Abstract
In an effort to understand mammalian olfactory processing, we have been describing the responses to systematically different odorants in the glomerular layer of the main olfactory bulb of rats. To understand the processing of pure hydrocarbon structures in this system, we used the [14C]2-deoxyglucose method to determine glomerular responses to a homologous series of alkanes (from six to sixteen carbons) that are straight-chained hydrocarbons without functional groups. We found two rostral regions of activity evoked by these odorants, one lateral and one medial, that were observed to shift ventrally with increasing alkane carbon chain length. Furthermore, we successfully predicted that the longest alkanes with carbon chain length greater than our previous odorant selections would stimulate extremely ventral glomerular regions where no activation had been observed with the hundreds of odorants that we had previously studied. Overlaps in response profiles were observed in the patterns evoked by alkanes and by other aliphatic odorants of corresponding carbon chain length despite possessing different oxygen-containing functional groups, which demonstrated that hydrocarbon chains could serve as molecular features in the combinatorial coding of odorant information. We found a close and predictable relationship among the molecular properties of odorants, their induced neural activity, and their perceptual similarities.
Keywords: alkanes, chemotopic organization, combinatorial coding, odor similarity, glomeruli, deoxyglucose, habituation
Studies by a number of laboratories using odorants that differ systematically in molecular structures continue to support a combinatorial model of olfactory processing initially proposed by Polak (1973), in which molecular features of an odorant are recognized and encoded by the system (Kauer and Cinelli, 1993; Friedrich and Korsching, 1997, 1998; Joerges et al., 1997; Johnson et al., 1998, 1999, 2002, 2004, 2005a, b; Malnic et al., 1999; Rubin and Katz, 1999; Sachse et al., 1999; Johnson and Leon, 2000a, b; Uchida et al., 2000; Belluscio and Katz, 2001; Fuss and Korsching, 2001; Meister and Bonhoeffer, 2001; Igarashi and Mori, 2005). According to this model, odorants that share a molecular feature should be recognized by the same type of olfactory receptors to produce overlapping neural activity. To test this prediction, and to understand how the olfactory system responds to various molecular features, our laboratory has been studying responses to hundreds of odorants in the glomerular layer of the rat main olfactory bulb (Johnson et al., 1998, 1999, 2002, 2004, 2005a, b; Johnson and Leon, 2000a, b; Linster et al., 2001a). In an effort to understand the processing of pure hydrocarbon structures in this system, we determined in this study the response to a series of alkanes, which are straight-chained hydrocarbons without functional groups.
The responses to the odorants studied thus far collectively involve most of the glomerular layer, with the marked exception of an area in the midventral region. From studies of numerous homologous series of odorants up to eleven carbons in the chain, it was clear that larger odorants systematically evoked responses that were progressively ventral (Johnson et al., 1999, 2004; Johnson and Leon, 2000b). We therefore hypothesize that the previously unresponsive ventral area might respond to even larger odorants than we had yet studied (Johnson et al., 2002). Since straight-chained alkanes remain volatile even at great molecular length, we used the [14C]2-deoxyglucose (2-DG) method developed by our laboratory to examine glomerular responses to a homologous series varying from six to sixteen carbons, and predicted that responses to the larger odorants in this series would be observed in the mid-ventral area of the bulb.
Even though glomerular responses have been observed to be organized topographically and have been found to be correlated with a number of molecular features of odorous stimuli (Imamura et al., 1992; Katoh et al., 1993; Friedrich and Korsching, 1997, 1998; Joerges et al., 1997; Johnson et al., 1998, 1999, 2002, 2004, 2005a, b; Johnson and Leon, 2000a, b; Fuss and Korsching, 2001; Meister and Bonhoeffer, 2001; Takahashi et al., 2004; Igarashi and Mori, 2005), it remains unclear whether such chemotopic information is maintained through downstream perceptual processing, so that odorant-evoked responses in the olfactory bulb would predict perceptual phenomena such as odor recognition and discrimination. Indeed, few studies have examined directly the relationship between the similarity of overall glomerular responses and perceived odor similarity (Linster et al., 2001a; Cleland et al., 2002). Nevertheless, these studies have shown a high correlation between the evoked glomerular patterns and odor perception. Aspects of odorant chemistry also have been found to correlate with odor discriminability in a number of psychophysical studies (Laska and Freyer, 1997; Laska and Teubner, 1999; Laska et al., 1999, 2000; Linster and Hasselmo, 1999; Laska and Galizia, 2001; Laska and Hübener, 2001; Linster et al., 2001a, b; Cleland et al., 2002). Together, these results suggest a close and predictable relationship among odorous stimuli, glomerular responses and perceived odors. In all of these cases, however, the odorants used involved molecules with oxygen-containing functional groups, which are well known to have profound impact on perceived odor. In fact, aliphatic odorants containing the same oxygen-containing functional group were found to be discriminable even with just a one-carbon difference in their chain length (Laska and Freyer, 1997; Laska and Teubner, 1999; Laska et al., 1999; Linster and Hasselmo, 1999; Laska and Galizia, 2001; Laska and Hübener, 2001; Cleland et al., 2002).
Odorants possessing oxygen-containing functional groups can interact with receptors by way of hydrogen bonds. In contrast, alkane odorants can only interact with receptors by way of Van der Waals forces, which are much weaker than hydrogen bonds. The weakness of these interactions would be expected to result in low-affinity binding. Given the likelihood of low-affinity receptor binding, it was not clear whether the pattern of glomerular activity evoked by these alkane odorants would reveal the same degree of specificity that has characterized the patterns evoked by the numerous oxygen-containing odorants that we have studied previously. It also was not clear whether these alkanes would be perceived to have significantly distinct odors. Indeed, these odorants often are given the same “gasoline-like” odor description by humans (see, for example, http://hazmap.nlm.nih.gov/). Therefore, we investigated the triad relationships among odorant chemistry, bulbar activation patterns, and perceived odors using a homologous series of pure alkanes.
MATERIALS AND METHODS
Odorants
Table 1 contains detailed information regarding all odorants included in this study, which consisted of four independently conducted experiments. The initial experiment used a homologous series of straight-chained alkanes varying from six to sixteen carbons. The experiments examining odorant concentration and odorant purity tested various concentrations of heptane and four samples of pentadecane from different manufacturers, respectively. 2-Dodecanone and 8-pentadecanone were examined in a separate experiment to be compared to the alkanes. For each alkane, 100 mL of neat odorant was placed in a 125-mL gas-washing bottle equipped with an extra-coarse porosity diffuser. The ketones were first dissolved in mineral oil at a 1/20 dilution, before 200 mL was placed in a 500-mL gas-washing bottle. During each exposure, highly pure research-grade nitrogen was passed through the odorant column at a rate of 250 mL/min (100 mL/min for ketones). The resulting vapor was mixed with ultra-zero grade air, after which the mixture was delivered to the exposure chamber at a rate of 2 L/min (1 L/min for ketones). The final vapor phase concentration listed in Table 1 for each odorant was calculated using the mode of vapor pressure data obtained from the following sources: PhysProp Database from Syracuse Research Corporation (http://www.syrres.com/esc/physdemo.htm), the Chemical and Physical Properties Database from the Pennsylvania Department of Environmental Protection (http://www.dep.state.pa.us/physicalproperties/CPP_Search.htm), Molecular Modeling Pro version 3.14 (ChemSW, Fairfield, CA), and ChemDraw Ultra version 6.0 (CambridgeSoft, Cambridge, MA).
Table 1.
Information and Exposure Conditions of Odorants.
| Odorant | CAS # | Molecular Formula | Vendor | Dilution in Air | Vapor Concentration (ppm) | Number of rats |
|---|---|---|---|---|---|---|
| Hexane | 110-54-3 | C6H14 | F | 1/8 | 24885 | 4 |
| Heptane | 142-82-5 | C7H16 | A | 1/8 | 7535 | 91 |
| Heptane | 142-82-5 | C7H16 | A | 1/24 | 2500 | 5 |
| Heptane | 142-82-5 | C7H16 | A | 1/75 | 800 | 5 |
| Heptane | 142-82-5 | C7H16 | A | 1/240 | 250 | 5 |
| Heptane | 142-82-5 | C7H16 | A | 1/750 | 80 | 5 |
| Octane | 111-65-9 | C8H18 | A | 1/8 | 2231 | 4 |
| Nonane | 111-84-2 | C9H20 | A | 1/8 | 705 | 4 |
| Decane | 124-18-5 | C10H22 | A | 1/8 | 229 | 4 |
| Undecane | 1120-21-4 | C11H24 | A | 1/8 | 66 | 4 |
| Dodecane | 112-40-3 | C12H26 | A | 1/8 | 29 | 4 |
| Tridecane | 629-50-5 | C13H28 | A | 1/8 | 6 | 4 |
| Tetradecane | 629-59-4 | C14H30 | A | 1/8 | 2 | 4 |
| Pentadecane | 629-62-9 | C15H32 | A | 1/8 | 0.4 | 92 |
| Pentadecane | 629-62-9 | C15H32 | AA | 1/8 | 0.4 | 5 |
| Pentadecane | 629-62-9 | C15H32 | S | 1/8 | 0.4 | 5 |
| Pentadecane | 629-62-9 | C15H32 | F | 1/8 | 0.4 | 5 |
| Hexadecane | 544-76-3 | C16H34 | A | 1/8 | 0.2 | 4 |
| 2-Dodecanone | 6175-49-1 | C12H24O | A | 1/10 | ND | 4 |
| 8-Pentadecanone | 818-23-5 | C15H30O | A | 1/10 | ND | 4 |
A: Acros; AA: Alfa Aesar; S: Sigma; F: Fluka; ND: Not determined because our calculation is not applicable to odorants diluted in mineral oil.
Four rats were used for the homologous series experiment, whereas five were used for the concentration experiment.
