [Google Scholar]
- 107.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575. [DOI] [PubMed] [Google Scholar]
- 108.Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, Matthay MA. Aerosolized beta(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med 2002;28:705–711. [DOI] [PubMed] [Google Scholar]
- 109.Manocha S, Salehifar E, Groshaus H, Brown G, Walley KR, Russell JA. Use of inhaled beta-2 agonist is associated with more days alive and free of acute lung injury. Am J Respir Crit Care Med 2004;169:A781. [Google Scholar]
- 110.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281–287. [DOI] [PubMed] [Google Scholar]
- 111.Pittet JF, Lu LN, Morris DG, Modelska K, Welch WJ, Carey HV, Roux J, Matthay MA. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol 2001;166:6301–6310. [DOI] [PubMed] [Google Scholar]
- 112.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP- dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 2003;284:L791–L798. [DOI] [PubMed] [Google Scholar]
- 113.Saldias FJ, Comellas A, Ridge KM, Lecuona E, Sznajder JI. Isoproterenol improves ability of lung to clear edema in rats exposed to hyperoxia. J Appl Physiol 1999;87:30–35. [DOI] [PubMed] [Google Scholar]
- 114.Modelska K, Matthay M, Brown L, Deutch E, Lu L, Pittet J. Inhibition of beta-adrenergic-dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock. Am J Physiol Lung Cell Mol Physiol 1999;276:L844–L857. [DOI] [PubMed] [Google Scholar]
- 115.Folkesson HG, Nitenberg G, Oliver BL, Jayr C, Albertine KH, Matthay MA. Upregulation of alveolar epithelial fluid transport after subacute lung injury in rats from bleomycin. Am J Physiol 1998;275:L478–L490. [DOI] [PubMed] [Google Scholar]
- 116.Sugita M, Ferraro P, Dagenais A, Clermont ME, Barbry P, Michel RP, Berthiaume Y. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lungs. Am J Respir Crit Care Med 2003;167:1440–1450. [DOI] [PubMed] [Google Scholar]
- 117.Nijkamp FP, Engels F, Henricks PA, Van Oosterhout AJ. Mechanisms of beta-adrenergic receptor regulation in lungs and its implications for physiological responses. Physiol Rev 1992;72:323–367. [DOI] [PubMed] [Google Scholar]
- 118.Barnes PJ. Beta-adrenergic receptors and their regulation. Am J Respir Crit Care Med 1995;152:838–860. [DOI] [PubMed] [Google Scholar]
- 119.Strasser RH, Stiles GL, Lefkowitz RJ. Translocation and uncoupling of the beta-adrenergic receptor in rat lung after catecholamine promoted desensitization in vivo. Endocrinology 1984;115:1392–1400. [DOI] [PubMed] [Google Scholar]
- 120.Morgan EE, Hodnichak CM, Stader SM, Maender KC, Boja JW, Folkesson HG, Maron MB. Prolonged isoproterenol infusion impairs the ability of beta(2)-agonists to increase alveolar liquid clearance. Am J Physiol Lung Cell Mol Physiol 2002;282:L666–L674. [DOI] [PubMed] [Google Scholar]
- 121.Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol 2003;43:381–411. [DOI] [PubMed] [Google Scholar]
- 122.Small KM, Rathz DA, Liggett SB. Identification of adrenergic receptor polymorphisms. Methods Enzymol 2002;343:459–475. [DOI] [PubMed] [Google Scholar]
- 123.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med 2000;162:75–80. [DOI] [PubMed] [Google Scholar]
- 124.Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax 2000;55:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish J, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004;364:1505–1512. [DOI] [PubMed] [Google Scholar]
- 126.Wechsler ME, Lehman E, Lazarus SC, Lemanske RF Jr, Boushey HA, Deykin A, Fahy JV, Sorkness CA, Chinchilli VM, Craig TJ, et al. β-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med 2006;173:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]