Abstract
Diminished stem-cell functions with age may be a major cause of anemias and other defects. Unfortunately, treatments that increase stem-cell function can also increase the incidence of cancers. Lifelong dietary restriction (DR) is known to decrease spontaneous cancers and lengthen lifespan. This study examines the effect of DR on the ability of bone marrow cells to repopulate irradiated recipients and produce erythrocytes and lymphocytes. In BALB/cByJ (BALB) mice, repopulating abilities decline with age; DR ameliorates this trend. In C57BL/6J (B6) and (BALB × B6) F1 hybrid (F1) mice, repopulating abilities increase with age; DR maintains this increase. Hematopoietic stem cell (HSC) numbers are highly variable in aged BALB mice; however, the observed loss of marrow function results from a major loss in repopulating ability per HSC. DR greatly ameliorates this loss of function with age. In contrast, function per HSC in B6 mice is affected neither by age nor by DR. Thus, DR increases or maintains increased marrow repopulating ability with age in the 3 different genotypes tested, but effects on function per HSC depend on genotype. That DR increases or maintains stem-cell function with age, while decreasing cancer, has far-reaching health implications.
Introduction
Decline in repopulating and differentiating abilities of hematopoietic stem cells (HSCs) is characteristic of human diseases such as aplastic anemia and bone marrow (BM) failure. The common unexplained anemias1 in elderly people can be caused by declining HSC functions with age. These anemias are serious diseases with high financial and personal costs.2,3 The functional defects found in HSCs may be an example of a more generalized intrinsic loss of proliferative capacity that occurs in all aged adult stem cells.4,5 Thus, interventions that maintain HSC function with age will help alleviate these serious diseases and benefit health.
Laboratory mice provide well-recognized models in which to study hematopoietic defects. Genetic regulation of the age-related decline in BM repopulating and differentiating abilities has been demonstrated by the in vivo comparison of these abilities among mouse strains.6–10 The ability of bone marrow cells (BMCs) to repopulate and produce erythrocytes and lymphocytes is reduced in old DBA/2J (D2) and old BALB/cByJ (BALB) compared with young controls. However, BM from old C57BL/6J (B6) mice actually has a greater repopulating ability than BM from young controls,6–10 although its ability to repopulate after serial transplantation diminishes.6,11,12 These strain-related differences are apparently the result of the underlying genetic differences in stem-cell exhaustion.11 Because the patterns of aging differ among mouse strains, it is important to examine HSC aging in more than a single strain of mice.
The maximal potential lifespan of an organism may in part be determined by the increased risk of cancer concomitant with mechanisms that can maintain stem-cell function in aging subjects.12,13 Thus, the loss of function in aging stem cells is a consequence of reducing the probability that cells will transform. This paradigm, which balances functional senescence and cancer risk, is supported by recent studies in which precursor ability and cancer were linked in the predicted manner to the expression of the tumor-suppressor p16INK4a.14–18
Dietary restriction (DR) appears to break this paradigm. It is well known that DR decreases incidences of murine cancer with age,19–21 counteracts most aging processes, and extends maximal lifespan in rodents.22–26 Initial work in BALB mice showed that DR prevents both the loss of HSC clonal stability and the loss of BMC functional abilities with age.27 Combined, these previous studies suggest that it is possible to both maintain function with age and decrease the risk of cancer. Under different genetic regulation in B6 mice, the repopulating ability of BMCs and the numbers of HSCs present are known to increase with age.6–10 This raised the question of whether lifelong DR would inhibit the age-related increase in repopulating ability found in B6 mice.
This study will examine the hypothesis that DR, known to decrease the incidence of cancer, can simultaneously increase or maintain increased HSC functions with age across various genetic backgrounds. The competitive repopulation assay (CRA) is combined with flow cytometric analysis to test not only how age and DR affect the functional ability of whole BMCs, but also whether the functional ability per HSC is altered. The ratio of erythrocyte percentage to lymphocyte percentage (E/L ratio) in peripheral blood 6 months after transplantation will test whether age or DR alters the ability of HSCs to proportionally produce multiple lineages of differentiated cells. Establishing the effects of DR in models under different genetic regulation is important for future studies that wish to investigate the relationship of in vivo responses to molecular mechanisms. If a candidate mechanism truly regulates the effects of DR, it will need to alter stem-cell function and the incidences of cancer according to the different patterns generated in these divergent models.
Methods
Mice and dietary management
Inbred female BALB/cByJ and C57BL/6J mice, along with male (BALB × B6) F1 hybrid mice (CByB6F1/J), were produced and maintained in a barrier colony at the Jackson Laboratory (Bar Harbor, ME). At weaning (3 weeks of age), they were housed 8 per box in weaning cages. These cages were assigned one of 2 feeding regimens using Purina LabDiet's 5LG6 irradiated formulation of the NIH-31 (4% fat) diet: (1) Ad libitum (AL) fed mice were given uninhibited access to the grain. They consumed on average 3.3 g food/mouse per day. (2) DR BALB mice were fed 90% of the AL rate (3 g food/mouse per day) at weaning and gradually restricted to 70% of the AL rate (2.3 g food/mouse per day) over the course of 3 months. B6 and F1 DR mice were fed at 70% of the AL rate from weaning. All long-term DR mice were maintained at 70% of the AL intake (2.3 g food/mouse per day) until they were 22 to 25 months of age, and short-term DR mice were maintained on this regimen until used at 6 to 7 months of age. The grams/mouse per day given is an average; the actual amounts fed DR mice were determined as a percentage of the food consumed by matched littermate controls. All mice were maintained on a 12:12 dark/light cycle; DR mice were mechanically fed pulverized grain once per day, 5 hours into their active dark cycle (23:00 hours).