Four rats were used for the homologous series experiment, and five were used for the purity experiment.
Odorant exposures
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Odorant exposures were conducted as described in our previous studies (Johnson et al., 1998, 1999, 2002, 2004, 2005a, Johnson et al., b; Johnson and Leon, 2000 a, b). Eighteen- to twenty-one-day-old Wistar rats, culled to eight per litter, were used for all experiments. In order to reduce odors carried over from the soiled housing cage, each litter was transferred to a clean cage with the dam at least one hour before an exposure. Prior to the introduction of an animal, the exposure system first was equilibrated with the odorant of interest for fifteen minutes. Individual pups were injected subcutaneously at the back of the neck with a dose of [14C]2-DG determined by body weights (1.6 μL/g, 0.1 mCi/mL, 52 mCi/mmol, Sigma Chemical Company, St. Louis) before being placed in a clean, 1-L glass jar used as the exposure chamber. Each exposure lasted for forty-five minutes, with the odorant entering and exiting the glass jar through two vents in the jar lid, so that odorant concentration increased steadily at the beginning of the exposure. Animals’ brains then were immediately removed, frozen in isopentane at −45°C, and stored at −80 °C.
The number of animals exposed to each odorant condition is shown in Table 1. The first pup obtained from each litter was always exposed to the vehicle, which was air for the alkane experiments and mineral oil for the ketones. No two rats from the same litter were exposed to the identical odorant condition. The order of odorant presentation was pseudo-randomized across litters to avoid systematic relationships among odorants, except for in the heptane concentration experiment, where exposures were done in order of increasing odorant concentration within each day.
Measurement and analysis of 2-DG uptake
Histology, autoradiography, and activity mapping were performed as described in our previous studies (Johnson et al., 1998, 1999, 2002, 2004, 2005a, Johnson et al., b; Johnson and Leon, 2000 a, b). Briefly, for each coronal bulb section, an appropriate selection from a set of polar grids was chosen according to the number of bulb sections between anatomical landmarks and was applied to the section’s autoradiographic image overlaid with the image of the adjacent Nissl-stained section. The grids guided the collection of 2-DG uptake measurements from the glomerular layer throughout the entire olfactory bulb into standardized matrices. After the subtraction of measurements from vehicle, each individual data matrix was converted to z-scores. Matrices from the same odorant condition within a study were averaged to yield an average z-score pattern for that condition. However, for statistical purposes, individual z-score patterns, rather than average z-score patterns from a single experiment were subjected to ANOVA followed by false discovery rate analyses (Curran-Everett, 2000; Johnson et al., 2002, 2004, 2005a, b) to test for statistical differences among odorant-induced responses in thirty predetermined areas termed glomerular modules, including twenty-seven of them that were described in our previous studies (Johnson et al., 2002, 2004, 2005a, Johnson et al., b) and three new response modules that were defined based on glomerular responses to the longer alkanes in this study. The use of these predetermined modules in the present study does not imply their functional significance within the olfactory system. Rather, it serves as a tool both to ensure an unbiased simplification of the activity patterns for statistical analysis and to facilitate comparisons with previous studies that were analyzed with respect to the same modules.
The centroid of activity within an outlined glomerular region was determined separately for the lateral and the medial aspects of each odorant’s response pattern. To examine whether a significant directional shift of glomerular activation existed, dorsal-ventral centroid coordinates for all alkanes were included in an ANOVA test.
Overall pattern similarity among glomerular responses was analyzed by using Pearson correlation and principal components analysis. Each average response pattern for an odorant actually represented a z-score array containing over 2,300 values. Using Pearson correlation (Johnson et al., 2002; 2004, 2005 a, b), point-to-point comparisons could be made between any two patterns, and the level of overall pattern similarity produced by two odorants was indicated by the correlation coefficient (r). Correlation results from all possible paired comparisons within this homologous series of alkanes were further subjected to principal components analysis (StatView®, SAS Institute, Cary, NC) to reveal the clustering of glomerular responses to these odorants. The components were extracted using the scree test and scree plot, with initial orthogonal solution matrices undergoing at least one oblique transformation to optimize their structures. These procedures also were repeated with individual response patterns.
Olfactory discrimination
Perceived odor similarity was assessed using a habituation assay similar to that described in a prior study (Linster et al., 2001a). Adult male Wistar rats were handled and shaped in the behavior testing apparatus for seven consecutive days prior to experimentation. The testing apparatus included a clean test cage similar to the home cages (30 cm L × 20 cm W × 19 cm H), and a clear, customized cage lid with evenly distributed 1-cm wide holes and a slit for a cage separator, which could be lowered vertically to separate the length of the cage into two compartments.
Animals were food-deprived for two days, through the completion of individual behavioral testing, which was conducted under dim light during the dark phase of a reverse light-dark cycle. An animal was placed in a clean cage, and a plastic cap lined with clean filter paper was positioned over one of the holes in the cage lid. A ten-minute control period then began immediately, during which animals were allowed to investigate the cage. Upon the termination of the control period, the cage separator was lowered to confine the rat to one side of the cage. An odorized cap was then put on top of the other side of cage, and the first experimental trial started after the separator was lifted. Each experimental trial lasted for two minutes, followed by a ten-minute inter-trial period.
The same odorant was presented for the first three trials to familiarize and habituate animals to this odorant. Test odorants then were alternated with the familiar odorant for the remaining trials. Three experimental series were conducted with different odorant sets (Table 2). The presentation order of test odorants was varied across animals in such a way to avoid systematic relationships among odorants. To control for performance fatigue, the final trial used an odorant different from the experimental odorant set based on molecular properties and glomerular activity patterns. An additional experiment was performed to test the discriminability between two samples of pentadecane, Fluka and Acros. All trials were recorded on videotape for subsequent analysis.
Table 2.
Odorants for the Three Series of Behavioral Experiments.
| Odorants | Series 1 | Series 2 | Series 3 |
|---|---|---|---|
| Familiar | Octane (C8) | Dodecane (C12) | Pentadecane (C15) |
| Test | Hexane (C6) | Decane (C10) | Undecane (C11) |
| Heptane (C7) | Undecane (C11) | Dodecane (C12) | |
| Octane (C8) | Dodecane (C12) | Tridecane (C13) | |
| Nonane (C9) | Tridecane (C13) | Tetradecane (C14) | |
| Decane (C10) | Tetradecane (C14) | Pentadecane (C15) | |
| Undecane (C11) | Pentadecane (C15) | Hexadecane (C16) | |
| Fatigue control | m-Anisaldehyde | L-Carvone | L-Menthone |
Behavioral data analysis
The total amount of time each animal spent investigating the odorant during each trial was determined. Investigation was defined as active sniffing within 1 cm of the odorized cap. Animals were excluded from further analysis if they failed to habituate to the familiar odorant by the third trial, or failed to investigate odorants during the first or the last trial. Habituation was indicated by the ratio of investigation time in the third trial to that in the first trial, and was considered insufficient if the ratio exceeded 0.15, which was the ratio averaged across animals from pilot studies. We imposed these criteria because habituation to the first odorant was required for it to serve as the reference odorant to be compared with all subsequent test odorants, whereas a lack of investigation in the first or the last trial may indicate different kinds of behavioral problems. A general low level of interest in or motivation for the behavioral task may be indicated if there were no odorant investigation during the first odorant presentation. Meanwhile, performance fatigue may cause a lack of investigative behavior in the final trial. These criteria were satisfied by 11–12 out of 24 animals for each odorant series. Investigation time was sorted by the carbon number difference between test and familiar odorants. Data from the same carbon-number difference group in each of the three experimental series were collapsed together, and analyzed for statistical differences with an overall ANOVA, followed by Dunnett’s post hoc tests.
Overall 2-DG uptake pattern similarity between test and familiar odorants also was examined. Pairs of individual z-score patterns were compared using Pearson correlation as described above. Similar to the behavioral data, the resulting Pearson coefficients were first sorted by the carbon number difference between test and familiar odorants, and then combined across experimental series before being subjected to the same statistical analyses.
RESULTS
Chemotopic progression of predicted but novel glomerular activation
Even though responses to this homologous series had not been examined systematically before the current study, based on our previous observations (Johnson et al., 1999, 2002, 2004; Johnson and Leon, 2000b), we predicted that these odorants would produce chemotopically organized responses, with increasingly ventral glomeruli being activated by increasing carbon chain length. The molecular length of the longer alkanes was greater than the odorants that we previously had studied, and we therefore expected that these alkanes would reveal a novel activation in the anterior to mid-ventral glomerular layer where other odorants had not been observed to evoke a response. Glomerular 2-DG uptake patterns evoked by the homologous series are shown in Figure 1, and were found to be significantly different using ANOVA tests (p < 0.05) for uptake in previously defined regions of the bulb followed by false discovery rate analysis (Curran-Everett, 2000; Johnson et al., 2002, 2004, 2005a, b). Presumably because these alkanes do not contain any functional groups with only their straight hydrocarbon backbone, none evoked observable activation in any glomerular regions previously identified to respond to various functional groups (Johnson and Leon, 2000a; Johnson et al., 2002, 2004. 2005a,b), except for hexane (C6) and heptane (C7). The lateral-medial paired dorsal glomerular clusters activated by hexane and heptane (Fig. 1A) have been activated in other studies by aliphatic ketones and some aromatic odorants that do not share apparent odorant features (Johnson and Leon, 2000a; Johnson et al., 2002, 2004, 2005a, b). Octane (C8) evoked responses in a lateral-medial pair of more ventrally located foci, which were enclosed by the black outline (Fig. 1A). The location of these foci corresponded well with octane-induced response predicted from studies of the olfactory epithelium (Mozell, 1966; Scott et al., 1996; Scott et al., 2000) and observed in studies of the olfactory bulb using electrophysiological recording of mitral/tufted cells (Imamura et al., 1992; Igarashi and Mori, 2005) as well as 2-DG (Johnson et al., 2005a). As we predicted, the activity foci shifted more ventrally towards the center of the black outline with longer alkanes, extending to more ventral paired areas with dodecane (C12), and continuing until activity merged at the ventral midline with tetradecane (C14). The extreme ventral activation can be appreciated more readily when a pattern (circled in pink) evoked by the 15-carbon alkane, pentadecane, was overlaid onto a 3-dimensional glomerular layer model that has been rotated to show the response on the ventral midline of the bulb (Fig. 1).
