Abstract
As part of our ongoing effort to relate stimulus to response in the olfactory system, we tested the hypothesis that the unique chemical structures and odors of various cyclic odorants would be associated with unique spatial response patterns in the glomerular layer of the rat olfactory bulb. To this end, rats were exposed to sets of odorants including monocyclic hydrocarbons, bicyclic compounds, and various heterocyclic structures containing oxygen or nitrogen in the ring. Relative activity across the entire layer was assessed by mapping uptake of 2-deoxyglucose into anatomically standardized data matrices. Whereas monocyclic hydrocarbons evoked patterns similar to those evoked by open-chained hydrocarbon odorants, a set of bicyclic compounds with structures and odors similar to camphor evoked uptake in paired ventral domains not previously associated with any other odorant chemical structures. Despite their unique odors as judged by humans, heterocyclic odorants either evoked uptake in previously characterized areas corresponding to their functional groups or stimulated weak or patchy patterns involving isolated glomeruli. While the patchiness of the patterns may be partly related to the rigidity of the compounds, which would be expected to restrict their interactions to only a few receptors, the weakness of the patterns suggests the possibility of species-specific odorant representations. We conclude that whereas some of the novel cyclic structures indeed were represented by unique patterns in the rat bulb, other unique structures were poorly represented, even when they evoked intense and unique odors in humans.
Indexing terms: 2-deoxyglucose, odors, imaging techniques, mapping
Axons of olfactory sensory neurons that express the same odorant receptor gene converge to terminate in small numbers of glomeruli in the olfactory bulb (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996). Each glomerulus appears to receive terminals associated with only one receptor type (Treloar et al., 2002). Given this anatomy, it is possible to get a read-out of receptor activation by measuring glomerular activity across the bulb. Studies using uptake of 2-deoxyglucose (2-DG) and optical imaging of endogenous signals have indicated that neighboring glomeruli in the rat olfactory bulb tend to be activated by odorants of similar chemical structure (Johnson et al., 1998; 1999; 2002; 2004; Johnson and Leon, 2000b; Farahbod et al., 2006; Ho et al., 2006a; Uchida et al., 2000; Takahashi et al., 2004). Certain clusters of glomeruli respond to odorants with particular functional groups such as carboxylic acids, alcohols, or ketones (Johnson et al., 1999; 2002; 2004; Johnson and Leon, 2000a,b; Uchida et al., 2000), while other clusters respond to odorants with particular hydrocarbon structures, such as benzene rings (Johnson et al., 2005b; Farahbod et al., 2006).
In our survey of 2-DG uptake across the entire glomerular layer in response to odorants of systematically different chemical structures, we have observed evoked activity in most regions of the bulb (Johnson et al., 2002; Ho et al., 2006a; http://leonserver.bio.uci.edu), but there are a few remaining areas in the glomerular layer where we have not yet documented differential activity to any odorants. We expected that these remaining areas would be stimulated by odorants that possess structural elements distinct from those previously investigated, and we have been engaged in a continuing effort to increase the structural variety of odorant chemicals in our archive to test this prediction.
In the present study, we have expanded our survey to include a number of cyclic odorants including monocyclic hydrocarbons, bicyclic compounds, and heterocyclic compounds (Table 1). Monocyclic hydrocarbons are both aliphatic and cyclic (alicyclic), in the sense that they are aliphatic in chemical behavior but their carbon atoms are organized in a closed-chain or ring structure without being aromatic. Bicyclic molecules contain two fused closed chains. Heterocyclic molecules contain ring structures that include atoms other than carbon within the ring. All of these cyclic odorants are comparatively rigid molecules that might be expected to activate a limited set of receptors and glomeruli compared to most open-chained aliphatic odorants because the rigidity of the cyclic molecules precludes the range of steric profiles afforded by the multiple potential conformations of the more flexible, open-chained odorants. Many of these cyclic compounds elicit unique and strong odor perceptions in humans, an observation that may be related to their unique structural features. We tested the hypothesis that these unique odorant structures would be associated with the activation of unique, focal glomerular activity patterns by studying 2-DG uptake across the entire glomerular layer of the rat olfactory bulb.
Table 1.
Odorant exposures in this study.
| Odorant | CAS#5 | Vendor | Purity (%) | Solvent | Solvent dilution | Air dilution | n |
|---|---|---|---|---|---|---|---|
| Methylcyclohexane (250 ppm) | 108-87-2 | Acros | 99 | None | None | 1/244 | 5 |
| Methylcyclohexane (1000 ppm) | 108-87-2 | Acros | 99 | None | None | 1/61 | 4 |
| Cyclohexane (250 ppm) | 110-82-7 | Acros | > 99 | None | None | 1/513 | 5 |
| Cyclohexane (1000 ppm) | 110-82-7 | Acros | > 99 | None | None | 1/128 | 5 |
| Cycloheptane (250 ppm) | 291-64-5 | Acros | 99 | None | None | 1/115 | 5 |
| Cycloheptane (1000 ppm) | 291-64-5 | Acros | 99 | None | None | 1/28.7 | 5 |
| Cyclooctane (250 ppm) | 292-64-8 | Alfa Aesar | > 99 | None | None | 1/36.8 | 5 |
| Cyclooctane (1000 ppm) | 292-64-8 | Alfa Aesar | > 99 | None | None | 1/9.2 | 5 |
| Heptane1 | 142-82-5 | Acros | 99 | None | None | 1/8 | 4 |
| Norbornane | 279-23-2 | Aldrich | 98 | Mineral oil | 1/20 | 1/10 | 5 |
| Norcamphor | 497-38-1 | Aldrich | 98 | Mineral oil | 1/20 | 1/10 | 5 |
| 7-Oxabicycloheptane | 279-49-2 | Aldrich | 98 | Mineral oil | 1/20 | 1/10 | 5 |
| Beta-pinene | 18172-67-3 | Aldrich | 99 | Mineral oil | 1/20 | 1/10 | 5 |
| Camphor | 464-49-3 | Aldrich | 98 | Mineral oil | 1/20 | 1/10 | 5 |
| Fenchone | 7787-20-4 | Aldrich | 98 | Mineral oil | 1/20 | 1/10 | 5 |
| Eucalyptol | 470-82-6 | Aldrich | 99 | Mineral oil | 1/20 | 1/10 | 5 |
| Bornyl acetate | 5655-61-8 | Aldrich | 95 | Mineral oil | 1/20 | 1/10 | 5 |
| Eucalyptol (2nd replicate) | 470-82-6 | Fluka | 97 | None | None | 1/8 | 3 |
| Eucalyptol (3rd replicate) | 470-82-6 | Fluka | 97 | None | None | 1/8 | 3 |
| Bornyl acetate (2nd replicate) | 5655-61-8 | Acros | 95 | None | None | 1/8 | 3 |
| Bornyl acetate (3rd replicate) | 5655-61-8 | Acros | 95 | None | None | 1/8 | 10 |
| Thujone | 546-80-5 | Aldrich | 96 | Mineral oil | 1/20 | 1/10 | 4 |
| Oxyoctaline formate | 65405-72-3 | Vigon | 95 | Mineral oil | 1/10 | 1/10 | 4 |
| Alpha-ionone2 | 127-41-3 | Aldrich | 90 | None | None | 1/8 | 3 |
| L-Menthone2 | 14073-97-3 | Aldrich | 96 | Mineral oil | 1/4 | 1/20 | 6 |
| L-Menthyl acetate2 | 29066-34-0 | Aldrich | 97 | Mineral oil | 1/4 | 1/20 | 6 |
| Ethyl chrysanthemumate | 97-41-6 | Acros | 95 | None | None | 1/8 | 3 |
| 3,3,5-Trimethylcyclohexanone | 873-94-9 | Acros | 97 | None | None | 1/8 | 3 |
| Pinacolone | 75-97-8 | Acros | 96 | None | None | 1/8 | 3 |
| Tert-butylbenzene3 | 98-06-6 | Acros | 99 | None | None | 1/8.4 | 4 |
| Acetylcyclohexane4 | 823-76-7 | Alfa Aesar | 96 | None | None | 1/8 | 4 |
| Tert-butanol | 75-65-0 | Acros | 99.5 | Mineral oil | 1/4 | 1/20 | 4 |
| 2,5-Dimethyl-2-octen-6-one | 2550-11-0 | Vigon | 96 | Mineral oil | 1/10 | 1/10 | 3 |
| Delta-valerolactone | 542-28-9 | Acros | 99 | None | None | 1/8 | 3 |
| Gamma-caprolactone | 695-06-7 | Aldrich | 98 | None | None | 1/8 | 4 |
| Alpha-angelica lactone | 591-12-8 | Acros | 98 | None | None | 1/8 | 3 |
| 4,6-Dimethyl-2-pyrone | 675-09-2 | Acros | 98 | Mineral oil | 1/10 | 1/19 | 4 |
| Abhexone | 698-10-2 | Acros | 98 | Mineral oil | 1/20 | 1/10 | 3 |
| 4,5-Dimethyl-3-hydroxy-2,5-dihydrofuran-2-one | 28664-35-9 | Aldrich | > 97 | Mineral oil | 1/1000 | 1/34 | 4 |
| Celeriax | 17369-59-4 | Alfa Aesar | 95 | None | None | 1/8 | 3 |
| Coumarin | 91-64-5 | Acros | > 99 | Mineral oil | 1/10 | 1/14 | 4 |
| 2-Ethylfuran | 3208-16-0 | Aldrich | 99 | None | None | 1/8 | 3 |
| 3-(5-Methyl-2-furyl)butanal | 31704-80-0 | Aldrich | 99 | None | None | 1/8 | 3 |
| Menthofuran | 17957-94-7 | Aldrich | 95 | None | None | 1/8 | 3 |
| Methyl 2-furoate | 611-13-2 | Aldrich | > 98 | Mineral oil | 1/10 | 1/10 | 4 |
| 3-Acetyl-2,5-dimethylfuran | 10599-70-9 | Aldrich | > 99 | Mineral oil | 1/100 | 1/10 | 4 |
| 5-Methylfurfural | 620-02-0 | Aldrich | > 98 | Mineral oil | 1/10 | 1/34 | 3 |
| 5-Hydroxymethylfurfural | 67-47-0 | Aldrich | > 99 | Mineral oil | 1/10 | 1/34 | 4 |
| Furaneol | 3658-77-3 | Acros | 95 | Mineral oil | 1/20 | 1/10 | 3 |