Colony management and animal husbandry followed standard protocols described elsewhere.25,27,28 All procedures were approved by the animal care and use committee of the Jackson Laboratory, and staff veterinarians monitored mice on a regular basis, finding no pathogens.29
Sample preparation
BMCs were removed from young AL mice at 2 to 7 months from young DR mice at 6 to 7 months, and from old AL or old DR mice at 22 to 25 months. The BMCs from each mouse were extracted from the tibias and femurs of both hind legs by washing the marrow out with 2 mL of Dulbecco modified Eagle medium (DMEM).8,11,27 Cells were mechanically dispersed and filtered through a 100μm Nytex (nylon) mesh (Tetko, Elmsford, NY) to remove debris and assure a single cell suspension. The concentration of nucleated cells was determined with a model Z1 Coulter counter (Coulter Electronics, Hialeah, FL) after cells were lysed with ZAP-OGLOBIN II lytic reagent (Coulter). Isolated BMCs were subsequently used for either fluorescence-activated cell sorting (FACS) analysis (Figure 1) or the CRA (Figures 2–4).
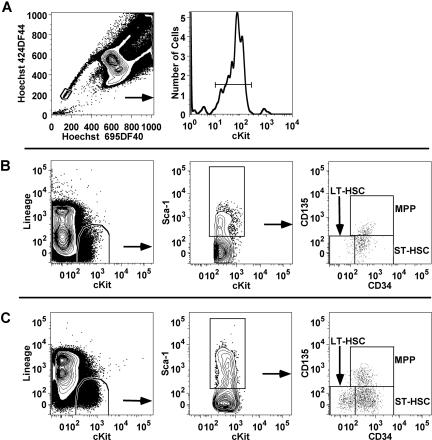
Figure 1.
Determining the frequencies of hematopoietic precursors in BALB and B6. The BMCs from old and young, DR- and AL-fed BALB mice were analyzed by 2 different flow criteria. (A) The gates used in the SP + Kit criterion, first gating for the Hoechst double low fluorescence side population,37,38,44,45 and then for c-kit+. (B) The series of gates (lin−, c-Kit+, Sca-1−, CD34±, CD135±) used in the mKSL criterion35 to determine LT-HSC, ST-HSC, and MPP subpopulations. (C) How the same mKSL criterion was used on B6 mice to determine the numbers of precursors.
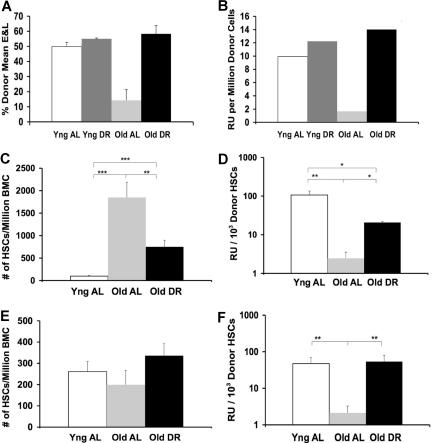
Figure 2.
BALB: Effects of DR and age on in vivo functional abilities of BMCs and numbers of HSCs. BMCs from young AL, young DR, old AL, and old DR, female BALB (Gpi1a) mice were each mixed 1:1 with BMCs from a standard competitor pool of young female congenic BALB (Gpi1b) mice. This mixture was injected intravenously into lethally irradiated young female BALB recipients. Donor (Gpi1a) contributions to erythrocytes and lymphocytes in the peripheral blood of replicates were averaged 6 months after transplantation. A total of 3-9 young AL, old AL, or old DR donors were used over the course of 3 experiments. (A) Total marrow functional ability based on the average composition of erythrocytes and lymphocytes (E & L). (B) Total marrow functional ability expressed in repopulating units. (C) Numbers of LT-HSCs present in the original donor marrow using the mKSL flow criterion. Counts from young DR were not available (**P < .01; ***P < .001). (D) Functional ability per LT-HSC in the subset of animals using mKSL flow criterion (*P < .05; **P < .01). (E) Numbers of HSCs present in the original donor marrow using the SP + Kit flow criterion. Counts from young DR were not available. (F) Functional ability per HSC in the subset of animals using the SP + Kit flow criterion (**P < .01). Functional ability of old AL versus others in panel A is significant (P < .001). Error bars represent SE.
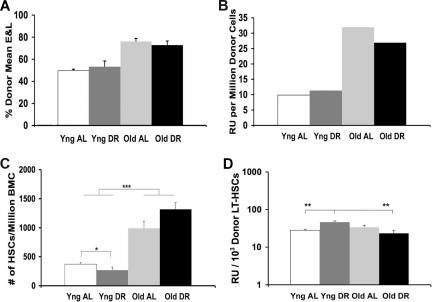
Figure 3.
B6: Effects of DR and age on in vivo functional abilities of BMCs and numbers of LT-HSCs. (A) Total marrow functional ability based on the average composition of erythrocytes and lymphocytes. (B) Total marrow functional ability expressed in repopulating units. (C) Numbers of LT-HSCs present in the original donor marrow using the mKSL criterion (*P < .05; ***P < .001). (D) Functional ability per LT-HSC in animals using the mKSL criterion (**P < .01). Assays were conducted as described in the legend to Figure 1, except that donors and recipients were B6 Gpi1b and competitors were B6 Gpi1a. A total of 5-7 donors of each type was used over the course of 2 experiments. Old versus young functional ability in Figure 3A was significant at P < .001. Error bars represent SE.