Figure 1.
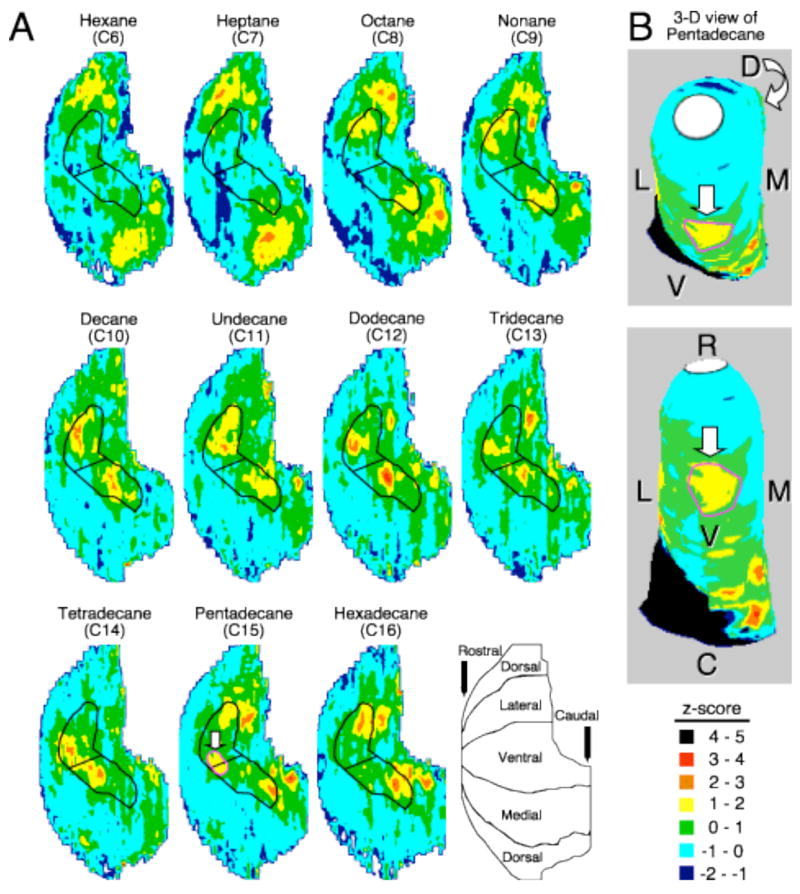
Glomerular responses to a homologous series of six- to sixteen-carbon straight-chained alkanes revealed novel activation in the hypothesized mid-ventral region. A: The glomerular 2-DG uptake pattern for each odorant was obtained by averaging across four individual responses that had undergone the subtraction of vehicle-induced activity and z-score transformation. The expressed z-score values were represented according to the color scale at the bottom right, next to the anatomical orientation. Both the color scale and the orientation are applicable to all 2-DG uptake patterns included in this article. The black outline on each pattern defines the glomerular region where the observed activity shift occurred across the homologous series. Shorter odorants, starting with octane (C8), generally stimulated more dorsal glomeruli in the outlined region both laterally and medially. With dodecane (C12), activation progressed into the mid-ventral glomerular layer that had previously remained unresponsive to hundreds of odorants we had studied. Activity started to merge at the ventral midline with tetradecane (C14), and became a single activity focus (outlined in pink and indicated by a white arrow) produced by pentadecane (C15). B: Pentadecane’s z-score pattern was overlaid onto a 3-D model of the glomerular layer. The single activity focus at the ventral midline was signified as in A. The two image frames depict different stages of a ventral-to-dorsal rotation. The second frame was captured after rotating the most rostral end upwards so that the entire ventral glomerular layer was facing outward; D: dorsal, V: ventral, L: lateral, M: medial, R: rostral, and C: caudal. Rotatable 3-D models and dorsal-centered perspectives containing responses to these and other odorants are available on our website (http://leonlab.bio.uci.edu).
The chemotopic progression of glomerular responses was examined further with a centroid analysis (Johnson and Leon, 2000a, b; Johnson et al., 2004, 2005a, b). The centroid of 2-DG uptake was calculated separately for the lateral and the medial aspects of individual matrices within the region outlined in black in Figure 1. The mean dorsal-ventral centroid coordinate for each alkane is shown in Figure 2. In both the lateral (Fig. 2A) and the medial (Fig. 2B) regions, an overall ventral shift of centroid position was observed from eight to fourteen carbons within the homologous series of alkanes. Centroids were confirmed to be statistically significant across the whole series in both the lateral (F(10,33) = 6.69, p < 0.001) and medial (F(10,33) = 8.01, p < 0.001) glomerular layer by an ANOVA analysis. However, we noticed that the most ventral centroid position was seen with tetradecane (C14), rather than the longest hexadecane (C16). In fact, the centroid position shifted back dorsally again with pentadecane and hexadecane (Fig 2). This dorsal progression could be explained by the presence of glomerular activation in the more dorsal region towards the top and bottom of the black outline similar to that observed with octane (Fig. 1A).
Figure 2.
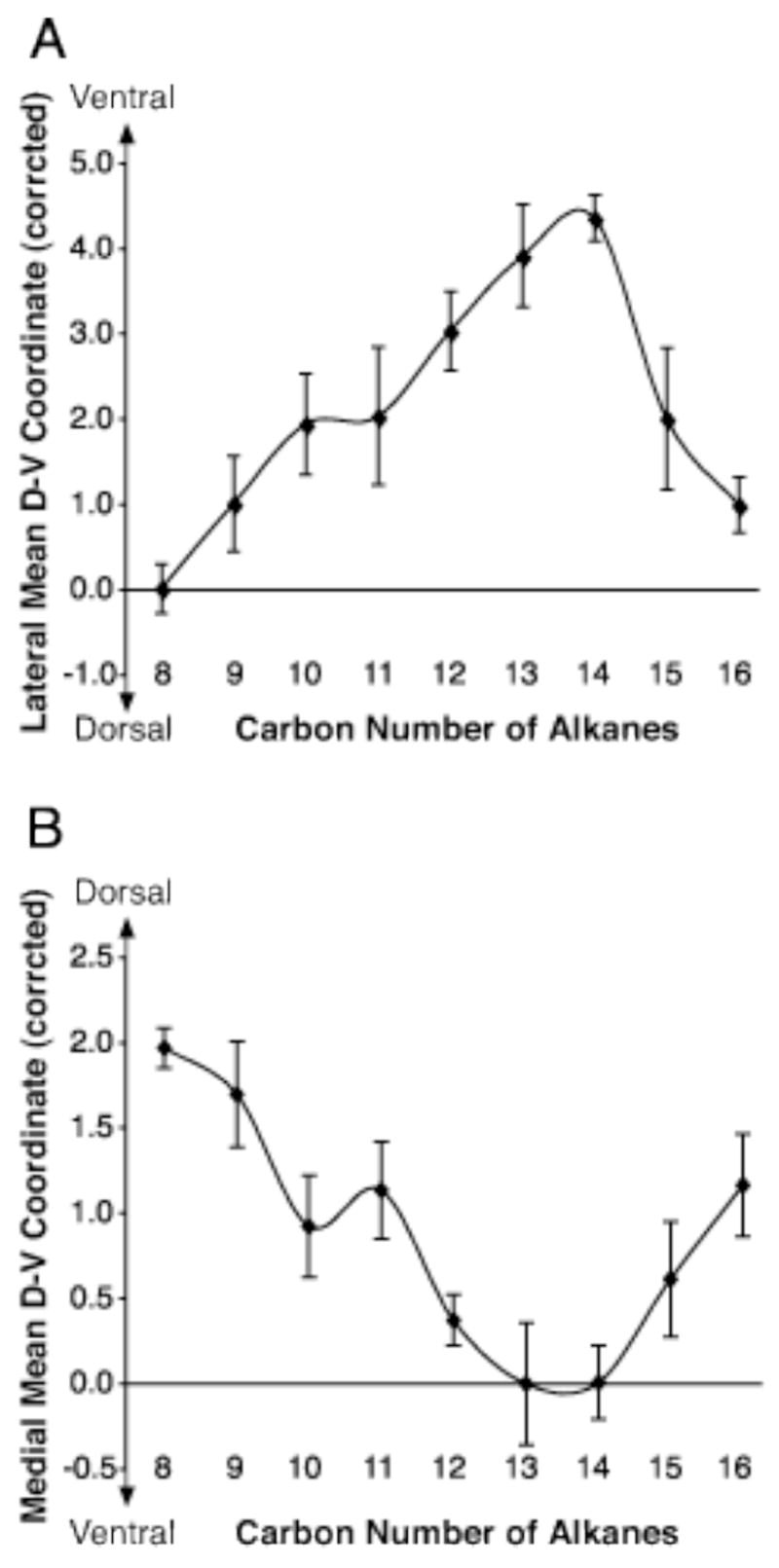
Centroid analyses of 2-DG uptake support a ventral shift of glomerular activation with increasing carbon chain length. A: The lateral part (top portion) of the glomerular region outlined in Fig. 1A was included in this centroid analysis, and the average centroid position along the dorsal-ventral axis was plotted for each alkane. Increases in the coordinates indicate increasingly ventral positions. Standard errors are represented here. As appeared to be the case from inspecting the 2-DG uptake patterns, centroids shifted ventrally from octane (C8) to tetradecane (C14), and became more dorsal again with pentadecane (C15) and hexadecane (C16). The observed centroid progression was found to be statistically significant (ANOVA, p < 0.001). B: The analyses described in A were applied to the medial aspect (bottom portion) of the outline (Fig. 1A), and results were similar to those observed in A (p < 0.001). In this panel, increasingly ventral positions are represented by smaller coordinates.