| 2-Acetylpyridine2 | 1122-62-9 | Acros | 98 | None | None | 1/8 | 3 |
| 4-Tert-butylpyridine4 | 3978-81-2 | Acros | 96 | None | None | 1/8 | 3 |
| Methyl nicotinate4 | 93-60-7 | Acros | 99 | Mineral oil | 1/20 | 1/10 | 3 |
| Quinoline | 91-22-5 | Acros | 99 | Mineral oil | 1/160 | 1/20 | 3 |
| 2-Benzylpyridine | 101-82-6 | Acros | 98 | None | None | 1/8 | 3 |
| 2,3,4,6-Tetramethylpyrazine3 | 1124-11-4 | Aldrich | 98 | Mineral oil | 1/10 | 1/10 | 4 |
| 2,6-Dimethylpyrazine3 | 108-50-9 | Aldrich | 98 | Mineral oil | 1/10 | 1/10 | 4 |
| 2-Isobutyl-3-methoxypyrazine | 24683-00-9 | Aldrich | > 99 | Mineral oil | 1/1000 | 1/10 | 4 |
| 2-Acetylpyrazine | 22047-25-2 | Aldrich | > 99 | Mineral oil | 1/100 | 1/34 | 4 |
| 5-Methylquinoxaline | 13708-12-8 | Aldrich | > 99 | Mineral oil | 1/1000 | 1/34 | 4 |
| 5,6,7,8-Tetrahydroquinoxaline | 34413-35-9 | Aldrich | > 97 | Mineral oil | 1/100 | 1/34 | 4 |
| Pyrrole | 109-97-7 | Acros | 99 | Mineral oil | 1/10 | 1/19 | 4 |
| Indoline | 496-15-1 | Acros | 99 | Mineral oil | 1/10 | 1/19 | 4 |
| Indole | 120-72-9 | Acros | > 99 | Mineral oil | 1/10 | 1/34 | 4 |
| 2-Pyrrolidinone | 616-45-5 | Acros | 98 | Mineral oil | 1/10 | 1/34 | 4 |
| 2,4,5-Trimethyloxazole | 20662-84-4 | Aldrich | 95 | Mineral oil | 1/10 | 1/34 | 4 |
| Thiazole | 288-47-1 | Acros | 99 | Mineral oil | 1/10 | 1/34 | 4 |
| 2-Acetylthiazole | 24295-03-2 | Aldrich | > 99 | Mineral oil | 1/100 | 1/34 | 3 |
First published in Ho et al. (2006a).
First published in Johnson et al. (2002).
First published in Farahbod et al. (2006).
First published in Johnson et al. (2005b).
CAS#, Chemical Abstract Services registry number.
MATERIALS AND METHODS
Odorants
The odorants used in this study are listed in Table 1, which includes both our own preferred name and the unique Chemical Abstract Services registry number associated with each compound. Structural drawings illustrating the molecules are provided within the individual figures showing 2-DG uptake evoked by the odorants. Table 1 also indicates the vendors and the reported purities of their products. The purities ranged from 90% to greater than 99.5%. We have recent evidence that even minor impurities can have a substantial impact on patterns of 2-DG uptake (Ho et al., 2006a). Therefore, one should view with caution any isolated instance of an odorant-evoked pattern and should look for corroborating information from related odorant chemicals. Table 1 further indicates the dilutions of each odorant. For two odorants, multiple independent exposures were performed, and the order in the table matches the order in which the evoked uptake patterns are presented in the text and figures. The final column of Table 1 displays the number of animals exposed to that exposure condition that were averaged together to generate the uptake pattern representing the odorant exposure.
The exposures comprising this study were organized into two independently executed experiments involving systematic differences in odorant chemistry as well as a series of exposures to sets of odorants that were less related in structure. In one systematic experiment, we presented four monocyclic hydrocarbons (cyclohexane, cycloheptane, cyclooctane, and methylcyclohexane) at two concentrations each. The vapor phase concentrations of these compounds were achieved by differential dilution of the vapor arising from neat material, and the initial saturated vapor pressure was calculated as a median of all unique values from two Internet databases (PhysProp Database from Syracuse Research Corporation at http://www.syrres.com/esc/physdemo.htm and the Chemical and Physical Properties Database from the Pennsylvania Department of Environmental Protection at http://www.dep.state.pa.us/physicalproperties/CPP_Search.htm) and two chemical modeling software packages (Molecular Modeling Pro version 3.14, ChemSW, Fairfield, CA and ChemDraw Ultra version 6.0, CambridgeSoft, Cambridge, MA).
The second systematic experiment involved a set of eight simple bicyclic odorants (norbornane, norcamphor, 7-oxabicycloheptane, β-pinene, camphor, fenchone, eucalyptol, and bornyl acetate). Some of these bicyclic odorants were solids, leading us to suspend the compounds in light mineral oil (Fisher Scientific) before use. Because the vapor pressure over the suspension was unknown, equal vapor phase concentrations were not used in this experiment. For each systematic experiment, we used a block design involving animals from the same litter for different odorant conditions, varying the order of odorant exposure between litters. Within each systematic experiment, the same blank pattern was subtracted from each individual animal’s pattern during data analysis (see details below), insuring a closer relationship between the patterns within a systematic experiment than typically exists across different experiments.
In addition to the odorants used for the two systematic experiments, we used 36 other new odorants and 10 previously published odorants (Johnson et al., 2002; 2005b; Ho et al., 2006; Farahbod et al., 2006) both to explore patterns evoked by cyclic odorants of more widely varying composition and to demonstrate 2-DG uptake in areas overlapping with those activated by cyclic odorants. When possible, we presented minimal dilutions of the saturated vapors from neat material, but many of these compounds were either solid, available in only small quantities, or very expensive, in which cases we employed dilutions in mineral oil. The degree of dilution differed among the compounds for a number of reasons. In some cases, the dilution factor was determined by the limited amount of material available. In other cases, dilutions were increased to avoid excessive foaming from the mineral oil suspension. Finally, in three other cases (2-isobutyl-3-methoxypyrazine, 5-methylquinoxaline, and 4,5-dimethyl-3-hydroxy-2,5-dihydrofuran-2-one), the odor of an already quite dilute (1/100) suspension in mineral oil was perceived to be so intense by the investigators that we feared stressing the animals and therefore reduced the concentration further. Not all odorant compounds were fully soluble in mineral oil. In these cases, we used a saturated suspension in the same way that we would have used a solution. Because different concentrations of certain odorants can evoke different patterns of 2-DG uptake (Johnson and Leon, 2000a; Johnson et al., 2004; Ho et al., 2006a), it remains possible that we would have obtained different results if we had used different concentrations of the odorants in the present study.
Odorant Exposures
All procedures involving rats were approved by the UC Irvine Institutional Animal Care and Use Committee (IACUC). Weanling rat pups between postnatal days 17 and 21 were given subcutaneous injections of [14C]2-DG (1.6 μL/g, 0.1 mCi/mL, 52 mCi/mmol, Sigma Chemical Company, St. Louis) immediately before being placed into an empty exposure chamber for the beginning of the odorant exposure. The exposure chamber was constructed from a 2-liter glass Mason jar with two holes bored in the lid for odorant entry and exhaust.
Odorants were volatilized by bubbling research-grade, high-purity nitrogen gas through the liquid odorant preparation in a gas-washing bottle. The nitrogen vapor then was mixed with ultra-zero grade, high-purity air prior to delivery into the exposure chamber. For the neat odorants, the total flow rate into the chamber was 2 L/min. For odorants suspended in mineral oil, the flow rate was 1 L/min. The odor delivery system was equilibrated for at least 15 minutes prior to an exposure. All tubing, regulators, and connectors were constructed from inert materials such as brass, Kynar, and fluorinated ethylene propylene. Each set of tubing was reserved for use with a single odorant and discarded after the experiment was complete. For each study, one rat from each litter was exposed to vehicle vapor as a blank condition before any of the odorant exposures. When an experiment employed an odorant suspended in mineral oil, mineral oil alone served as the blank condition, whereas a mixture of nitrogen and air was passed through an empty gas-washing bottle as a blank when the experiment involved neat odorant liquids.
Exposures continued for 45 minutes and were terminated by removing and killing the rat by decapitation, followed by rapid dissection of the brain and olfactory bulbs. Brains were frozen at about −45°C in 2-methylbutane cooled on dry ice. Brains were stored at −80°C until sectioning in a cryostat. Although there is one report that brief and intermittent odorant exposures result in a pattern of 2-DG uptake comparable to that obtained in a continuous 45-minute exposure such ours (Slotnick et al., 1989), we cannot exclude the possibility that the timing of the odorant exposure might affect the patterns of activity evoked by particular odorants.