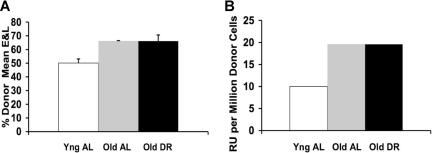
Figure 4.
(BALB × B6) F1: Effects of DR and age on in vivo functional abilities of BMCs. (A) Total marrow functional ability based on the average composition of erythrocytes and lymphocytes. (B) Total marrow functional ability expressed in repopulating units. Assays were performed as described in the legend to Figure 1, except that recipients and donors were (BALB × B6) F1 hybrids with Gpi1a/b and Gpi1a competitors. A total of 3 to 5 donors was used for each type. Old versus young were significantly different (P < .01). Error bars represent SE.
Flow cytometry analysis
BMCs from B6 mice were stained with specific antibodies for fluorescence analysis using Lineage-low (lin−), cKit+, Sca1+, CD34−, and Flk2− cell surface markers, as this identifies a subpopulation that is greatly enriched for long-term HSC (LT-HSC).30–35 Lin− is actually the lack of response to a cocktail of antibodies against granulocytes (Ly6G/Gr-1, clone RB6-8C5), macrophages (CD11b/Mac-1, clone M1/70), B cells (CD19, clone ID3), T cells (CD3, clone 145-2C11; CD4, clone GK 1.5; CD8, clone 53-6.72), NK cells (NK1.1, clone PK136), and erythrocytic cells (Ly76, clone Ter119). BMCs that were lin−, c-Kit+ (CD117, c-Kit clone 2B8) and Sca-1+ (Ly6A/E, clone E13-161 or clone D7) were further gated with respect to CD34 (clone RAM34) and Flk2 (CD135, clone A2F10) to identify and count subpopulations enriched for LT-HSCs (CD34−, Flk2−), short term-HSCs (ST-HSC; CD34+, Flk2−) and multipotent progenitor cells (MPP; CD34+, Flk2+; Figure 1C; Table 1). All antibodies were purchased from BD Biosciences/PharMingen or eBiosciences (San Diego, CA) and were pretitered to determine optimal staining concentration under identical conditions. Each antibody was uniquely labeled with a specific fluorochrome, except for the antibodies in the lineage cocktail, which were biotinylated. These fluorochromes included Pacific blue, fluorescein-isothiocyanate, phycoerythrin, allophycocyanin, or phycoerythrin-cyanine 7. Biotinylated antibodies were counter stained with allophycocyanin-cyanine 7–conjugated streptavidin. Propidium iodide (Sigma-Aldrich, St Louis, MO) was used to gate out dead cells.
Table 1.
Effects of DR and age on HSC frequencies using the mKSL criterion
| Donor type | LT-HSC | ST-HSC | MPP |
|---|---|---|---|
| BALB | |||
| Young AL | 99 ± 19 | 24 ± 5 | 1.9 ± 0.8 |
| Old AL | 1847 ± 341* | 251 ± 112† | 112.7 ± 80.8† |
| Old DR | 752 ± 145* | 87 ± 5‡ | 23.1 ± 4.8‡ |
| B6 | |||
| Young AL | 373 ± 26 | 852 ± 147 | 517 ± 146 |
| Old AL | 991 ± 122* | 1242 ± 341 | 787 ± 294 |
| Old DR | 1317 ± 117* | 810 ± 197 | 472 ± 135 |
BMCs from a total of 3–8 female BALB or B6 mice per group (Young AL, Young DR, Old AL, and Old DR) were analyzed by FACS before intravenous injection into lethally irradiated recipients. LT-HSCs, ST-HSCs, and MPPs present in the original donor marrow are given as number per 106 cells ± SE. After gating for lin−, cKit+, Sca1+, these BMCs were identified as LT-HSCs (CD34−, Flk2−), ST-HSCs (CD34+, Flk2−) and MPPs (CD34+, Flk2+).
P < .001 versus young AL controls.
P < .01 versus young AL controls.
P < .05 versus young AL controls.
In BALB, 2 different flow criteria were used to identify HSCs. The first was exactly the same as used in Figure 1B to identify LT-HSCs in B6 (lin−, cKit+, Sca1+, CD34−, and Flk2−). However, the Sca cell-surface marker, although present in BALB marrow, has an altered phenotype compared with B6 marrow.36 As an alternative, a second flow criterion identified HSCs in BM by the ability of cells to efflux the Hoechst 33342 stain, resulting in the least fluorescence at 450 nm and 675 nm. These cells, defined as the side population (SP),37–39 were then gated with respect to cKit+ to further enrich the HSC population (Figure 1A).
BALB BMCs to be analyzed by the SP flow criterion were first stained for 90 minutes at 106 cells/mL of a prewarmed (37°C) buffer containing DMEM, 2% fetal bovine serum, and 5 μg/mL Hoechst 33342. Cells were then spun at 350g for 5 minutes at 10°C, decanted, and the pellet resuspended in the residual DMEM. Subsequent staining of BALB samples was done at 4°C, with 2 μg/mL Hoechst added to all buffers.