Minor impurities greatly impact uptake patterns
According to the reliable chemotopic organization previously observed (Johnson and Leon, 2000b; Johnson et al., 1999, 2002, 2004, 2005a, b), and found here for smaller alkanes, the more dorsal glomerular activation by the longest alkanes in the series was surprising. Given the low vapor concentrations of these large odorants, we considered the possibility that the dorsal responses were due to an odorous contaminant in pentadecane and hexadecane. To test this possibility, we examined glomerular responses to pentadecane samples of different purity grades. In addition to the original 99% pure material, two other samples of the same purity grade from different vendors were tested along with a 99.8% pure odorant.
All three 99% pure samples produced activity both at the ventral midline and in the more dorsal and caudal regions outlined in Figure 3. However, the more dorsal and caudal activation was absent in the response to the 99.8% pure pentadecane, leaving only activation at the ventral midline (Fig. 3). Modular ANOVAs followed by false discovery rate analyses revealed a significant difference among these activity patterns (p < 0.05). Specifically, when responses to the Acros (99% pure, our original source) and the Fluka (99.8% pure, the highest purity) samples were compared across glomerular modules using paired t-tests, significant differences (p < 0.05) were found only in areas included by the outlines (Fig. 3). In addition, a Pearson correlation comparison of these responses indicated a relatively low level of overall pattern similarity (r = 0.42). These results supported the hypothesis that the unexpected dorsal activation observed in the responses to the longest alkanes was produced by impurities present in odorants from different sources. Indeed, further analysis of these odorant samples using gas chromatography-mass spectrometry (GC-MS) detected minor contaminants in the 99% pure materials that were absent from the purer pentadecane sample. Moreover, different contaminants identified among the 99% pure samples suggest that they may have been obtained by using distinct starting materials and/or production processes, so that subsequent differences in impurities may have contributed to the observed differences in their evoked neural responses. Given that there was a significant difference and a low correlation between the glomerular patterns evoked by the 99% pure pentadecane and the 99.8% pure pentadecane, we predicted that rats might discriminate spontaneously between these two odorant samples. A discrimination experiment was conducted to confirm this prediction, and its results will be discussed below.
Figure 3.
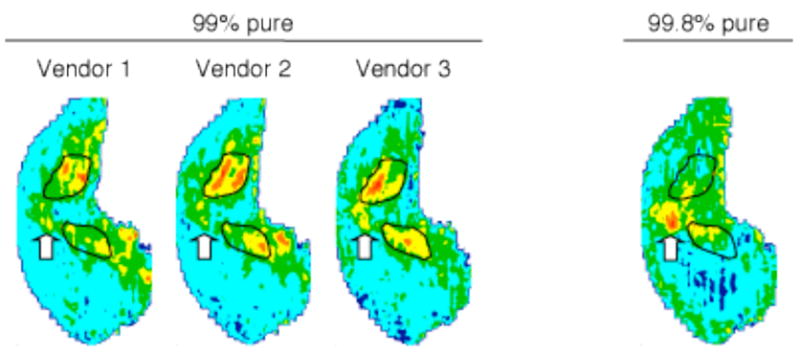
Glomerular responses to pentadecane of different purity grades revealed effects of chemical impurities on activity patterns. Three samples of 99% pure pentadecane from different vendors (1: Acros, 2: Alfa Aesar, 3: Sigma) produced similar z-score transformed activity patterns in both the extreme mid-ventral and more dorsocaudal areas. However, dorsocaudal activation (black outlines) was not observed in response to the purer (99.8%) pentadecane from Fluka, suggesting that the activation may be produced by higher amounts of contaminants present in the less pure odorant samples.
Overall pattern similarity among alkane-evoked glomerular responses
Studies of other homologous series of odorants have shown that they evoke neural responses in the olfactory bulb that become increasingly dissimilar with greater differences in carbon number between odorants (Imamura et al., 1992; Katoh et al., 1993; Johnson et al., 1999, 2002, 2004; Rubin and Katz, 1999; Belluscio and Katz, 2001; Meister and Bonhoeffer, 2001). Using Pearson correlation (Johnson et al., 2002, 2004, 2005a), point-to-point comparisons can be made between glomerular responses elicited by any two of the odorant conditions to examine their overall pattern similarity, which is indicated by a resulting single value, the correlation coefficient (r). The relationship among glomerular responses to the homologous series of alkanes can be appreciated more easily by subjecting a correlation matrix containing all possible paired comparisons of these responses to a principal components analysis. Three major clusters of odorants were revealed consequently in the olfactory space represented by these glomerular responses (Fig. 4A). Hexane and heptane constituted one of the clusters, and were accounted for mostly by the second component (Fig. 4A). A second group was associated with the first as well as the second components, and included octane, nonane, and decane, which were not closely clustered with any other alkanes (Fig. 4A). Longer alkanes with at least 11 carbons in their hydrocarbon structures formed the last cluster, and were separated from the other odorants largely by the first component (Fig. 4A). The demonstrated dissimilarity between neighboring alkanes and odorant clusters corresponded to the glomerular activity patterns described above (Fig. 1A). Hexane and heptane evoked high levels of uptake in the dorsal bulb both laterally and medially in regions where octane and all other odorants did not (Fig. 1A), a finding that probably explained both the dissimilarity between heptane and octane and the clustering of hexane and heptane (Fig. 4A). Meanwhile, the clustering of the glomerular response for longer alkanes (Fig. 4A) may be a consequence of the more ventral glomerular stimulation shared by the responses to these odorants (Fig. 1A).
Figure 4.
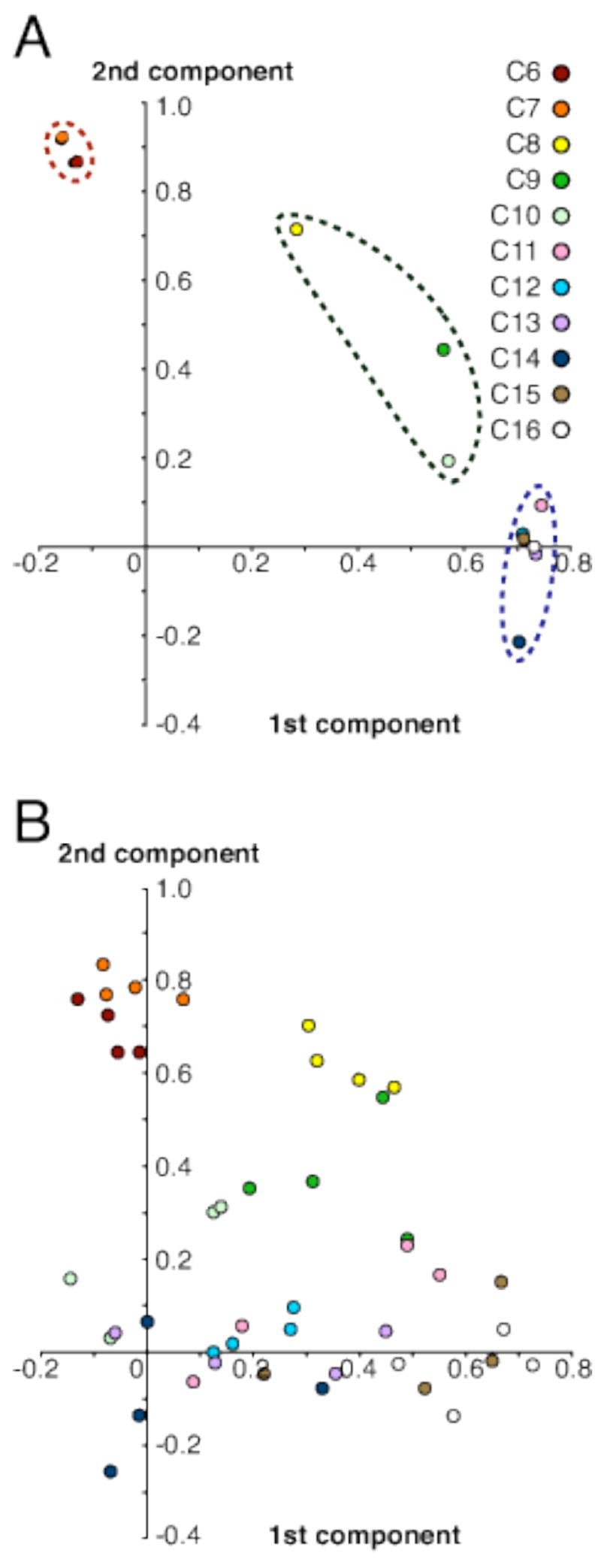
Principal component analyses of glomerular response patterns evoked by the homologous series of alkanes. The results illustrated here have undergone at least one oblique transformation to optimize the interpretation of the factor matrix structure. A: Analysis of the average pattern correlations yielded three primary groups of response patterns consistent with the chemotopic progression described earlier. Hexane (C6) and heptane (C7) clustered closely and were accounted for mostly by the 2nd component. Octane (C8), nonane (C9), and decane (C10) were not particularly associated with any other alkanes. The third cluster contained alkanes with 11 carbons and more, and correlated highly with the 1st component. Approximately 65% of the variance represented by the alkane series was explained by these two components. B: The same analysis also was performed on individual response patterns (n = 4 per odorant), and a factor matrix structure similar to A was observed. In general, greater variance between individuals exposed to the same odorant condition was associated with longer and more hydrophobic alkanes. Here, the two components accounted for 40% of the variances, with most of the remaining components that were extracted each explaining only about 1%.