Data analysis
Tissue sectioning, histological processing, imaging, and data analysis were performed as detailed previously (Johnson et al., 1999; 2004). Uptake in the glomerular layer was sampled by way of autoradiographic images of 20-μm coronal sections that were separated by 120 μm. The layer was located in these sections by overlaying images of adjacent cresyl violet-stained sections. Sampling was standardized across bulbs and animals in reference to: (1) anatomical landmarks encountered along the anterior-posterior dimension in the stained sections and (2) a set of polar grids that were positioned on each section in relation to the location of the glomerular layer and that were chosen on the basis of the position of the section between the anterior-posterior landmarks. Data were converted from grayscale values to values of nCi/mg tissue on the basis of radioactivity standards exposed to each sheet of autoradiography film. Data from individual sections were concatenated into matrices that then were further standardized for differences in size by compression or by insertion of new data to equalize the number of sections between pairs of landmarks.
The matrices from the left and right bulbs of each animal were averaged, and the units of uptake were converted to a ratio of glomerular layer uptake to uptake measured in the subependymal zone of the bulb. For each experiment, the matrices of all vehicle blank-exposed animals were averaged and subtracted from each other matrix. Then, units in each matrix were converted to z-scores relative to the mean and standard deviation of values across that matrix. Statistical analyses in the cyclic hydrocarbon experiment involved these matrices. Within each experiment, the z-score matrices involving the same odorant exposure condition were averaged together and plotted as contour charts. We prefer to orient these charts in a ventral-centered format to marginalize the missing values along the dorsal surface that occasionally result from loss of tissue during sectioning; however, both dorsal-centered charts and 3-dimensional, rotatable images of the activity patterns can be seen at http://leonsever.bio.uci.edu and at [URL to be completed by publisher].
Generation of images
Digital images of individual autoradiographic sections were collected by using a Sony XC-77 CCD video camera, a lens stack, and trans-illumination by way of a Northern Lights light box. The camera was connected to a frame grabber card (LG-3 Scientific PCI from Scion Corporation) in a Macintosh G3 computer running NIH IMAGE 1.62 software. The autoradiographic images were false-colored using a 32-color look-up table that was adjusted to show the highest uptake as red and the lowest uptake as blue. All sections from the same brain were imaged using the same look-up table. In some cases, adjacent Nissl-stained sections were imaged at the same magnification but using a grayscale look-up table. Composites and overlays were created using Adobe Photoshop. Images of Nissl-stained sections were enhanced in contrast and brightness and then overlaid using the “multiply” blending mode.
RESULTS
Monocyclic hydrocarbons
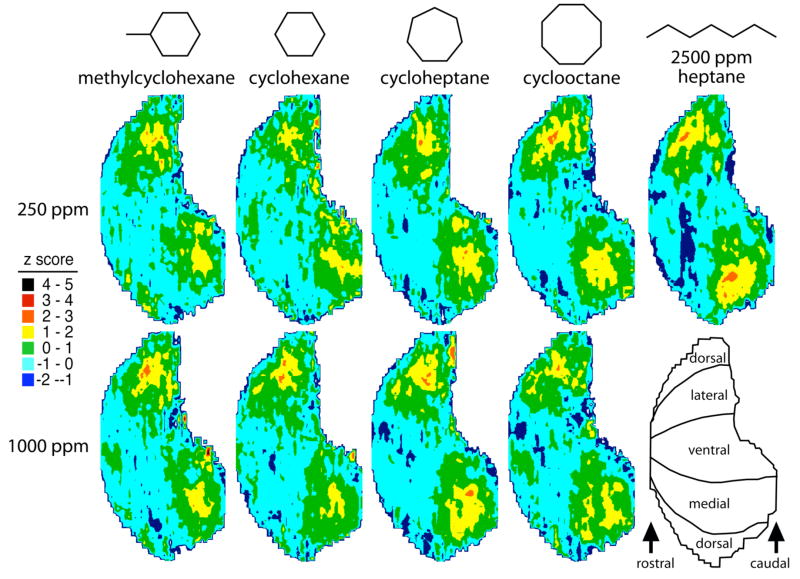
To address the possibility of a special representation of cyclic odorants in the olfactory bulb, we began by studying a series of closed-chained hydrocarbons of differing carbon number. We planned to compare these patterns to each other and to open-chained hydrocarbon odorants that we had investigated recently for evoked activity patterns (Ho et al., 2006a,b). Because representations of the hydrocarbon heptane had been found to differ with increasing concentration (Ho et al., 2006a), we used a lower (250 ppm) and higher (1000 ppm) concentration of each of the cyclic hydrocarbons in this study.
As shown in Figure 1, each of the cyclic hydrocarbons evoked activity primarily in the posterior third of the lateral and medial quadrants of the bulb. The activity was largely symmetrical with respect to the lateral and medial aspects, in agreement with most of the activity patterns studied using 2-DG to assess activity across the entire glomerular layer (Johnson et al., 2002) and reflecting the paired medial and lateral projection of sensory neurons expressing the same odorant receptor genes (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996).
Fig. 1.
Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in response to a series of cyclic hydrocarbon odorants at two concentrations. Also included is a previously published chart (Ho et al., 2006a) showing uptake evoked by the straight-chained hydrocarbon odorant heptane, which activated similar parts of the bulb. Charts are oriented as shown at bottom right. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition.
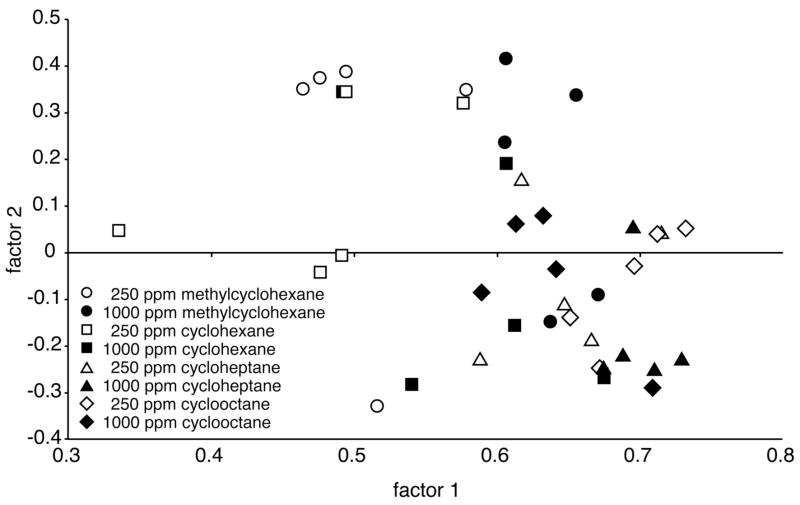
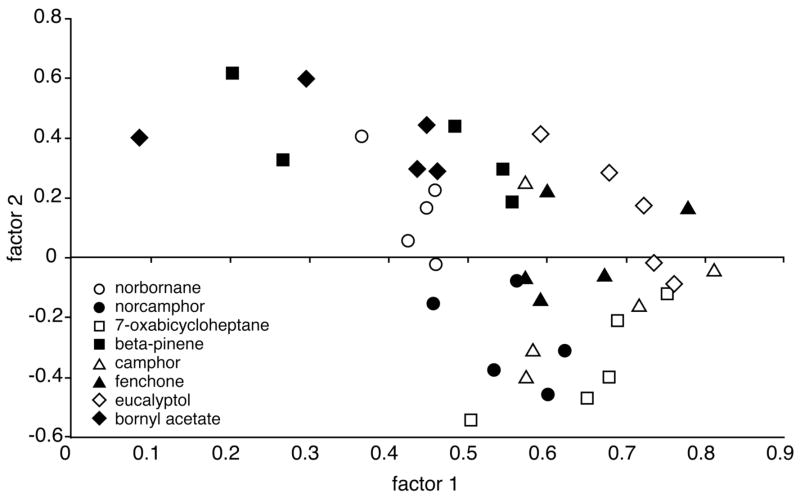
The possibility of systematic differences in patterns across the different cyclic odorants and concentrations was addressed statistically. We analyzed the individual animal’s overall activity patterns by calculating pair-wise Pearson correlations involving each of the 39 patterns (4 odorants × 2 concentrations × 5 replicates, except for 4 replicates in one condition; Table 1). The matrix of correlation coefficients then was used as input data for a principal components analysis (Fig. 2). Loadings on the first factor, which accounted for 39% of the variance, were found to be significantly different across both odorants (2-way ANOVA, F = 18.77, P < 0.0001) and concentrations (F = 13.12, P = 0.0010), also displaying a significant interaction (F = 6.51, P = 0.0015). Loadings on the second factor (5% of the variance) were significantly different only across odorants (F = 4.15, P = 0.014). Post-hoc analyses revealed that the first factor of the principal components analysis segregated both cycloheptane and cyclooctane from both cyclohexane and methylcyclohexane, whereas the second factor further segregated methylcyclohexane from both cycloheptane and cyclooctane.
Fig. 2.
Scatter plot of the first two factors extracted by principal components analysis of the individual activity patterns involved in the cyclic hydrocarbons experiment. Individual uptake matrices standardized using z scores were subjected to pair-wise Pearson correlation analysis, and the resulting matrix of correlation coefficients was used as input data for a principal components analysis. Each point in the scatter plot represents an individual animal’s pattern of 2-DG uptake. Relative clustering of points indicates overall similarity of pattern. Subsequent analyses revealed that both factors contained significant information regarding odorant identity, and that the first factor also contained information regarding odorant concentration.