B6 and BALB BMCs, with or without Hoechst 33342, were stained for cell surface markers after filtering with a 100 μm Nytex (nylon) mesh (Tetko) to ensure a single cell suspension. BMCs (40 μL, of 2.5 × 107 cells/mL) were incubated at 4°C for 30 minutes with 10 μL of the appropriate antibody cocktail (either cKit for the SP flow criterion or the full set of antibodies described previously in this section) in FACS buffer.40 Filter sterilized (0.22 μm) FACS buffer consisted of NaCl (137 mM), KCl (2.7 mM), KH2PO4 (1.5 mM), Na2HPO4 (8 mM), Na4EDTA (2 mM), NaN3 (3 mM), fetal bovine serum (2%), and Phenol red (0.05 g/L). Cells were then washed with 2 mL of FACS buffer, spun at 350g for 5 minutes at 10°C, sharply decanted, and resuspended in residual buffer. Biotinylated antibodies were then counterstained with the streptavidin conjugate using the same incubation/wash procedure as used with the primary antibodies. Stained BMCs were finally resuspended in 250 μL of FACS buffer per 106 cells. Propidium iodide (10 μL of 20 μg/mL) was added immediately before counting.
Analyses were performed on a FACSVantageSE/DiVa (BD Biosciences) using standard techniques described elsewhere.25 Cellular debris was excluded based on forward scatter and size scatter profiles. Approximately 1 million viable cell events were collected per sample. Assays included fluorochrome minus one controls, and single fluorochrome controls for compensation.
Competitive repopulation
In the long term CRA, 2 × 106 BMCs from each treated donor were mixed with 2 × 106 BMCs from a standard competitor pool, prepared from young AL-fed mice congenic for the nondonor allele at the glucose 6-phosphate isomerase (Gpi) locus. This allowed donor and competitor cells to be distinguished. In the BALB study, donors were Gpi1a/Gpi1a; competitors were Gpi1b/Gpi1b. In the B6 study, donors were Gpi1b/Gpi1b; competitors were Gpi1a/Gpi1a. F1 donors and recipients were Gpi1a/Gpi1b; competitors were Gpi1a/Gpi1a. Mixtures of donor and competitor cells were injected into the tail veins of 2- to 6-month-old recipients that were lethally irradiated (9-11 Gy) with a Shepherd Mark 1 137cesium gamma source (J.L. Shepherd, Glendale, CA) 6 hours before injection. Each assay was replicated 2 to 4 times using donors, competitors, and recipients that were matched for strain and sex. Peripheral blood was collected via orbital sinus bleeding of recipient mice 1, 4, and 6 months after transplantation. Lymphocytes and erythrocytes were analyzed separately after isolation using LSM medium (ICN, 50494). The percentage of donor to standard competitor in lymphocytes or erythrocytes was determined from the band densities of Gpi1 alleles separated by cellulose-acetate gel electrophoresis, given in detail elsewhere.6–8 The percentage of donor-type Gpi-1 represents the ratio of the donor-contributed repopulating ability to the total (donor plus competitor) repopulating ability of the marrow transplanted, in repopulating units (RUs). We define 1 RU as the repopulating ability of 100 000 standard competitor BMCs; thus, % donor = 100 × donor RU/(donor RU + competitor RU). To calculate donor RU values: donor RU = % donor × competitor RU/(100 − % donor).6–8,11,27 In this study, the competitor dose was 2 × 106 cells; thus, the competitor RU value was 20. In each experiment, a group of control mice received only competitor BMCs. This group was monitored to insure that irradiated host HSCs did not produce detectable amounts of circulating erythrocytes and lymphocytes. Using a mixture of donor and competitor cells minimizes stress to the mouse and assures rescue from lethal irradiation, regardless of the functional ability of the donor cells because the competitor cells alone are sufficient to maintain recipient health.6–8
Data analysis
Statistical analyses of variances in flow cytometry and CRAs were performed using the JMP statistical discovery software on the Fit model platform (SAS Institute, Cary, NC). To adjust for heterogeneity of variance, data were transformed using the logit protocol or analyzed using pair-wise comparisons assuming unequal variances. Data are presented as mean plus or minus SE. Of the 52 donors that started the study, 6 were eliminated from the data analysis: 2 donors were eliminated because all recipients died before analysis, 6 months after transplantation. In addition, 4 donors were eliminated because their data were more than 3 SDs from the mean. Donors, determined to be outliers, also had unusually high variability among their recipients.
Results
BALB HSCs
Using the CRA, we compared functional ability of donor BMCs, derived from young and old BALB mice that were fed AL or DR, relative to a genetically distinguishable, standard competitor BMC pool, coinjected into lethally irradiated recipients. The functional ability of BALB BMCs, measured in RUs, decreased approximately 5-fold with age in AL-fed mice (Figure 2A,B).