A separate principal components analysis was performed on another Pearson correlation matrix containing paired comparisons of individual, rather than average, response patterns (Fig. 4B). Overall, clustering of odorants was comparable to that observed in Figure 4A. However, individual differences between subjects exposed to the same odorant condition were greater with longer, more hydrophobic alkanes.
Concentration-dependent glomerular activation
Differences in the activation of the dorsal glomerular layer led to a more pronounced separation of the hexane-heptane cluster from the rest of the alkanes than was expected for odorants in a homologous series, especially between neighboring members such as heptane and octane. In this study, however, odorants were presented at equal dilutions rather than at equal vapor concentrations in order to assure that there would be responses to odorants at both ends of the series. Therefore, differences in odorant concentration may have contributed to differences in evoked activity patterns. In particular, we had previously observed stimulation of the dorsal glomerular layer, similar to the area activated by heptane, with higher concentrations of 2-hexanone, which did not evoke activity in the same area at lower concentrations (Johnson and Leon, 2000a; Johnson et al., 2004). Based on those results, we hypothesized that lower heptane concentrations might induce significantly less activity in the dorsal bulb, resulting in higher pattern similarity with octane.
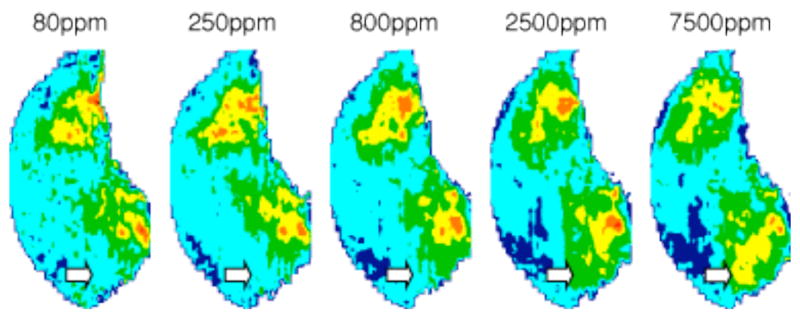
We tested five concentrations of heptane, with the highest level identical to the concentration used in the original homologous series experiment (Table 1). Modular ANOVA tests followed by the false discovery rate analysis as described above revealed dorsal areas, paired laterally and medially, to be the only regions giving significantly different responses to different concentrations of heptane (Fig. 5). Indeed, strong activation of these dorsal areas was only evident at 7500 ppm, the highest concentration that we tested (Fig. 5). This effect appeared to be more robust in the medial bulb, as indicated by the white arrows, than in the lateral aspect (Fig. 5A). Interestingly, the response pattern evoked by 2200 ppm of octane (Fig. 1A, Table 1) showed the highest correlation (r = 0.82) with the activity pattern produced by the most closely matched heptane concentration of 2500 ppm.
Figure 5.
Glomerular responses to heptane at various concentrations. Significantly different (p < 0.05) patterns of activation were observed in the dorsal bulb, both laterally and medially, with different concentrations of heptane. This effect was more pronounced in the medial aspect as indicated by white arrows in the average z-score transformed patterns, with higher concentrations producing a higher level of activation.
Combinatorial coding of molecular features
Combinatorial models of olfactory processing predict that odorants with a shared molecular feature should be encoded by the same neural elements to elicit overlapping neural responses (Friedrich and Korsching, 1997, 1998; Joerges et al., 1997; Johnson et al., 1998, 1999, 2002, 2004, 2005a, b; Malnic et al., 1999; Rubin and Katz, 1999; Sachse et al., 1999; Uchida et al., 2000; Belluscio and Katz, 2001; Fuss and Korsching, 2001; Meister and Bonhoeffer, 2001; Leon and Johnson, 2003). Because straight-chained alkanes do not contain any functional groups other than their simple hydrocarbon structures, the molecular features that were recognized by the olfactory system to produce the observed responses must exist solely within the carbon chain. Many aliphatic odorants that contain functional groups also possess the same straight hydrocarbon backbone, so that the alkanes would be predicted to evoke a similar glomerular activation as other aliphatic odorants that have different functional groups, as long as they share a similar hydrocarbon chain.
We therefore compared glomerular responses to the eight-, twelve-, and fifteen-carbon alkanes with the responses to the corresponding aliphatic alcohol or ketone in separate experiments (Fig. 6). Similar ventral responses were observed for each paired comparison, and are indicated by white arrows of similar orientations. Octane (eight-carbon alkane) and 2-octanol (eight-carbon alcohol; Johnson et al., 2004) elicited overlapping responses in the octane activity foci described above (Fig. 1A). Overlapping areas of activation were also observed more ventrally between the twelve-carbon dodecane (alkane) and 2-dodecanone (ketone), and at the ventral midline between the fifteen-carbon pentadecane (alkane) and 8-pentadecanone (ketone; Fig. 6). Since the shared carbon chain length elicited a common glomerular response, despite differences in functional groups, this result indicated that molecular features recognized by the system were indeed contained within the aliphatic carbon chain.
Figure 6.
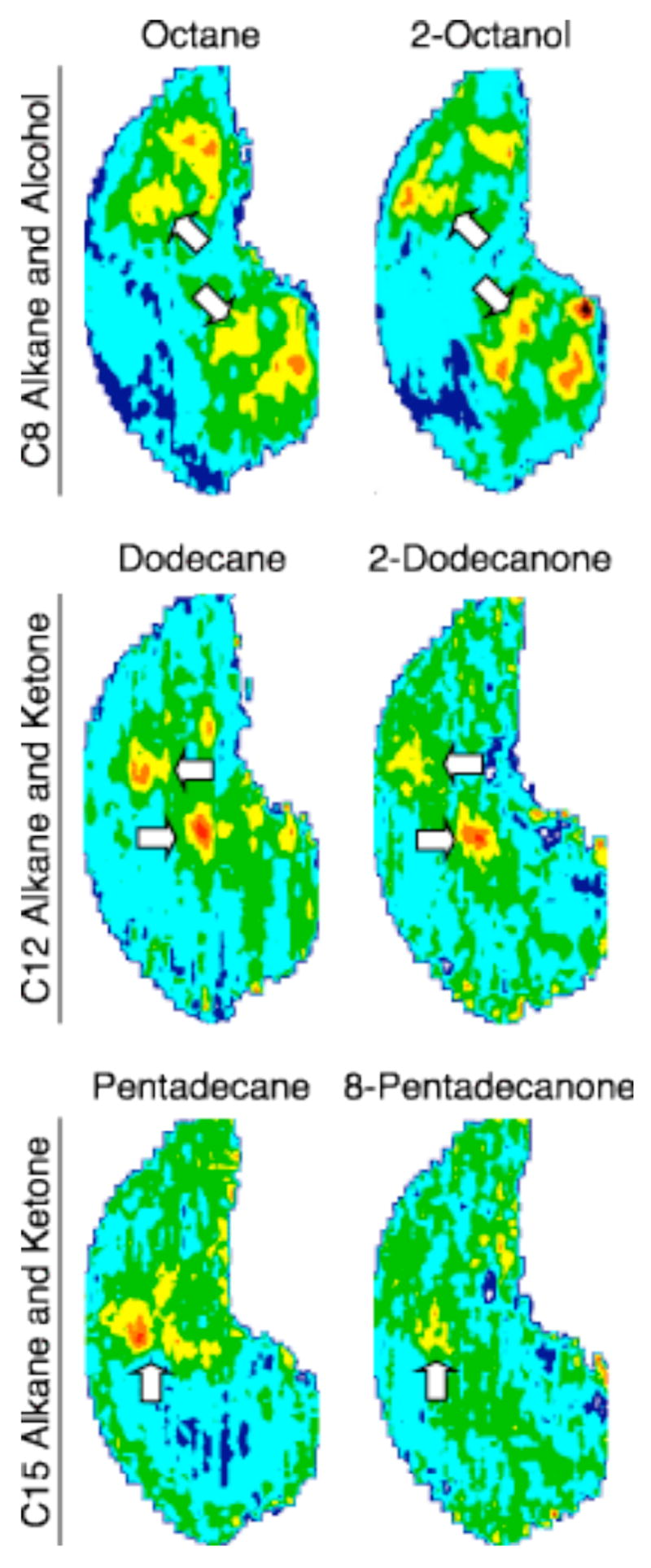
Aliphatic odorants with similar hydrocarbon structures, regardless of functional group, stimulate overlapping glomeruli. Glomerular responses to eight- (top row), twelve- (middle row), and fifteen-carbon (bottom row) alkanes were compared with the alcohol or ketone of corresponding carbon numbers. Overlapping areas of activation are indicated by white arrows of similar orientations, and were observed for all three pairs of comparisons, shifting more ventrally with greater carbon chain length.
It is noteworthy to mention that light mineral oil, in which the ketones were diluted, can contain about 2% of fifteen- and sixteen-carbon alkanes (Fisher Scientific Technical Support). However, the volatility of these long alkanes is so low that their minor presence in mineral oil is unlikely to result in high enough vapor concentrations to evoke an olfactory response. Moreover, the subtraction of the mineral oil vehicle response from the responses produced by these ketones should have removed any possible effects of long alkanes that may exist in the mineral oil. Therefore, the presence of fifteen- and sixteen-carbon alkanes in mineral oil cannot account for the shared glomerular representation at the ventral midline produced by 8-pentadecanone and pentadecane.