Despite statistical evidence for differences among the patterns evoked by the four monocyclic hydrocarbons, their patterns appeared quite similar to one another (Fig. 1). Indeed, analyses of pair-wise correlations between the average patterns evoked by 1000-ppm odorants yielded Pearson correlation coefficients ranging from high values of 0.56 to 0.69. In addition, the patterns were quite similar to patterns evoked by open-chained hydrocarbons. Table 2 shows the highest correlations between patterns evoked by the four 1000-ppm monocyclic odorants and our previously published patterns (264 patterns representing 184 unique odorants); the highest correlations for cyclooctane, cycloheptane, and cyclohexane all involved an open-chained hydrocarbon. Correlations with 2-octyne and heptane (Ho et al., 2006a,b) were high for all three of these unsubstituted cyclic hydrocarbons. Correlations with ethylbenzene (Johnson et al., 2005b; Farahbod et al., 2006), an aromatic cyclic hydrocarbon, also were high for most of the odorants. The high pattern correlations between alicylic hydrocarbons and open-chained hydrocarbons can be taken as evidence that despite their unique molecular characteristics, monocyclic hydrocarbons surprisingly do not elicit unique glomerular responses in the rat olfactory bulb. Therefore, we would not consider the ring structure itself to be a molecular feature recognized by the olfactory system.
Table 2.
Similarities between cyclic hydrocarbon-evoked patterns and other patterns1
| Most closely correlated odorant-evoked patterns
|
||||||
|---|---|---|---|---|---|---|
| Odorant | #1 | #2 | #3 | #4 | Highest r | Heptane r |
| Cyclooctane | 2-Octyne | Heptane | Ethylbenzene | 1,7-Octadiene | 0.63 | 0.62 |
| Cycloheptane | Heptane | 2-Octyne | 2-Hexanone | Ethylbenzene | 0.71 | 0.71 |
| Cyclohexane | 2-Octyne | cis-4-Octene | Heptane | trans-2-Octene | 0.58 | 0.56 |
| Methylcyclohexane | Ethylbenzene | Butyl propionate | trans-4-Octene | Butyl acetate | 0.57 | 0.51 |
The activity pattern evoked by each alicyclic hydrocarbon at 1000 ppm was compared to all other previously published evoked patterns in our database at the time of writing (264 patterns representing 184 unique odorants) by way of pair-wise cell-by-cell correlation analyses of the underlying data matrices. Shown here are the odorants associated with the highest Pearson correlation coefficients (r). Also listed for each odorant is the correlation coefficient describing the similarity in pattern between the odorant and the odorant listed as #1, as well as the correlation coefficient for each odorant compared to heptane.
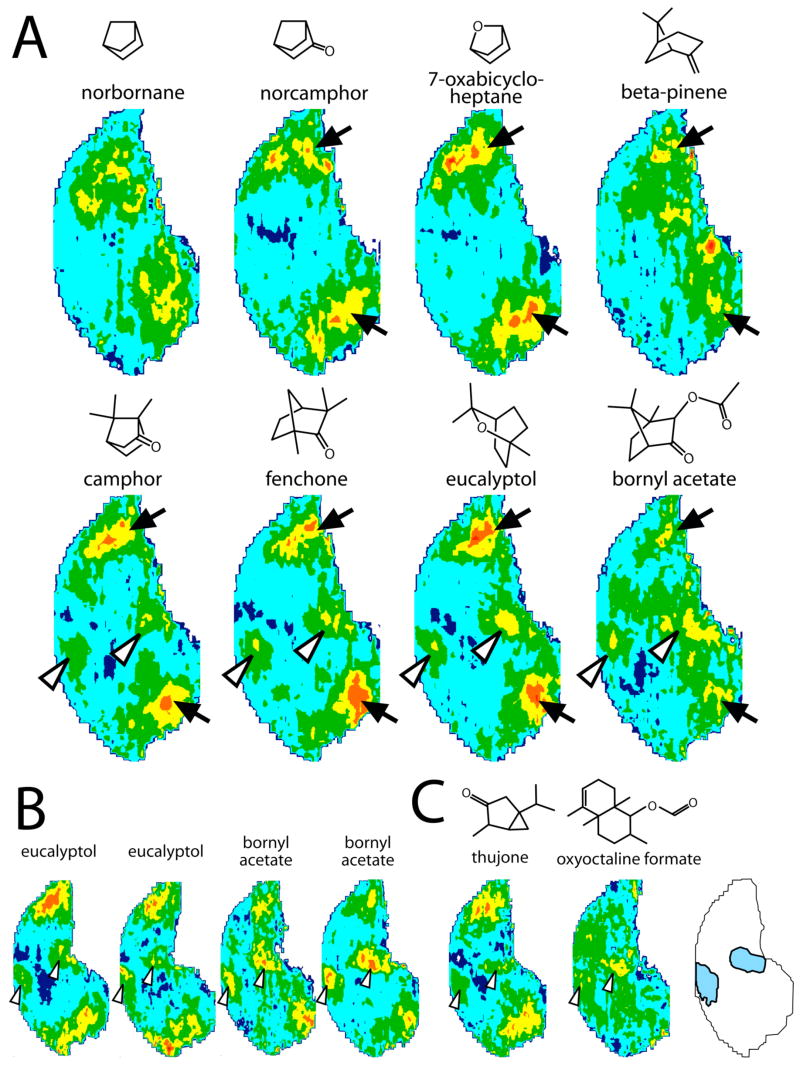
Bicyclic odorants
To address further the possibility that other cyclic structures might be recognized as unique odorant features, we investigated a series of bicyclic odorants, all of which possessed a cyclohexyl backbone with bridging atoms across carbons within this ring (Fig. 3A). The odorants included the relatively simple hydrocarbons norbornane and beta-pinene as well as the oxygen-containing molecules norcamphor and 7-oxabicycloheptane, and more complex, methyl-substituted structures such as camphor, eucalyptol, fenchone, and bornyl acetate that are found in various fragrant herbs and oils derived from tree leaves and wood.
Fig. 3.
Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in animals exposed to bicyclic odorants. Orientation and color scale are the same as for Figure 1. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition. A: Patterns from a single experiment involving exposures to a series of related bicyclic odorants first suspended in mineral oil. Most of the odorants activated similarly positioned clusters of glomeruli in the dorsolateral and dorsomedial quadrants of the posterior part of the glomerular layer (solid arrows). The latter four odorants in the series also activated a pair of novel glomerular clusters situated at the ventral extremity of the bulb (open arrowheads). B: Patterns resulting from independent exposures involving eucalyptol and bornyl acetate odorants volatilized from neat liquid. Similar patterns were obtained for the independent exposures, although the ventral uptake (open arrowheads) evoked by bornyl acetate appeared to be more robust when the neat liquid was used as the odorant source. C: Patterns resulting from exposures to two bicyclic odorants that did not contain bridging atoms. These odorants also showed evidence of activating the pair of ventrally positioned glomerular clusters (open arrowheads). The schematic at bottom right indicates the location of the novel paired ventral activity foci.
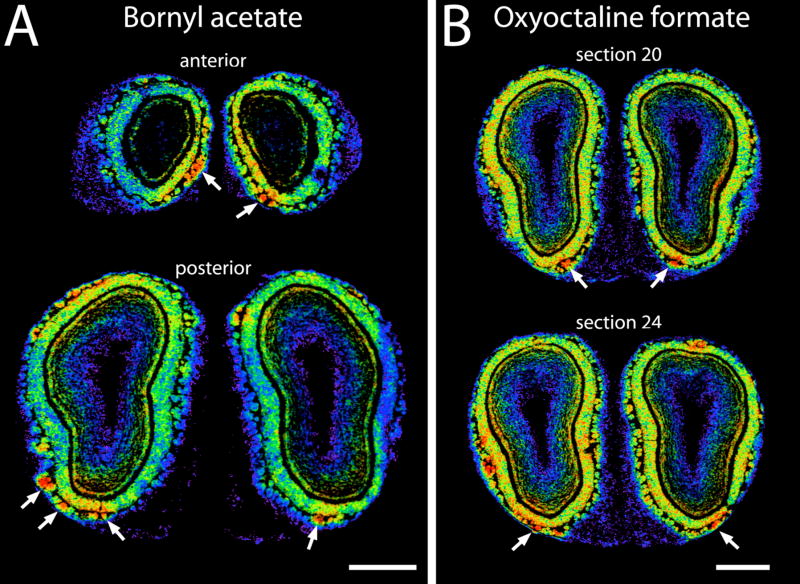
As shown in Figure 3A, most of the bicyclic odorants stimulated uptake in lateral and medial regions overlapping with areas activated by the monocyclic odorants and open-chained hydrocarbons (solid arrows). However, the last four odorants in the series also all activated a novel pair of extremely ventral clusters of glomeruli (open arrowheads, Fig. 3A). The paired response was comprised of one anterior and one posterior focus, a pattern that is quite different from our typical results involving pairs of activated glomeruli located in lateral and medial aspects of the bulb. The extreme ventral location of the glomeruli activated by these four bicyclic odorants can be seen in Figure 4A, which includes pseudocolor-enhanced images of the 2-DG uptake evoked by bornyl acetate overlaid with images of adjacent cresyl violet-stained sections.
Fig. 4.
Digitized images of ventral 2-DG uptake evoked by two bicyclic odorants. To produce these composites, enhanced-contrast, grayscale images of adjacent Nissl-stained sections have been superimposed on pseudocolor images of autoradiographic sections. The color scale has been adjusted to display the full range of uptake across the sections. A: Sections through the anterior (top) and posterior (bottom) responses to bornyl acetate in one animal. B: Two sections through the posterior response region to oxyoctaline formate in one animal. Scale bars = 1 mm.