In flow cytometric analyses, we used 2 different criteria in independent experiments to estimate concentrations of HSCs in marrow from BALB donors. The first was a modified KSL system (mKSL) using lin−, Sca+, cKit+, CD34−, and Flk2− antigenic markers to separate the primitive precursors of hematopoiesis (Table 1). The second, given the lower expression of Sca in BALB mice,36 used the Hoechst 33342 dye excluding side population combined with cKit+ (SP + Kit). The mKSL flow criterion indicated a greater than 18-fold increase with age in the concentration of LT-HSCs from BALB marrow (Figure 2C). This, combined with the nearly 2-fold functional loss in this experiment (Table 2), reveals a greater than 40-fold loss of functional ability with age per BALB LT-HSC (Figure 2D). The observed increase in BALB stem cells with age was not merely a loss of cell surface marker recognition increasing the double negative LT-HSC subpopulation, as all classes of cells increased with age and DR (Table 1). Using the SP + Kit flow criterion, HSC numbers differed from those found by the mKSL criterion (Figure 2E; Table 2) and did not change with age. However, BMCs from old AL mice showed a very severe loss in function (Table 2) and a 24-fold decrease in functional ability per HSC with age (Figure 2F). Although functional abilities of BMCs from old AL mice varied in the 2 experiments, as did numbers of HSCs using the 2 criteria, in both cases there was an enormous loss of function per HSC with age, more than 40- and 24-fold, respectively (Figure 2D,F). This loss has not been reported previously.
Table 2.
HSC frequency and function using different flow criteria
| HSC measure | Young AL | Old AL | Old DR |
|---|---|---|---|
| mKSL criterion | |||
| LT-HSC/106 viable BMC | 99 ± 19 | 1847 ± 341* | 752 ± 145* |
| RU/106 viable BMC | 10 ± 2 | 6 ± 3 | 15 ± 4 |
| RU/103 LT-HSC | 107 ± 29 | 2 ± 1† | 21 ± 2‡ |
| SP+Kit criterion | |||
| HSC/106 viable BMC | 261 ± 48 | 199 ± 68 | 336 ± 56 |
| RU/106 viable BMC | 11 ± 3 | 0.3 ± 0.1† | 17 ± 7 |
| RU/103 HSC | 47 ± 21 | 2 ± 1† | 54 ± 26 |
For each flow criterion, the frequency (± SE) of HSCs and functional abilities (RU) were determined in separate groups of BALB BMC donors that are subsets of those presented in Figure 2A,B. The mKSL flow criterion48 used lineage−, Sca-1+, c-Kit+, CD34−, and Flk2−, whereas the SP+Kit criterion used the Hoescht effluxing, double-negative side population,37,38,44,45 plus c-Kit+. Because HSCs were defined per viable BMC and functional ability (RU) per total BMCs, the ratio is a lower limit. However, flow cytometry viabilities were about the same in both experiments, so relative ratios are correct.
P < .001 versus young AL controls.
P < .01 versus young AL controls.
P < .05 versus young AL controls.
Using the mKSL criterion, DR reduced the age–related increase in LT-HSC number by approximately 2.5-fold while obviating the decrease in function with age. Thus, BMCs from old donors with DR treatment had a function per LT-HSC more than 10-fold greater than LT-HSCs from old AL donors (Table 2; Figure 2C,D). Using the SP + Kit criterion, HSC numbers were similar in young, old AL and old DR donors; however, the meager function of old AL donors was increased more than 50-fold in old DR donors. This resulted in BMCs from old donors on DR having a function per stem cell approximately 27-fold better than those from old AL donors (Table 2; Figure 2E,F). Thus, in both cases, the experiments showed that, despite the high variability in function and number of HSCs from old donors, DR treatment in BALB mice substantially or completely alleviated the age-related losses in overall BM function (Figure 2A,B) and function per HSC (Figure 2D,F).
B6 HSCs
In parallel CRA studies, we compared functional abilities of donor BMCs from young and old B6 mice that were fed AL or DR, relative to a genetically distinguishable, standard competitor BMC pool coinjected into lethally irradiated recipients. In stark contrast to BMCs from BALB mice, the functional ability of BMCs from B6 mice increased approximately 3-fold with age (Figure 3A,B).
In cytometric analysis, we used mKSL with lin−, Sca+, cKit+, CD34−, and Flk2− antigenic markers to assay concentrations of HSCs (Table 1) in marrow from young and old, AL and DR fed, B6 donors. Numbers of LT-HSCs increased approximately 3-fold with age in B6 mice (Figure 3C). As in BALB, the observed increase in the number of B6 LT-HSCs is not merely a loss of marker recognition increasing the double negative LT-HSC subpopulation, as numbers of both ST-HSCs and MPPs are increased as well (Table 1). When the functional ability is expressed per LT-HSC, there is no significant difference between young and old (Figure 3D). In B6 mice, the increase in functional ability of the whole marrow with age is accomplished by increasing the number of LT-HSCs present, not by altering functional abilities of stem cells.
Although DR in BALB mice reduces the loss of function with age, DR did not alter the increase in repopulating ability (Figure 3A,B) or LT-HSC number (Figure 3C) observed in B6 mice with age. LT-HSC numbers were slightly reduced in young DR B6 mice compared with similarly aged controls. Even though, in young mice, DR did have a statistically significant effect on HSC numbers and function per stem cell (RU/LT-HSC; Figure 3D), this may not have any biologic significance, as it may merely reflect a temporary acclimation to reduced food intake.
F1 HSCs
In the F1, the CRA compared donor BMCs of young AL, old AL, and old DR fed mice relative to the genetically distinguishable F1 standard competitor pool coinjected into lethally irradiated F1 recipients. The functional abilities of BMCs from the F1 significantly increased with age, with approximately a 2-fold increase in repopulating ability (Figure 4A,B). DR did not alter the age-related effects, giving the F1 mice an overall pattern similar to that of B6 mice. The increase with age was, however, less pronounced.