Neurobehavioral correlates of straight-chained alkanes
To determine if there were a meaningful relationship between odor perception and the patterns of 2-DG uptake evoked by alkanes, we compared glomerular activity patterns evoked by the alkane homologous series with the behavioral responses to these odorants using an odor habituation assay that depends on the reliable willingness of rats to investigate novel odorants in their environment (Linster et al., 2001; Cleland et al., 2002). With repeated exposure, however, the novelty of the odorant dissipates and rats are less and less likely to investigate that odorant (Fig. 7A). If a second odorant is then placed in their environment, rats are likely to investigate it if they regard the new odor as being different from the habituated odor. Using this assay, we tested whether the perceived odors became more different with greater differences in alkane carbon number. Behavioral results including all animals from each experimental series were presented after being sorted into disqualified and qualified groups based on our criteria that were determined a priori (Fig. 7). Major differences in the behavior between the qualified and disqualified groups of animals were exhibited during the habituation trials (Fig. 7A). However, both groups of animals expressed similar levels of investigation of the control odorant that tested for motor fatigue (Fig. 7B), so that very few animals in this study were excluded for statistical analyses due to a lack of responses to control odorants. These animals also exhibited comparable behavior during test odorant trials (Fig. 7C). Together, these observations support the notion that the analysis criteria were unlikely to have selected for a group of animals that were systematically different from the excluded group. Using only data from the qualified group (Fig. 8A), statistical significance was demonstrated with an ANOVA (F(4, 197) = 5.64, p = 0.00026), and Dunnett’s post hoc tests revealed that the mean investigation times for test odorants that differed by at least one carbon were significantly greater than investigation times for the familiar odorant with zero carbon difference. This pattern of response was also evident when each habituated odorant was considered separately (Fig. 7C). The mean investigation time for the two- and three-carbon difference groups also was found to be significantly different from the one-carbon difference group. Overall, longer investigation time, presumably indicating more dissimilar perceived odors, was observed with larger carbon differences between test and familiar odorants.
Figure 7.
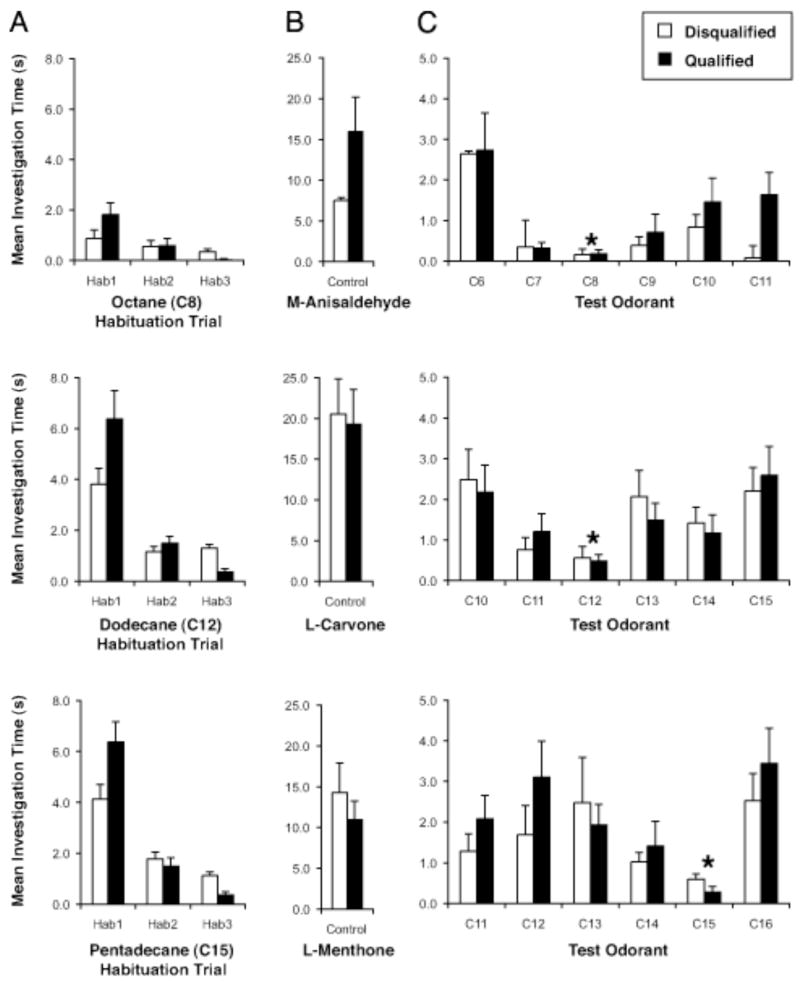
Behavioral results from animals that satisfied the criteria to be included for statistical analysis (black bar) and those that were excluded (white bar). A: The three habituation trials revealed major differences in behavior between these two groups of animals. B: Both groups of animals responded to the control odorant, which is consistent with the fact that few animals were disqualified in this study for the lack of investigation during this control trial. C: Similar behavior exhibited by both groups of animals during the test trials. For each experimental series, the alkane that was used as the habituated odorant was marked with an asterisk. Standard errors were shown in all panels.
Figure 8.
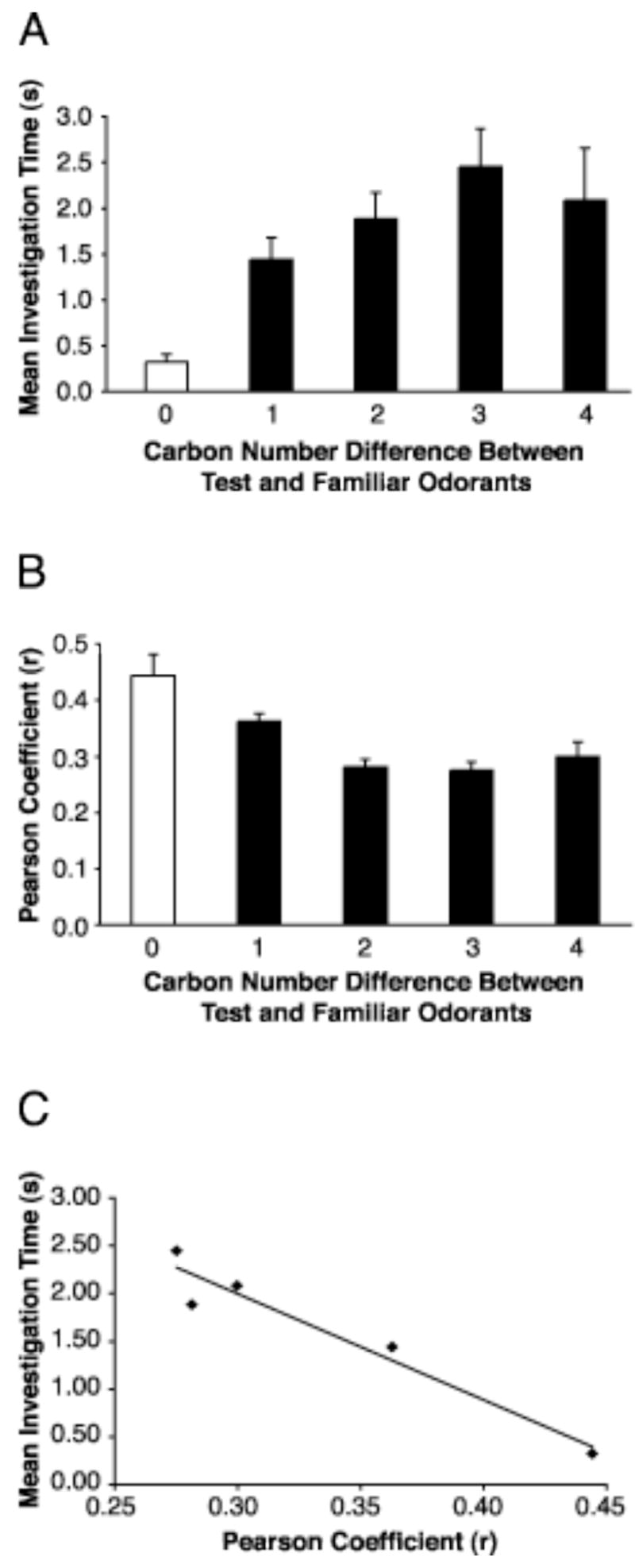
Behavioral responses based on an odor habituation assay supported a close relationship among odorant properties, odorant-induced glomerular activities, and perceptual similarities. A: Data from three series of behavioral experiments (Fig. 7), including different sets of alkanes (see Table 2), were combined for analyses. The total amount of investigation time for each test trial was sorted by the carbon number difference between the test and familiar odorants. A mean investigation time was then calculated and plotted for each carbon number difference. Zero carbon difference refers to test trials where the familiar odorant was used. Standard errors are shown. An ANOVA (p = 0.00026) followed by Dunnett’s tests revealed significant differences between the zero-carbon versus all other groups, as well as between the one-carbon versus all but the four-carbon groups. In general, longer investigation time, which indicated increasing odor dissimilarity, was observed with larger carbon number difference between test and familiar odorants. B: The overall similarity of glomerular activity patterns between test and familiar odorants used in the behavior experiments was analyzed using Pearson correlations. The resulting correlation coefficients (r) were sorted similarly to the investigation time data, and then were averaged for each carbon number difference group. Standard errors are shown. All groups were found to be significantly different from the zero-carbon group using the same statistical tests mentioned in A (p = 0.0021). Overall, decreasing pattern similarity, represented by decreasing Pearson coefficients, was observed with increasing carbon number difference. C: The investigation time and Pearson coefficient data from Panels A and B were included in a regression analysis, which revealed a significantly negative correlation (p = 0.0067), supporting a strong relationship between neural activity pattern and perceptual similarities.
Our behavioral results have supported a relationship between the molecular similarity of odorants and their perceptual similarity. However, to be able to compare neural activity and behavior results meaningfully, we also had to determine whether a relationship existed between odorant properties and odorant-induced activity patterns. To test for this relationship, individual z-score patterns representing odorants involved in the behavioral experiments were compared for overall pattern similarity using Pearson coefficients (Johnson et al., 2002, 2004). The resulting Pearson coefficients were sorted according to the carbon number difference between test and familiar odorants as with the behavioral data, and then were averaged for each group (Fig. 8B). Again, ANOVA followed by the Dunnett’s tests showed that odorants differing by at least one carbon from the familiar odorants produced significantly less correlated glomerular response patterns (F(4,253) = 10.56, p = 0.0021). In general, decreasing pattern similarity was observed with increasing carbon number difference, except for a slight increase observed for the four-carbon group. Interestingly, the four-carbon group showed a similar reversal in the behavior data (Fig. 8A). This group, with the largest carbon number difference in the current study, included only comparisons between pentadecane and undecane. Both of these odorants elicited glomerular activation (Fig. 1A) in the dorsal regions outlined in Figure 3. Their considerably overlapping activation in that particular region may be responsible for higher pattern similarity, as well as perceptual similarity (Fig 8A), between the two odorants.