Statistical analyses yielded clear indications that the activity patterns differed greatly across the eight bicyclic odorants. Principal components analysis (Fig. 5) revealed significant differences (P < 0.0001) across odorants in the first three extracted factors (factor 1, 33% of variance, F = 8.1; factor 2, 10% of variance, F = 12.2; factor 3, 6% of variance, F = 14.8).
Fig. 5.
Scatter plot of the first two factors extracted by principal components analysis of the individual activity patterns involved in the bicyclic odorants experiment (Fig. 3A). Individual z-score matrices were subjected to pair-wise Pearson correlation analysis, and the matrix of correlation coefficients served as input data for principal components analysis. Each point represents an individual animal’s pattern of 2-DG uptake. Subsequent statistical analyses revealed that both factors contained highly significant information regarding odorant identity.
As illustrated in Figure 3B, the reliability of the evoked patterns across different animals within the bicyclic odorant experiment also extended across exposures conducted in different experiments. Uptake was detected in the two ventral regions in two additional, independent experiments involving eucalyptol and bornyl acetate (Fig. 3B). The relative intensity of uptake in the ventral versus more dorsal response regions, however, differed somewhat from experiment to experiment. For example, on two different occasions when bornyl acetate was volatilized by bubbling nitrogen through neat liquid (Fig. 3B), we detected greater uptake in the ventral modules than when the odorant was first mixed with mineral oil prior to volatilization (Fig. 3A). The slight differences in patterns across these studies may be related to differences in odorant concentration, as it is likely that the evaporation of bornyl acetate from a 1/20 dilution in mineral oil results in a different vapor phase concentration than does evaporation from neat liquid.
The stimulation of paired overlapping ventral modules by a set of four bicyclic odorants suggested that these rigid, compact, oxygen-containing molecules might share some structural motif necessary for stimulating a class of odorant receptors expressed by sensory neurons projecting to this region. To explore this possibility further, we exposed other rats to a number of other bicyclic odorants. As shown in Figure 3C, two such odorants, thujone and oxyoctaline formate were found to stimulate the same regions (open arrowheads). These two compounds are bicyclic, but do they not possess bridging carbons such as those in the first series (Fig. 3A), suggesting that bridges are not necessary for activation of these receptors. The anterior, ventral response to oxyoctaline formate was less pronounced than the posterior, ventral response, and the posterior, ventral response appeared to be comprised of multiple glomeruli as detailed in Figure 4B.
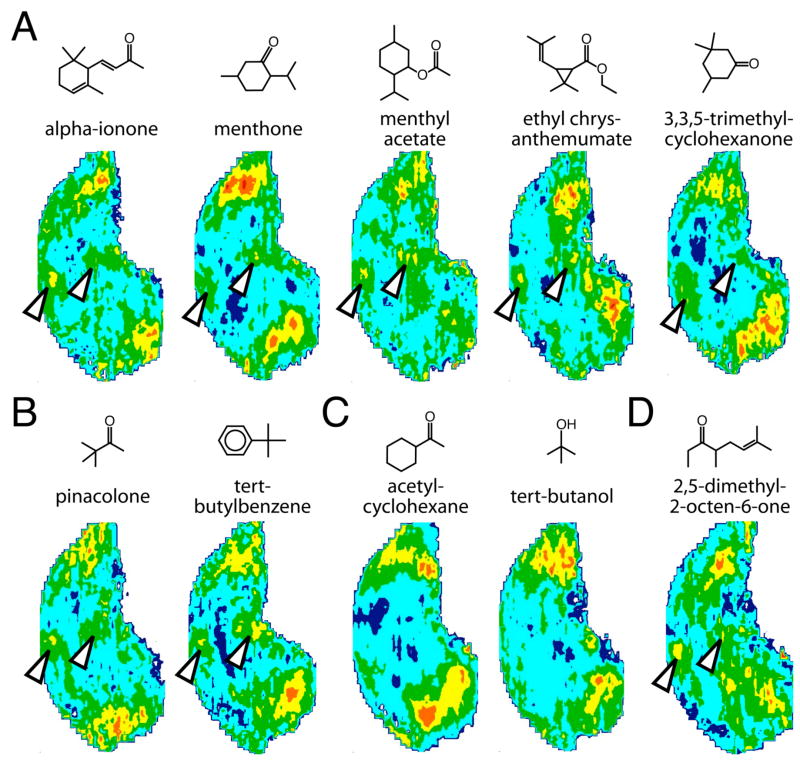
As another approach to understanding the structural features required for stimulation of these paired ventral glomeruli, we surveyed our archive of patterns for other odorants evoking uptake in the anterior, ventral region. Several of these odorants at least superficially resembled the effective bicyclic odorants in chemical structure, in that they were cyclic with highly substituted rings (Fig. 6A, open arrowheads). These odorants, alpha-ionone, menthone, menthyl acetate, and ethyl chrysanthemumate, also were found to be effective in stimulating glomeruli in the posterior, ventral region involved in the paired response to the bicyclic compounds (Fig. 6A, open arrowheads).
Fig. 6.
Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in animals exposed to odorants found to stimulate ventral modules (A, B, D) and/or sharing the camphoraceous odor quality that is characteristic of the effective bicyclic odorants (B, C). Orientation and color scale are the same as for Figure 1. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition. Uptake within the paired ventral regions is indicated using open arrowheads. A: Monocyclic odorants in our database that were found to stimulate uptake in the anterior, ventral region at levels comparable to bicyclic odorants. B: Odorants that both stimulate the anterior ventral module and share the camphoraceous odor quality elicited by effective bicyclic odorants, despite having very different chemical structures. C: Odorants that possess a camphoraceous odor quality, but that do not appear to stimulate the ventral response areas. D: An odorant that was identified as stimulating the anterior, ventral region that is neither cyclic nor described as camphoraceous in odor.
Many of the odorants in the bicyclic series that stimulated the paired ventral regions have odors considered to be camphoraceous in quality (Dravnieks, 1985). The camphoraceous odor descriptor in turn has been associated with particular compact shapes involving very different functional groups and hydrocarbon structures (Rossiter, 1996). Interestingly, there were two odorants in our archive, pinacolone and tert-butylbenzene (Fig. 6B), that were identified as stimulating the anterior module and that are described as being camphoraceous in odor, but that differ from the cyclic theme established by the bicyclic odorants and the odorants in Figure 6A. The response to cyclooctane, which was observed in our original cyclic hydrocarbon series (Fig. 1), also has been described as having a camphoraceous odor (Rossiter, 1996). Review of the uptake patterns in Figure 1 reveals a slight stimulation of the anterior, ventral module by the higher concentration of this odorant (Fig. 1). However, there were two other odorants in our archive (acetylcyclohexane and tert-butanol) that also are described as camphoraceous in odor, but that did not stimulate the ventral modules (Fig. 6C). Finally, there was a single odorant (2,5-dimethyl-2-octen-6-one) that was identified as stimulating the anterior ventral region that was neither camphoraceous in odor nor cyclic in structure (Fig. 6D).
Lactones
In addition to cyclic structures involving only carbon atoms in the ring, there are a number of simple heterocyclic chemicals with important odor qualities that possess rings containing other elements, especially oxygen, nitrogen, and sulfur. We hypothesized that the characteristic odors and chemistries possessed by certain subsets of these odorants might be associated with the activation of unique glomerular regions or unique patterns involving unusual combinations of glomerular modules. We therefore collected sets of these compounds overlapping in aspects of chemistry and investigated their evoked activity patterns.
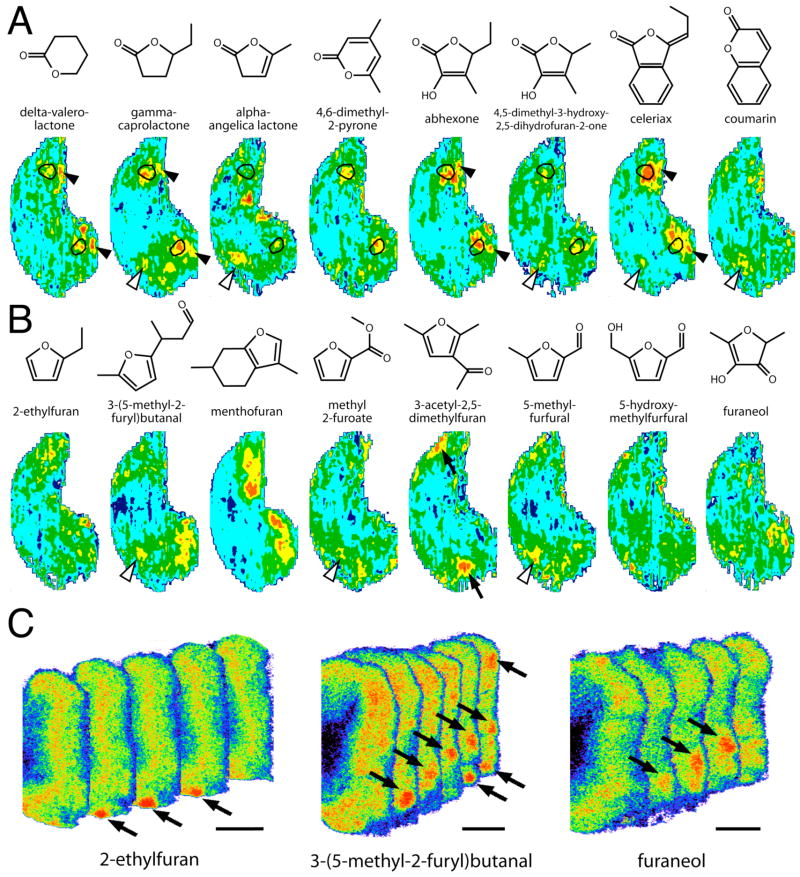
Figure 7A shows patterns of 2-DG uptake evoked by a set of lactones, which are heterocyclic structures in which an ester bond is part of the ring. Most of these compounds evoked robust uptake, and all but coumarin overlapped in the activation of a pair of small areas in the mid-lateral and mid-medial regions of the bulb. These areas corresponded quite well to odorant response locales (Fig. 7A, outlined regions) that are an important part of the representation of a broad range of open-chained esters (Johnson et al., 2002; 2004). A subset of the lactones including γ-caprolactone, α-angelica lactone, 4,5-dimethyl-3-hydroxy-2,5-dihydrofuran-2-one, celeriax, and coumarin also stimulated a small amount of 2-DG uptake in a part of the anterior, dorsomedial olfactory bulb (Fig. 7A, open arrowheads). Many aliphatic methyl and ethyl esters stimulate this region to some degree (Johnson and Leon, 2000a; Johnson et al., 2002; 2004; 2005a), perhaps due to the tendency of these compounds to become hydrolyzed to the corresponding carboxylic acids that very robustly activate the same dorsomedial region (Johnson et al., 1999; 2002; Johnson and Leon, 2000a,b). It therefore seems possible that the responses to lactones in this area are due to hydrolysis of the ester bonds to produce carboxylic acids.