Multilineage function
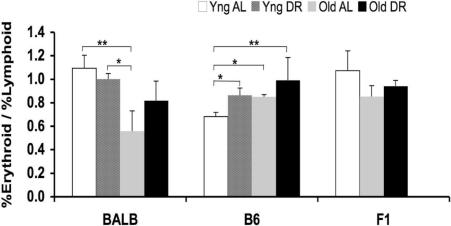
Besides repopulating ability, HSCs are defined by their ability to produce both myeloid and lymphoid lineages. We tested whether age or DR alters the ability of HSCs to proportionally produce multiple lineages of differentiated cells by measuring the ratio of erythrocyte percentage to lymphocyte percentage (E/L ratios) in peripheral blood 6 months after transplantation. By 6 months, the peripheral blood is composed of cells descended from the injected marrow, as demonstrated by experiments in which platelets, granulocytes, B cells, and T cells were produced proportionally from the same precursors by 8 weeks after transplantation.41 The E/L ratio was determined for donors of all 3 genotypes (Figure 5). In BALB mice, there is a 2-fold decrease in E/L with age, which is partly restored by DR. In contrast, in B6 mice, the E/L ratio significantly increases in the young DR mice as well as in both old AL and old DR mice but is consistently less than 1.0. The F1 hybrid pattern has a greater similarity to the BALB than the B6 pattern. This correlation between parent and F1 hybrid (Figure 5) is opposite that found in the CRA data, suggesting that the B6 HSC aging phenotype is not entirely dominant.
Figure 5.
Multilineage repopulation 6 months after transplantation. Data are analyses of the ratios of percentage donor erythroid cells/percentage donor lymphoid cells (E/L) in CRA recipients of BMCs from the same donors examined in Figures 1–3 (*P < .05; **P < .01). Error bars represent SE.
Discussion
The aging of BALB HSCs is a good example of how adult stem cells can lose function with age and how DR can reverse these effects. The fact that DR has little effect on HSCs from young BALB mice, but a large effect on HSCs from old BALB mice, suggests that DR does not alter a regulatory setting but protects against degradation of functional capacity over the lifespan. The ability of lifelong DR in BALB mice to retard the loss of functional abilities per aging HSC is especially dramatic (Table 2; Figure 2D,F), and has not been reported previously. The changes in HSC repopulating abilities are no doubt the consequence of several cellular functions that can be altered by genotype, age, and diet. This study, like most that have measured HSC ability in vivo, does not identify which cellular functions are defective. Thus, to mitigate the severe loss of function per HSC in old BALB mice, DR may alter the ability of HSCs to home, engraft, renew, or differentiate. The differences observed in old AL mice between experiments using different flow criteria (Table 2) are in part because of the large natural variations in function and health found in aged AL mice and in part because of the inherent differences in the flow criteria.
The dramatic age-related decrease in function per cell in BALB mice, shown when using the mKSL flow criterion (Figure 2D), is in part because of the large increase in LT-HSC number with age (Figure 2C). Previous studies examining flow cytometry criteria indicate that cell marker fidelity changes with age in some subpopulations.42 Using the mKSL flow cytometry criterion, we found that concentrations of all classes of progenitors, both positive and negative for CD34 and Flk2, increase 1-2 orders of magnitude with age. Thus, the huge increase in LT-HSC with age cannot merely result from a loss of CD34 and Flk2 recognition in other classes of progenitors (Table 1). The substantial differences in HSC number in young, old, and old DR marrow, depending on which criterion was used (Table 2), demonstrate that other flow cytometry criteria may be useful in determining HSC concentration in aging mice. Future studies will take advantage of the Slam family,42,43 or other new markers, which minimize changes in marker fidelity with age and can be used across strains of mice.
We considered, as well, whether DR might alter the expression of cell markers used in flow cytometry. There is no evidence for this in B6, where lifelong DR alters neither HSC number nor function (Figure 3). In BALB, where DR has dramatically different effects on HSC numbers and function, it results in values for both that are closer to those found in young controls (Figure 2C,E). The trend is consistent with the effect of DR on hundreds of disparate changes with age and suggests that DR does not merely alter cell markers.
B6 mice exhibit a completely different pattern of marrow and HSC aging than BALB mice; overall functional ability of marrow increases with age, and DR does not alter this effect (Figure 3). Although DR increases lifespan and decreases cancer in B6 mice,19–23,25,26 DR probably fails to increase HSC functional ability in aged B6 donors because levels are already higher than that found in young controls (Figure 3). In the F1, functional ability of marrow also increases with age and is not altered by DR (Figure 4), suggesting that this B6 phenotype is genetically dominant. In addition, in stark contrast to BALB, the functional abilities per HSC in B6 mice are nearly constant throughout the lifespan (Figure 3D). Thus, the increase in marrow function with age in B6 mice can be directly attributed to the increased numbers of HSC in the marrow. Previous studies of the effects of age on functional ability per HSC in B6 mice used a variety of flow cytometry markers and, although having similar trends, gave differing results.7,10,42 The discrepancies between laboratories may be the consequence of the different flow criteria, as well as differences in the exact techniques used to measure repopulating ability. The current study is the first to define effects of DR on HSCs from B6 mice and shows that DR had little effect on either HSC number or functional abilities per HSC (Figure 3C,D).