In Figure 8C, the mean investigation time (Fig. 8A) was plotted against the pattern similarity data (Fig. 8B). Increasing pattern similarity was associated with decreasing investigation time, which indicated increasing perceptual similarity. Indeed, a regression analysis yielded a significant negative correlation between the two (r = −0.96, p = 0.0067).
In addition, we tested the ability of rats to discriminate between the 99.8% and the 99.0% pentadecane in a separate experiment. Rats (n = 15) habituated to the purer sample were found to spend significantly longer time (t = 2.25, p = 0.016) investigating the 99.0% test odorant (mean investigation time = 1.67s, s.e.m. = 0.65) than the 99.8% sample (mean investigation time = 0.18s, s.e.m. = 0.09), indicating that they could spontaneously discriminate between different purity grades of the same compound. Again, differences in glomerular response patterns evoked by these odorants predicted differences in perception (Fig. 5B). It is also interesting that such a minor contaminant dramatically alters an odor.
DISCUSSION
Predictions of a combinatorial code
If odorants are coded by the combination of their molecular features, then one should predict that shared features would evoke a shared neural response. Indeed, the glomerular responses evoked by pure straight-chained hydrocarbons were shared by odorants with that feature accompanied by different combinations of other molecular features. These data therefore strongly support the notion of a combinatorial code for olfaction.
Glomerular response patterns may emerge from a combination of the inherent and imposed interactions between the olfactory epithelium and odorants
We have observed a chemotopic organization of responses to a homologous series of straight-chained alkanes that extended into a ventral glomerular region (Fig. 1) that had not been stimulated by any of the hundreds of odorants examined in our previous studies (Johnson et al., 1998, 1999, 2002, 2004, 2005a, Johnson et al., b; Johnson and Leon, 2000a, b). Consistent with prior results, increasingly ventral glomeruli tended to respond to longer odorous molecules (Johnson et al., 1999, 2004; Johnson and Leon, 2000b). Alkanes of greater length than were used in this homologous series probably would not be sufficiently volatile for receptor activation, which may explain why pentadecane stimulated the most ventral region of the bulb. It also may explain why hexadecane did not stimulate the extreme ventral aspect of the bulb (Fig. 1A); that molecule may not be volatile and the glomerular activity that was seen may have been evoked by volatile contaminants. Larger alkanes, such as octadecane (C18), are actually solids at room temperature (PhysProp Database from Syracuse Research Corporation: www.syrres.com/esc/physdemo.htm). It is interesting to note that mouse urine, which evoked activity in partially overlapping ventral glomerular regions (Schaefer et al., 2002), have been shown recently to contain non-volatile components probably of great molecular length (Kimoto and Touhara, 2005).
Among odorants in a homologous series, a number of molecular properties usually covary, so that the exact property associated with the change in glomerular response often becomes difficult to pinpoint. Thus far, only one study (Johnson and Leon, 2000b) has been conducted to demonstrate that molecular length, rather than other molecular properties, correlated with the ventral shift of glomerular activation observed with a homologous series of carboxylic acids (Johnson et al., 1999). Studies that further examine the molecular features responsible for chemotopic responses produced by other homologous series are warranted.
This chemotopy along the dorsal-ventral axis, which exists locally within response modules and globally throughout the olfactory bulb, may be explained by the distribution of olfactory receptor types in the olfactory epithelium (Hornung and Mozell, 1977; Schoenfeld and Knott, 2004), as well as the maintenance of that topography by way of axonal projections to the glomerular layer (Astic and Saucier, 1986; Ressler et al., 1994; Schoenfeld et al., 1994; Vassar et al., 1994; Schoenfeld and Knott, 2002; Schoenfeld and Knott, 2004). Indeed, preferential responses of the dorsal glomerular layer to small odorants with an oxygen moiety (Johnson et al., 1998, 1999, 2002, 2004, 2005a, Johnson et al., b; Johnson and Leon, 2000a, b) coincides with the dorsal epithelial expression of Class I olfactory receptors (Conzelmann et al., 2000; Zhang and Firestein, 2002; Zhang et al., 2004), which appear to have high affinity for small and/or hydrophilic odorants (Malnic et al., 1999; Sanz et al., 2005). Such odorant-evoked responses due to the intrinsic response specificity of olfactory receptors have been referred to as inherent responses (Moulton, 1976; Mozell et al., 1987).
However, it appears that mucosal sorption of odorous molecules also may contribute to the observed chemotopy within the olfactory bulb. Mozell and colleagues (Mozell, 1964, 1970; Mozell and Jagodowicz, 1973; Hornung and Mozell, 1977; Mozell et al., 1987; Hahn et al., 1994; Kent et al., 1996; Keyhani et al., 1997) have demonstrated that smaller and more hydrophilic odorants are absorbed rapidly by the aqueous mucosa upon entry into the nasal epithelium, and therefore are likely to have limited access only to the dorsal central channels towards the entrance of the nasal cavity (Schoenfeld and Knott, 2004). On the other hand, larger and more hydrophobic odorants, including octane, were found to be able to disperse more freely throughout the olfactory epithelium than the hydrophilic ones. This way, increasingly hydrophobic odorants that are sufficiently volatile, such as longer alkanes in this study, may be more likely to access the entire epithelium, even the most peripheral parts that contain receptor neurons projecting to the most ventral glomerular layer. Our results therefore support possible chromatographic properties of the epithelium, and the resulting epithelial responses have been referred to as imposed responses (Moulton, 1976; Mozell et al., 1987).
Responses to various odorants may be affected differently by their inherent and imposed interactions with the epithelium. More hydrophilic, and therefore more rapidly absorbed odorants were found to produce responses dominated by the imposed activity, whereas more hydrophobic odorants evoke activity that is more reflective of their inherent interactions (Mozell et al., 1987). However, both the inherent and imposed components may work together to facilitate the processing of odorous molecules at this level (Moulton, 1976; Mozell et al., 1987). An example of this synergism would be the affinity of Class I receptors for hydrophilic molecules and their localization in the dorsal olfactory epithelium where the majority of such molecules are likely to be absorbed upon entry into the aqueous mucosa (Hornung and Mozell, 1977; Schoenfeld and Knott, 2004). This intrinsic organization of the receptors may allow a subset of the receptors to respond optimally to smaller and/or hydrophilic odorants due to both their location and their high-affinity responses to odorant features (Malnic et al., 1999; Mezler et al., 2001; Zhang and Firestein, 2002). Odorant-evoked epithelial responses examined by others also suggest more peripheral responses to more hydrophobic odorants (Ezeh et al., 1995; Bozza and Kauer, 1998; Araneda et al., 2000; Scott et al., 2000; Scott-Johnson et al., 2000; Bozza et al., 2002). Together, these results support the notion that olfactory receptors with high affinity for specific molecular features may be expressed in an epithelial location that maximizes each receptor’s interaction with its preferential odorous ligands (Scott et al., 2000; Schoenfeld and Knott, 2004).
Only at the highest concentration tested here did heptane stimulate the dorsal glomerular layer that corresponds to projections from the central channels (Fig. 5). Similar concentration-dependent activation of the same dorsal regions also had been reported with 2-hexanone (Johnson and Leon, 2000a) and 2-octanone (Johnson et al., 2004). These dorsal glomerular regions, previously defined as modules c (lateral) and C (medial), were thought initially to respond preferentially to ketones (Johnson and Leon, 2000a; Johnson et al., 2002). Subsequently, reliable activation in these areas has been observed with a wide variety of chemicals including aromatic odorants, some cyclohexyl molecules, secondary alcohols, an ester, as well as the smaller alkanes in this study (Johnson et al., 2002, 2004, 2005a, Johnson et al., b). Even though these odorants possess different functional groups and appear to be structurally distinct, there is clear evidence of response specificity within these regions (Johnson et al., 2002, 2004, 2005a, Johnson et al., b; Takahashi et al., 2004). Furthermore, higher levels of activation in these dorsal modules were generally evoked by odorants of six to eight carbons in chain length (Johnson and Leon, 2000a; Johnson et al., 2002, 2004, 2005a, b). These particular dorsal modules receiving input from the central channel of the olfactory epithelium may be particularly responsive to high concentrations of odorants that possess specific molecular features. Again, the intrinsic differential responsiveness of the olfactory epithelium to odorant features appears to interact with the imposition of the physical characteristics of odorant molecules to produce the observed glomerular response patterns.
Odorant hydrophobicity as a source of variance in inter-individual responses
Our principal components analysis suggested that inter-animal variability was greater for the larger alkanes. The possible inherent and imposed interactions between the olfactory epithelium and odorants discussed above also may account for this overall greater individual variance. If these odorants could gain access to more areas in the epithelium, then their chances to interact with more types of receptors distributed throughout the epithelium would be increased, even though some of these interactions may be weaker and more inconsistent due to lower affinity binding that may produce individual variability.