Fig. 7.
A, B: Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in animals exposed to heterocyclic odorants in which oxygen participates in the ring. Orientation and color scale are the same as for Figure 1. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition. A: Activity patterns evoked by lactones, which are cyclic compounds having an ester bond within the ring. Most lactones activated paired regions (outlines) that we had previously identified as being responsive to open-chained esters. Some lactones also stimulated a dorsomedial area (open arrowheads) stimulated by methyl and ethyl esters that are prone to hydrolysis into corresponding carboxylic acids. Another subset of lactones stimulated very posterior, midlateral and midmedial areas (solid arrowheads) that also are stimulated by other small, hydrophilic odorants. B: Activity patterns evoked by furyl odorants. Most of these patterns were weak and apparently unrelated to each other. Certain responses appeared to be associated with functional groups present in substituents, including aldehydes (open arrowheads) and ketones (solid arrows). C: Pseudocolor-enhanced images showing stimulation of what appear to be isolated glomeruli (arrows) in the posterior, medial regions of response to each of three furyl odorants. There are 100 μm between the consecutive, 20-μm sections. Scale bars = 1 mm.
Another subset of the lactones stimulated very posterior glomeruli located in the mid-lateral and mid-medial sectors of the bulb (Fig. 7A, solid arrowheads). Stimulation near these areas has been seen before for odorants such as acetone, ethyl acetate, formic acid, 1-propanol, and acetic acid (Johnson et al., 2002), which share with the lactones a relatively low hydrophobicity.
In addition to these responses that were shared among various lactones, the odorant α-angelica lactone (Fig. 7A) stood alone in its stimulation of a pair of posterior, ventral glomerular clusters that resembled in location those activated by β-pinene (Fig. 3A; Johnson et al., 2002) and santalol (Johnson et al., 2002). The odorant γ-caprolactone, which is structurally quite similar to α-angelica lactone, showed no evidence of activating those areas, suggesting the possibility of a relatively narrow tuning of the underlying odorant receptors.
Furans
As a second series of heterocyclic odorants that have oxygen in the ring, we used various furans (Fig. 7B). The activity patterns evoked by these odorants varied greatly, so that there seemed to be no area of response attributable to the molecular feature they shared (Fig. 7B). Whereas some odorants such as 3-(5-methyl-2-furyl)butanal, menthofuran, and 3-acetyl-2,5-dimethylfuran stimulated relatively high levels of 2-DG uptake, others such as 5-hydroxymethylfurfural were associated with uptake patterns not readily distinguishable from background (Fig. 7B).
Most of the clearly organized areas of uptake in the activity patterns evoked by the furans could be attributed to already well-characterized relationships between functional groups and glomerular modules. For example, two of the odorants that possessed aldehydic substituents, 3-(5-methyl-2-furyl)butanal and 5-methylfurfural, stimulated a glomerular region (Fig. 7B, open arrowheads) that also is activated by open-chained aldehydes (Johnson and Leon, 2000a; Johnson et al., 2004), probably by virtue of the oxidation of the aldehyde functional group to produce a carboxylic acid (Johnson et al., 2004). Similarly, the methyl ester, methyl 2-furoate, showed slight stimulation of an acid-preferring region (Fig. 7B, open arrowhead), which also is characteristic of open-chained methyl and ethyl esters (Johnson et al., 1998; 2004; 2005a; Johnson and Leon, 2000a). Finally, the odorant menthofuran evoked a pattern of activity that was similar to other cyclic, terpene odorants of related structure that are found together with menthofuran in peppermint (e.g., correlation with the pattern evoked by α-phellandrene yielded r = 0.74) (Johnson et al., 2002).
Some of the furans, including 2-ethylfuran and 3-(5-methyl-2-furyl)butanal, as well as the related odorant furaneol, stimulated scattered foci of uptake in the posterior parts of the bulb that did not line up perfectly from one bulb to another. Variance in the exact position of homologous glomeruli can result both from natural variation in the projection of homologous sensory neurons (Royal and Key, 1999; Strotmann et al., 2000; Schaefer et al., 2001) and from variance in tissue handling. These differences in the position of isolated, activated glomeruli can result in a deceptively blurred or patchy, low level of apparent activation in averaged charts such as those shown in Figure 7B. Examples of such scattered foci of uptake are given in Figure 7C, where we show series of pseudocolor-enhanced images of autoradiograph sections taken from the posterior, medial parts of three bulbs from animals exposed to 2-ethylfuran, 3-(5-methyl-2-furyl)butanal, or furaneol. Individual, high-uptake patches that are about the size expected for single glomeruli or clusters of a very few glomeruli (Fig. 7C, arrows) were either entirely isolated or separated from one another by unlabelled spaces. This pattern of response is distinct from the activation of larger clusters of adjacent glomeruli that typifies responses to many open-chained odorants (Johnson et al., 1998; 1999; 2004). Perhaps the rigidity of the cyclic structure restricts the odorants to interact with fewer receptors compared to the far more flexible open-chained aliphatic odorants.
Pyridines and pyrazines
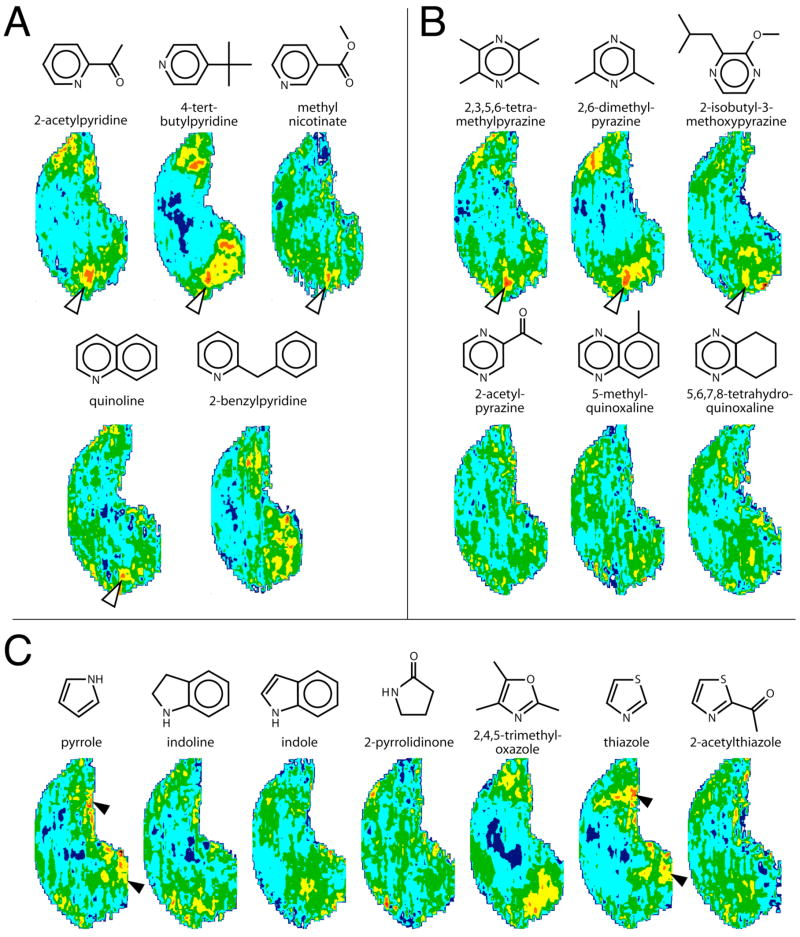
Another class of heterocyclic odorants includes pyridines (Fig. 8A) and pyrazines (Fig. 8B), which are aromatic compounds with nitrogen atoms in the ring. The odors of these compounds are characterized by descriptors such as roasted nuts, coffee, cocoa, baked potato, and popcorn. Patterns of uptake evoked by five pyridines and six pyrazines are shown in Figure 8A and 8B, respectively. Many of these compounds activated a small cluster of glomeruli in the dorsomedial portion of the bulb (open arrowheads) that overlaps with a region stimulated by both ketones and aromatic odorants possessing only carbons in the ring (Johnson and Leon, 2000a; Johnson et al., 2004, 2005a,b; Farahbod et al., 2006).