The typical set of FACS markers historically used to isolate LT-HSCs from B6 proved to be a useful criterion in BALB (Figure 2C,D). BALB marrow is, though, known to have a lower expression of the cell surface marker Sca.36 In B6 mice, 99% of the marrow repopulating ability is from cells expressing Sca. This is reduced in BALB marrow to 25%; however, the cells expressing Sca have the same marrow repopulating ability as Sca+ cells found in B6 marrow.36 Therefore, the number of LT-HSCs determined with the mKSL criterion in BALB should be proportional to the total pool of marrow repopulating cells. We verified our results using the cKit marker in conjunction with Hoechst SP. CD117, cKit, is present on both LT-HSCs and ST-HSCs; however, selecting hematopoietic cells that efflux the Hoechst dye more effectively (toward the tip of the SP) enriches the subpopulation for the more primitive LT-HSCs (Figure 1A).36,37,44,45 Interestingly, with this SP + Kit criterion, DR completely prevents loss of function per HSC (Table 2). The differences in subpopulations between strains preclude a direct statistical comparison of RU/HSC values. This does not alter the thrust of this study, which focuses on the effect of lifelong DR on HSC function within a given strain. Regardless of which flow criterion is used, DR in BALB mice partially or entirely prevents the age-related loss of function per HSC. In B6 mice, DR does not alter function where no loss in ability with age is observed.
Although the overall repopulating ability of B6 BM increases with age and the functional ability per stem cell (RU/LT-HSC) appears to be constant (Figure 3), this does not mean that HSCs from B6 avoid aging. We have previously shown that B6 HSCs have a decreased ability to repopulate lethally irradiated recipients after serial transplantation,6,11 which has been confirmed by an independent group.15 It is also known that there is a decrease in homing and engraftment with age.46 In the current study, HSC aging in B6 mice can be seen in the alteration of the E/L ratio, which measures another aspect of HSC function, multilineage differentiation. The observed increase with age in the E/L ratio found in B6 mice (Figure 5) is consistent with previous reports that show a relative increase with age in myeloid production and/or a decrease in lymphoid production.46–50 This effect of aging in B6 may reduce the ability to fight infections by lowering the overall immune capabilities of the animal.49,50 This is the first report of how aging affects the E/L ratio in BALB or F1 mice, where the trend with age is opposite that found in B6 mice. In BALB mice, the E/L ratio exhibits a pattern of stem cell aging that is retarded by DR (Figure 5), consistent with the pattern of repopulating abilities (Figure 2). In contrast, the E/L ratio in BMCs from young B6 donors is altered by DR in a manner similar to that associated with aging. The trends in the E/L ratio with age and DR in F1 mice are similar to the BALB parent. This is in contrast to data on repopulating ability, where F1 mice were similar to B6; thus, differences in HSC aging between BALB and B6 mice appear to be regulated by at least 2 independent genetic loci. In general, effects of age and DR on multilineage function (Figure 5) are smaller than on overall repopulating abilities (Figures 2–4); however, the strain difference is striking.
These results indicate that DR contradicts the current paradigm that increasing adult stem cell function to levels found in the young, to avoid senescence, must be balanced against the risk of higher incidences of cancer.12–14 In BALB mice, DR, a treatment that is known to increase life span and reduce cancer risk, increased the marrow repopulating ability of old mice to at least levels found in young mice, and greatly improved function per HSC (Table 2; Figure 2). DR decreased the risk of developing a wide variety of spontaneous cancers in B6 mice,19–21 a strain in which HSC functional ability increases with age. DR in B6 mice does not alter the functional ability with age (Figure 3A,B), which is already at levels greater than in the young. Therefore, DR breaks the paradigm in both BALB and B6 mice because it decreases the risk of developing cancer yet maintains or reduces the loss of marrow function and function per HSC with age.
This study demonstrates the importance of examining different strains when considering the effects of treatments on the aging of stem cells. The strikingly divergent responses of BALB and B6 mice in all 3 measures of HSC function make them useful models for determining the underlying mechanisms regulating the HSC proliferation, aging, and the DR effect. The hypothesis that effects of age or DR on HSCs are caused by a particular molecular change can be easily tested, as it must affect BALB and B6 mice in these different ways. For example, recent studies of HSCs,15 neural stem cells,16,17 and islet stem cells18 show an increase in P16ink4a expression with age that leads to a reduction of stem cell proliferation. This regulation of stem-cell function by tumor suppressors may be the mechanism by which aging cells balance proliferative ability against the increasing risk of cancer with age.14 Here, we show that DR reduces the loss of stem-cell proliferation with age in BALB mice. If tumor suppressors are regulating the DR effect on functional ability, it would predict that levels of p16INK4a would be reduced in BALB mice with lifelong DR compared with similarly age AL controls. In contrast, p16INK4a levels in B6 HSCs should remain constant regardless of DR. BALB mice on lifelong DR may be able to maintain low levels of p16INK4a, and thus more normal levels of proliferative ability, by reducing the mutation rate obviating the need for tumor suppressors. This would maintain or reduce the loss of function with age, reduce the incidence of cancer, and increase lifespan as observed in mice on DR. Learning how to maintain or increase function of stem cells in aging mammals while reducing cancer rates will have important implications for human health.
Acknowledgments
The authors thank Luanne Peters, Edward Leiter, and Joanne Currer for their critical editorial assistance, as well as Karen Davis and Pam Krason for their technical assistance.
This study was supported by NIH grants AG025007, AG026074, AG022308, AG025707 (D.E.H.) and NIH National Research Service Award DK07449 (D.E.H.), along with the Jackson Laboratory Cancer Core grant HL63620.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.P.E. helped with experimental design, performed research, analyzed data, and wrote the paper; J.C. helped with experimental design and preliminary research; C.M.A. helped with experimental design and performed research; T.M.D. did FACS analyses; and D.E.H. obtained funding, provided oversight, designed experiments, and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David E. Harrison, Jackson Laboratory, 600 Main St, Bar Harbor, ME 04609; e-mail: david.harrison@jax.org.
References
- 1.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 2.Robinson B. Cost of anemia in the elderly. J Am Geriatr Soc. 2003;51(suppl):14–17. doi: 10.1046/j.1532-5415.51.3s.5.x. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BW, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DE, Astle CM, Stone M. Numbers and functions of transplantable primitive immunohematopoietic stem cells: effects of age. J Immunol. 1989;142:3833–3840. [PubMed] [Google Scholar]
- 8.Harrison DE, Jordan CT, Zhong RK, Astle CM. Primitive hematopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp Hematol. 1993;21:206–219. [PubMed] [Google Scholar]
- 9.Van Zant G, Holland BP, Eldridge PW, Chen JJ. Genotype-restricted growth and aging patterns in hematopoietic stem cell populations of allophenic mice. J Exp Med. 1990;171:1547–1565. doi: 10.1084/jem.171.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 11.Yuan R, Astle CM, Chen J, Harrison DE. Genetic regulation of hematopoietic stem cell exhaustion during development and growth. Exp Hematol. 2005;33:243–250. doi: 10.1016/j.exphem.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Pardal R, Molofsky AV, He S, Morrison SJ. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of proto-oncogenes and tumor suppressors. Cold Spring Harb Symp Quant Biol. 2005;70:177–185. doi: 10.1101/sqb.2005.70.057. [DOI] [PubMed] [Google Scholar]
- 13.Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 14.Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- 15.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 16.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 19.Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 20.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed National Institutes of Health-31 open formula diet. Toxicol Pathol. 1995;23:570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 21.Turturro A, Duffy P, Hass B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol A Biol Sci Med Sci. 2002;57:379–389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- 22.Masoro EJ. Dietary restriction and aging. J Am Geriatr Soc. 1993;41:994–999. doi: 10.1111/j.1532-5415.1993.tb06767.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, Harrison DE. Delayed reduction in T cell precursor frequencies accompanies diet-induced lifespan extension. J Immunol. 1985;134:1426–1429. [PubMed] [Google Scholar]
- 24.Luan X, Zhao W, Chandrasekar B, Fernandes G. Calorie restriction modulates lymphocyte subset phenotype and increases apoptosis in MRL/lpr mice. Immunol Lett. 1995;47:181–186. doi: 10.1016/0165-2478(95)00091-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Astle CM, Harrison DE. Delayed immune aging in diet-restricted B6CBAT6F1 mice is associated with preservation of naive T cells. J Gerontol A Biol Sci Med Sci. 1998;53:330–337. doi: 10.1093/gerona/53a.5.b330. [DOI] [PubMed] [Google Scholar]
- 26.Effros RB, Walford RL, Weindruch R, Mitcheltree C. Influences of dietary restriction on immunity to influenza in aged mice. J Gerontol. 1991;46:142–147. doi: 10.1093/geronj/46.4.b142. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Exp Hematol. 2003;31:1097–1103. doi: 10.1016/s0301-472x(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 28.Jackson Laboratory Staff. Bar Harbor, ME: Jackson Laboratory; 1997. Handbook on Genetically Standardized JAX® Mice. [Google Scholar]
- 29.Jackson Laboratory. JAX Mice & Services. http://jaxmice.jax.org/health. (see the C-1 animal room from 2002-2006). Accessed quarterly.
- 30.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [Erratum in Science. 1989;244:1030] [DOI] [PubMed] [Google Scholar]
- 31.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida A, Zeng H, Ogawa M. Expression of lineage markers by CD34+ hematopoietic stem cells of adult mice. Exp Hematol. 2002;30:361–365. doi: 10.1016/s0301-472x(01)00795-0. [DOI] [PubMed] [Google Scholar]
- 34.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi DJ, Bryder D, Weissman IL. Hematopoeitic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- 37.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KK, Goodell MA. Purification of hematopoietic stem cells using the side population. Methods Enzymol. 2006;420:255–264. doi: 10.1016/S0076-6879(06)20011-9. [DOI] [PubMed] [Google Scholar]
- 39.Pearce DJ, Anjos-Afonso F, Ridler CM, Eddaoudi A, Bonnet D. Age dependent increase in SP distribution within Hematopoiesis: implications for our understanding of the mechanism of aging. Stem Cells. 2007;25:828–835. doi: 10.1634/stemcells.2006-0405. [DOI] [PubMed] [Google Scholar]
- 40.Sharma Y, Flurkey K, Astle CM, Harrison DE. Mice severely deficient in growth hormone have normal hemaotopoiesis. Exp Hematol. 2005;33:776–783. doi: 10.1016/j.exphem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Harrison DE, Zhong RK. The same exhaustible multilineage precursor produces both myeloid and lymphoid cells as early as 3-4 weeks after marrow transplantation. Proc Natl Acad Sci U S A. 1992;89:10134–10138. doi: 10.1073/pnas.89.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Goodell MA. Introduction: focus on hematology. CD34(+) or CD34(−): does it really matter? Blood. 1999;94:2545–2547. [PubMed] [Google Scholar]
- 45.Goodell MA, Rosenzweig M, Kim H, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 46.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- 49.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]