Fig. 8.
Anatomically standardized contour charts showing relative 2-DG uptake across the glomerular layer in animals exposed to heterocyclic odorants in which nitrogen participates in the ring. Orientation and color scale are the same as for Figure 1. Charts represent z-score standardized data matrices averaged across different rats exposed to the same odorant condition. A: Patterns evoked by pyridine odorants, many of which activated glomeruli in a region also stimulated by benzene-containing aromatic odorants (open arrowheads). B: Patterns evoked by pyrazine odorants, which also activated glomeruli within the general aromatic-responsive region (open arrowheads). C: Patterns evoked by heterocyclic odorants containing nitrogen in five-membered rings. Two of these odorants activated posterior, midlateral and midmedial glomeruli (solid arrowheads) in a similar location to those activated by some lactones (Fig. 7A) and other small, hydrophilic odorants.
The pyrazine-containing odorants, 2-acetylpyrazine, 5-methylquinoxaline, and 5,6,7,8-tetrahydroquinoxaline, yielded activity patterns that we could not distinguish from background activity (Fig. 8B). The absence of evoked glomerular uptake was surprising to us given that we perceived the odors of 2-acetylpyrazine and 5-methylquinoxaline to be very intense. It may be that these large rigid aromatic structures do not present molecular features that can bind to rat olfactory receptors.
Other nitrogen-containing heterocyclics
We tested activity patterns evoked by a number of other heterocyclic odorants containing nitrogen in the ring, some of which also incorporated oxygen or sulfur in the ring structure (Fig. 8C). The evoked activity patterns differed across the different specific structures. Similar to the case with the pyrazines, given the intense and unpleasant odors associated with some of these compounds, we were surprised at the low levels of 2-DG uptake many of them evoked. The small and hydrophilic odorants pyrrole and thiazole stimulated very posterior glomeruli in both the lateral and the medial aspect of the bulb (Fig. 8C, solid arrowheads), similar to what was observed for the small and hydrophilic lactones (Fig. 7A). Oddly, the odorant indole appeared to stimulate regions of the bulb previously shown to be activated by alcohols, aldehydes, and phenols (Johnson et al., 2002; 2004; Farahbod et al., 2006), whereas 2,4,5-trimethyloxazole stimulated glomeruli in dorsal regions that respond to ketones and benzyl compounds (Johnson et al., 2002; 2005b; Farahbod et al., 2006), as well as to pyridines and pyrazines (Fig. 8A,B).
DISCUSSION
Patterns of activity measured across the entire glomerular layer of the olfactory bulb have been shown to be predictive of perceived odor in a number of ways. Relative similarities in overall patterns of 2-DG uptake correlate with the extent that rats spontaneously discriminate between odorant enantiomers (Linster et al., 2001), as well as among straight-chained odorants differing in carbon number (Linster et al., 2002; Johnson et al., 2004; Ho et al., 2006a) and hydrocarbon odorants differing in either branch structure or the number and position of double or triple bonds (Ho et al., 2006b). Furthermore, maps of activity across the bulb in response to odorants differing systematically in chemical structure have helped to identify the molecular features that are recognized separately by odorant receptors and that then may be recombined to produce the perceived odor of the compound (Johnson et al., 1998; 1999; 2002; 2004; 2005a,b; Johnson and Leon, 2000a,b; Uchida et al., 2000; Takahashi et al., 2004). These maps also have revealed organizational principles within the olfactory bulb, such as systematic arrangements of glomeruli responding to odorants differing in molecular length, which may be used to enhance contrast between discriminable odorants that tend to co-activate many of the same receptors (Johnson et al., 1999, 2004; Johnson and Leon, 2000b).
The olfactory system is capable of detecting and distinguishing a very great variety of chemical structures as odorants, and these odorants in turn elicit a great variety of perceived odors (Arctander, 1994). To understand the neurobiology underlying these multidimensional relationships between stimulus and response, it is imperative to study a large sample of odorant stimuli, because different principles of organization might be used to identify and discriminate different classes of odorant chemicals. In the present work, we have extended our ongoing investigation to include a variety of cyclic structures that have not typically been utilized in neurobiological studies of olfaction. Because some of these odorants have odors of particular significance to humans, we had hypothesized that these unique odorants might be correlated with unique responses. While a novel response was indeed identified for a series of bicyclic odorants, we were surprised that cyclic structures in general were not recognized as separate molecular features by the rat olfactory system.
Our results with simple cyclic hydrocarbons demonstrated that there is no obvious glomerular module representing cyclic structures in the bulb. Simple monocyclic hydrocarbons evoked patterns that closely resembled those evoked by aliphatic, noncyclic hydrocarbons. This finding suggests that an overlapping set of receptors probably recognizes aliphatic (non-cyclic) and alicyclic structures. The absence of specific recognition of alicyclic structures stands in sharp contrast to our results with aromatic compounds, which typically stimulated a common dorsal region of the bulb, as though the benzene ring served as an extractable molecular feature recognized by a set of receptors in sensory neurons projecting together to a glomerular cluster in the dorsal bulb (Johnson et al., 2005b; Farahbod et al., 2006).
Representations of bicyclic, camphoraceous odorants
On the other hand, certain bicyclic odorants with related structures and odors did evoke activity in a pair of ventral regions that do not respond to most aliphatic odorants. The fact that a number of chemicals with similar structures activate overlapping glomerular regions is further evidence for a chemotopic organization, wherein responses are organized spatially in relation to odorant chemical structure. At the same time, we found evidence for a number of other compounds that did not have the bicyclic structure and yet also stimulated the same ventral regions. These results recall our findings with ketones and aromatic compounds, which overlap in their stimulation of dorsal bulbar regions and yet have no overt chemical similarities (Johnson et al., 2005b). It seems possible, as we proposed for the dorsal bulbar region, that the ventral regions contain glomeruli responding to the bicyclic odorants interspersed with glomeruli that are stimulated by odorants of different chemistry. Alternatively, there may be some less obvious similarity in the molecular properties and/or shapes of these disparate compounds that might lead to the activation of an overlapping set of receptors.
Many of the compounds that were effective in stimulating the novel ventral regions, despite their differing chemical structures, also overlapped in their stimulation of more dorsal regions. The presence of overlapping activity in multiple parts of the bulb indicates an overall pattern similarity that in past experiments has predicted a high degree of perceptual similarity (Linster et al., 2001; Cleland et al., 2002; Ho et al., 2006a,b). Indeed, some of the chemically distinct odorants that shared activation of the ventral modules also share an evoked camphoraceous odor quality as judged by humans. Some of the compounds such as menthone and menthyl acetate that are effective in stimulating these areas are described as minty in odor rather than as camphoraceous, which might relate to the similarity between mint and camphor in multidimensional analyses of human odor descriptors (Madany Mamlouk et al., 2003). However, some effective compounds were associated with odors apparently different than camphor, and some odorants with camphoraceous odors failed to activate the ventral regions. In evaluating these mismatches, one should consider the possibility that the human odor descriptor “camphoraceous” may not correspond exactly to a similar category of odor generalization by rats.
We recently found that a ventral region of the rat olfactory bulb located about half-way between the rostral pole and the accessory olfactory bulb responds to very long hydrocarbon chains such as possessed by straight-chained alkanes and ketones of 14 or more carbons (Ho et al., 2006a). The two ventral regions responding to the camphoraceous bicyclic compounds are located just anterior and just posterior to the region responding to the long hydrocarbon chains. These ventral regions are targets for an unusual class of olfactory sensory neuron defined by a unique set of related odorant receptors (Strotmann et al., 1999). Homologous sensory neurons of this class are distributed in clusters at the tips of turbinates rather than being distributed in zones along the anterior-posterior axis of the olfactory epithelium (Strotmann et al., 1992; 1999), and they project to the ventral aspect of the bulb rather than obeying the dorsal-ventral topography that is typical of other sensory neurons (Strotmann et al., 2000).
Our series of bicyclic odorants overlapped with odorants chosen by Scott and colleagues (2000) in their mapping of electrophysiological responses across the olfactory epithelium. Unlike many of the open-chained and aromatic odorants in their study, which gave linear gradients of responsiveness across the dorsal-ventral extent of the epithelium, they found that responses to a group of the bicyclic odorants were remarkably nonlinear (Scott et al., 2000). Consistent with (1) the presence of both ventral and dorsal bulbar 2-DG uptake in response to camphor and eucalyptol and (2) the absence of ventral 2-DG uptake for norbornane, norcamphor, and 7-oxabicycloheptane in our maps, they found that camphor and eucalyptol gave nonlinear epithelial responses in comparison to the more linear gradients of response to norbornane, norcamphor, and 7-oxabicycloheptane (Scott et al., 2000).
Heterocyclic odorants
Responses to heterocyclic odorants in our studies could be classified into three categories. Some patterns were explainable in terms of the detection of previously characterized molecular features such as functional groups, some patterns were highly focal, involving few glomeruli, and some patterns were weak and variable.
Patterns explainable from our previous characterizations of responses to odorant molecular features include lactones, pyrazines, pyridines, and a variety of heterocyclic compounds substituted with aliphatic moieties containing well-characterized functional groups. Lactones, which have an ester bond as part of their ring structures, stimulated 2-DG uptake in paired areas of the bulb that correspond in part to the glomerular response to other odorants containing ester bonds within open-chained structures (Johnson et al., 1998; 2002; 2004; 2005a,b). Some of the lactones also stimulated the acid-preferring glomerular regions that are activated by methyl and ethyl esters, perhaps because of the hydrolysis of these esters into corresponding carboxylic acids (Johnson et al., 2004; 2005a). Particular lactones activated posterior regions of the bulb previously found to respond to small, hydrophilic odorants such as propanol, ethyl acetate and acetone. Given that the lactones also have limited molecular length and low hydrophobicity, these properties might be important determinants of the specificity of the posterior glomeruli.
Pyrazines and pyridines containing various substituents stimulated a portion of the bulb that also responds to aromatic compounds possessing benzene rings (Johnson et al., 2005b; Farahbod et al., 2006). The area stimulated by these nitrogen-containing odorants appeared to be more restricted than the large area activated by many of the benzyl odorants (Farahbod et al., 2006), suggesting the possibility that fewer receptors might recognize the heterocyclic compounds. Other pyrazine odorants did not activate a clear glomerular response in the rat olfactory bulb, despite the fact that we perceived the odors to be very intense at the concentrations that we used.
Patterns that involved very punctate stimulation of isolated glomeruli were seen for a few oxygen-containing heterocyclic odorants with furyl rings. Such highly focal responses would be predicted if an odorant were an efficacious stimulus for only a few odorant receptors involved in projections to glomeruli that were not adjacent to each other. This pattern of response differs from the stimulation of large clusters of neighboring glomeruli that have typified responses to many open-chained, unbranched, flexible aliphatic odorants that we have studied previously (Johnson et al., 1998; 1999). We have considered the co-activation of sets of neighboring glomeruli to be evidence for the clustering of projections from receptors of related molecular specificity (Johnson et al., 1998; 1999; 2004). The flexibility of an open-chained odorant ligand would be expected to be associated with a number of subtly different molecular shapes that might stimulate a number of related, neighboring odorant receptors. However, the rigid furyl odorants would not provide such a spectrum of conformations and thereby might not activate as many receptors. Rigidity cannot be the only factor determining the punctate nature of the response to furyl odorants, because other very rigid odorants such as benzene-containing compounds stimulate large clusters of glomeruli (Johnson et al., 2005b; Farahbod et al., 2006). Probably, the specificity of response is an interaction between molecular rigidity and the presence of a limited repertoire of receptors for the ligand.
Many of the lactone and furyl odorants that we investigated in this study elicited such powerful, sweet, caramellic odors as to be unpleasantly intense to the investigators. Similarly intense roasted nut or tobacco-type odors were evoked by pyrazine and pyridine odorants. Also, many of the five-membered nitrogenous heterocyclic odorants had unpleasant, strong odors. We were surprised that these powerful odorants in most cases did not evoke clear patterns of 2-DG uptake in the glomerular layer of the rat olfactory bulb. This mismatch involving weak glomerular response patterns and strong odors as perceived by the researchers might be explained if the rat olfactory system is specialized to detect different odorants than some of those that are important to humans. Furans and pyrazines, for example, arise via intermolecular reactions during cooking of vegetables and meat. Whereas the detection of odors arising from cooking would serve as a selective advantage to humans in identifying a relevant food source, such an odorant may not have as much significance to a species such as the rat that typically consumes food without such preparation. Indeed, evidence that humans have undergone a positive selection for a subset of odorant receptors has recently emerged from genetic analyses (Gilad et al., 2005).
Previously, we made a number of observations that also were consistent with species-specific olfactory sensibilities. In our survey of carboxylic acids of different hydrocarbon structures, we found that 2-methylbutyric acid had an unusually strong and distinct posterior representation in the rat olfactory bulb compared to other five- and six-carbon acids (Johnson and Leon, 2000b). This odorant did not smell particularly different to us than other five-carbon acids, all of which elicited distinctive patterns of glomerular activity in rats. However, because 2-methylbutyric acid is a major odorant produced in rat ceca (Allison, 1978), it seemed likely that it has a special biological significance to rats that corresponds to its intense glomerular response (Johnson and Leon, 2000b).
In a recent study on bond saturation and branching in hydrocarbon odorants, we found that our own judgments of differences in perceived odor did not predict differences in either bulbar uptake patterns or behavioral discriminability by the rats, suggesting the possibility of a species difference in perception and neural coding of these odorants (Ho et al., 2006b). Finally, in our wider survey of patterns evoked by odorants of distinct structure and perceived odor, we have encountered numerous other examples of subjectively intense odor stimuli including flavoring ingredients (e.g., vanillin and eugenol) and perfume ingredients (e.g., the musks, ω-pentadecalactone and ketone moschus) that do not evoke appreciable 2-DG uptake in the rat bulb. Species differences in olfactory responses therefore may be both widespread and dramatic. Sometimes humans appear to be more sensitive to specific odors than rats and sometimes rats appear to be more sensitive to specific odors that humans, suggesting that the concepts of macrosmatic and microsmatic organisms should be used with caution (Laska et al., 2000).
CONCLUSIONS
In past work, we investigated the spatial distributions of activity evoked by numerous aliphatic and aromatic odorants differing systematically in chemical structure. Certain structural themes such as chemical functional groups were found to be associated with activity in particular glomerular regions, whereas others, such as double or triple bonds, only affected more subtle details of the overall activity pattern (Johnson and Leon, 2000a; Johnson et al., 2002; 2004; Farahbod et al., 2006; Ho et al., 2006b). The present work has shown that certain bicyclic structures are represented as unique molecular features in a manner similar to functional groups, in that multiple individual odorants possessing the same molecular features activated common regions of the bulb that had not been associated previously with any other structural theme. However, other cyclic elements (e.g., simple monocyclic hydrocarbons and heterocyclic structures) did not appear to be detected as unique features. We also found additional evidence that the type of molecular information that is detected by odorant receptors and that is transposed into spatial patterns of activity in the olfactory bulb may depend on the species being studied. Finally, the data reinforce the notion that the olfactory system is not a general chemical detection system, but has instead evolved to be specifically sensitive to some odorants and not others.
Acknowledgments
We thank Edna Hingco, Joanne Yihan, Tanya Riedel, Sepideh Saber, Mickel Gerges, and Talla Motakef for their technical assistance in performing odor exposures, sectioning tissue, and image analysis. We also thank Spart Arguello for writing and maintaining our analysis software and our Internet archive (http://leonserver.bio.uci.edu).
Supported by United States Public Health Service Grant DC03545
LITERATURE CITED
- Arctander S. Perfume and flavor chemicals (aroma chemicals) Carol Stream, Illinois: Allured Publishing Corporation; 1994. [Google Scholar]
- Allison MJ. Production of branched-chain volatile fatty acids by certain anaerobic bacteria. Appl Environ Microbiol. 1978;35:872–877. doi: 10.1128/aem.35.5.872-877.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Dravnieks A. ASTM Data Series DS 61. Philadelphia: 1985. Atlas of odor character profiles. [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006 doi: 10.1002/cne.20982. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Man O, Glusman G. A comparison of the human and chimpanzee olfactory receptor gene repertoires. Genome Res. 2005;15:224–30. doi: 10.1101/gr.2846405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Leon M. Long hydrocarbon chains serve as unique molecular features recognized by ventral glomeruli of the rat olfactory bulb. J Comp Neurol. 2006a doi: 10.1002/cne.20973. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 2006b doi: 10.1002/cne.21139. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M. Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol. 2005a;483:192–204. doi: 10.1002/cne.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005b;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska M, Seibt A, Weber A. ‘Microsmatic’ primates revisited: olfactory sensitivity in the squirrel monkey. Chem Senses. 2000;25:47–53. doi: 10.1093/chemse/25.1.47. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madany Mamlouk A, Chee-Ruiter C, Hofmann UG, Bower JM. Quantifying olfactory perception: mapping olfactory perception space by using multidimensional scaling and self-organizing maps. Neurocomputing. 2003;52–54:591–597. [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rossiter KJ. Structure-odor relationships. Chem Rev. 1996;96:3201–3240. doi: 10.1021/cr950068a. [DOI] [PubMed] [Google Scholar]
- Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J Neurosci. 1999;19:9856–64. doi: 10.1523/JNEUROSCI.19-22-09856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001;436:351–362. [PubMed] [Google Scholar]
- Scott JW, Brierley T, Schmidt FH. Chemical determinants of the rat electro-olfactogram. J Neurosci. 2000;20:4721–4731. doi: 10.1523/JNEUROSCI.20-12-04721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Panhuber H, Bell GA, Laing DG. Odor-induced metabolic activity in the olfactory bulb of rats trained to detect propionic acid vapor. Brain Res. 1989;500:161–168. doi: 10.1016/0006-8993(89)90310-7. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Wanner I, Krieger J, Raming K, Breer H. Expression of odorant receptors in spatially restricted subsets of chemosensory neurones. Neuroreport. 1992;3:1053–6. doi: 10.1097/00001756-199212000-00005. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Hoppe R, Conzelmann S, Feinstein P, Mombaerts P, Breer H. Small subfamily of olfactory receptor genes: structural features, expression pattern and genomic organization. Gene. 1999;236:281–91. doi: 10.1016/s0378-1119(99)00275-9. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–38. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Treloar HB, Feinstein P, Mombaerts P, Greer CA. Specificity of glomerular targeting by olfactory sensory axons. J Neurosci. 2002;22:2469–77. doi: 10.1523/JNEUROSCI.22-07-02469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nuñez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]