The importance of odorant volatility and purity
In this study, we observed an apparent exception to the typical chemotopic progression with responses to the longest alkanes. Because chemotopically organized glomerular responses have been so reliable at both the local and global level, such a surprising exception led us to examine alternative explanations. Here, the unexpected glomerular activation was explained by the impurity of the odorant preparations. A similar contaminant effect has been demonstrated previously with the stimulation of the extreme rostral glomerular layer by carboxylic acids present in the aldehyde preparations as a byproduct of oxidation (Johnson et al., 2004). It may be surprising at first that such a small difference in purity between the two grades of pentadecane used in our experiment would be significant for this system. Nevertheless, if the contaminant were appreciably more volatile than the label compound, then a large impact would be expected. At the dilution that pentadecane was presented to animals, there would be essentially no change in its vapor concentration (approximately 0.4 ppm) from 99% to 99.8% pure. However, a contaminant that were approximately 5000 fold more volatile than pentadecane, for example, would have changed in its vapor concentration from 20 ppm when using the less pure pentadecane to only 4 ppm with the purer preparation.
The finding that hexadecane evoked very little response in the extreme ventral aspect of the bulb is consistent with the notion that hexadecane has a low enough volatility to preclude a glomerular response. Given that hexadecane is even less volatile than pentadecane, its evoked activity pattern is likely to be dominated by the same dorsal responses to contaminants more volatile than itself.
Correlations among odorous stimuli, neural responses, and odor perceptions
After studying hundreds of odorants, we are gaining enough of an understanding of the relationship between odorant properties and their induced neural activities to make successful predictions of glomerular responses based on odorant molecular structure. Correlations between activity patterns and odorant chemistry also have been reported in other studies using a variety of methods (Friedrich and Korsching, 1997, 1998; Joerges et al., 1997; Johnson et al., 1998, 1999, 2002, 2004, 2005a, b; Malnic et al., 1999; Rubin and Katz, 1999; Sachse et al., 1999; Johnson and Leon, 2000a; Uchida et al., 2000; Belluscio and Katz, 2001; Fuss and Korsching, 2001; Meister and Bonhoeffer, 2001). Similarly, a close relationship between odorant properties and odor discriminability has been demonstrated through a number of psychophysical studies using various homologous series (Laska and Freyer, 1997; Laska and Teubner, 1999; Laska et al., 1999; Linster and Hasselmo, 1999; Laska and Galizia, 2001; Laska and Hübener, 2001; Linster et al., 2001b; Cleland et al., 2002). Fewer studies have been conducted in intact animals to compare both neural and perceptual responses to odorants that varied systematically in their molecular structures, although a recent study using an odor habituation paradigm to test odor similarity explored the relationship between glomerular and behavioral responses to a homologous series of carboxylic acids (Cleland et al., 2002). Since this behavioral assay does not require extensive training of animals, it can reveal spontaneous discriminability among odorants that may correlate more meaningfully with glomerular responses observed in animals naïve to the odorants presented. A significant correlation between the two types of responses observed in that study suggested that glomerular pattern similarity could predict perceived odor similarity (Cleland et al., 2002). This relationship was further supported by a later study comparing epithelial and discrimination responses to an aldehyde homologous series (Kent et al., 2003). Similarly, the strong correlation between neural and behavioral responses in our current study is consistent with the idea that chemotopic information represented within the olfactory bulb may be used for perceptual processing. Indeed, our results also are consistent with the response specificity to alkanes recorded from neurons in the anterior piriform cortex, the primary projection area of the olfactory bulb (Wilson, 2000). In conclusion, these data indicate that odorant molecular features, their evoked glomerular activity, and their odor perception are functionally related.
In summary, we have demonstrated that even pure hydrocarbons, which can use only weak Van der Waals forces to bind to olfactory receptors, evoke highly specific responses that can be seen at the glomerular layer. Systematic differences in these spatially specific responses were predicted based on systematic differences in the molecular features of the hydrocarbon chain. Indeed, the glomerular responses were systematic enough to allow us to pick out anomalies that were based on odorant contaminants, rather than the target odorant. We then went on to show that the degree of difference in glomerular responses was proportional to the magnitude of perceptual difference in rats. Our data further suggest that intrinsic differences in responsiveness to odorants by different olfactory receptor neurons interact with differences imposed by the physical characteristics of specific molecules to produce the olfactory code. Finally, odorants with oxygen-containing functional groups that shared the same hydrocarbon feature also shared portions of the glomerular response that were present for the alkane alone, a finding that is consistent with the idea of a combinatorial olfactory code.
Acknowledgments
We thank the following individuals for their technical assistance: Andrew Chen for 2-DG activity mapping, Talla Motakef and Sakura Minami for their work on the behavioral tests, and Espartaco (Spart) Arguello for the development and maintenance of a database for our activity patterns, analysis software, and our laboratory’s website. We also would like to recognize Dr. John Greaves, Director of the Mass Spectroscopy Facility at the University of California, Irvine, for his expert advice on our GC-MS analyses.
This research is supported by grants DC03545 and DC006516 from NIDCD.
References
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Astic L, Saucier D. Anatomical mapping of the neuroepithelial projection to the olfactory bulb in the rat. Brain Res Bull. 1986;16:445–454. doi: 10.1016/0361-9230(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza TC, Kauer JS. Odorant response properties of convergent olfactory receptor neurons. J Neurosci. 1998;18:4560–4569. doi: 10.1523/JNEUROSCI.18-12-04560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Ezeh PI, Davis LM, Scott JW. Regional distribution of rat electroolfactogram. Neurophysiol. 1995;73:2207–2220. doi: 10.1152/jn.1995.73.6.2207. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci. 1998;18:9977–9988. doi: 10.1523/JNEUROSCI.18-23-09977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss SH, Korsching SI. Odorant feature detection: activity mapping of structure response relationships in the zebrafish olfactory bulb. J Neurosci. 2001;21:8396–8407. doi: 10.1523/JNEUROSCI.21-21-08396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn I, Scherer PW, Mozell MM. A mass transport model of olfaction. J Theor Biol. 1994;167:115–128. doi: 10.1006/jtbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- Hornung DE, Mozell MM. Factors influencing the differential sorption of odorant molecules across the olfactory mucosa. J Gen Physiol. 1977;69:343–361. doi: 10.1085/jgp.69.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Mori K. Spatial representation of hydrocarbon odorants in the ventrolateral zones of the rat olfactory bulb. J Neurophysiol. 2005;93:1007–1019. doi: 10.1152/jn.00873.2004. [DOI] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Joerges J, Küttner A, Galizia G, Menzel R. Internal representations for odours and combinatorial coding of odour mixtures visualized by optical imaging. Nature. 1997;387:285–288. [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M. Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol. 2005a;483:192–204. doi: 10.1002/cne.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005b;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Koshimoto H, Tani A, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. II. Aromatic compounds. J Neurophysiol. 1993;70:2161–2175. doi: 10.1152/jn.1993.70.5.2161. [DOI] [PubMed] [Google Scholar]
- Kauer JS, Cinelli AR. Are there structural and functional modules in the vertebrate olfactory bulb? Microsc Res Tech. 1993;24:157–167. doi: 10.1002/jemt.1070240207. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Murphy SJ, Hornung DE. The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. J Neurosci. 1996;16:345–353. doi: 10.1523/JNEUROSCI.16-01-00345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Youngentob SL, Yurco P. Mucosal activity patterns as a basis for olfactory discrimination: comparing behavior and optical recordings. Brain Res. 2003;981:1–11. doi: 10.1016/s0006-8993(03)02512-5. [DOI] [PubMed] [Google Scholar]
- Keyhani K, Scherer PW, Mozell MM. A numerical model of nasal odorant transport for the analysis of human olfaction. J Theor Biol. 1997;186:279–301. doi: 10.1006/jtbi.1996.0347. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Touhara K. Induction of c-fos expression in mouse vomeronasal neurons by sex-specific non-volatile pheromone(s) Chem Senses. 2005;30:i146–i147. doi: 10.1093/chemse/bjh156. [DOI] [PubMed] [Google Scholar]
- Laska M, Freyer D. Olfactory discrimination ability for aliphatic esters in squirrel monkeys and humans. Chem Senses. 1997;22:457–465. doi: 10.1093/chemse/22.4.457. [DOI] [PubMed] [Google Scholar]
- Laska M, Galizia CG. Enantioselectivity of odor perception in honeybees (Apis mellifera carnica) Behav Neurosci. 2001;115:632–639. doi: 10.1037/0735-7044.115.3.632. [DOI] [PubMed] [Google Scholar]
- Laska M, Hübener F. Olfactory discrimination ability for homologous series of aliphatic ketones and acetic esters. Behav Brain Res. 2001;119:193–201. doi: 10.1016/s0166-4328(00)00348-x. [DOI] [PubMed] [Google Scholar]
- Laska M, Teubner P. Olfactory discrimination ability for homologous series of aliphatic alcohols and aldehydes. Chem Senses. 1999;24:263–270. doi: 10.1093/chemse/24.3.263. [DOI] [PubMed] [Google Scholar]
- Laska M, Galizia CG, Giurfa M, Menzel R. Olfactory discrimination ability and odor structure-activity relationships in honeybees. Chem Senses. 1999;24:429–438. doi: 10.1093/chemse/24.4.429. [DOI] [PubMed] [Google Scholar]
- Laska M, Ayabe-Kanamura S, Hübener F, Saito S. Olfactory discrimination ability for aliphatic odorants as a function of oxygen moiety. Chem Senses. 2000;25:189–197. doi: 10.1093/chemse/25.2.189. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66:497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001a;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001b;115:826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezler M, Fleischer J, Breer H. Characteristic features and ligand specificity of the two olfactory receptor classes from Xenopus laevis. Exp Biol. 2001;204:2987–2997. doi: 10.1242/jeb.204.17.2987. [DOI] [PubMed] [Google Scholar]
- Moulton DG. Spatial patterning of response to odors in the peripheral olfactory system. Physiol Rev. 1976;56:578–593. doi: 10.1152/physrev.1976.56.3.578. [DOI] [PubMed] [Google Scholar]
- Mozell MM. Evidence for sorption as a mechanism of the olfactory analysis of vapours. Nature. 1964;203:1181–1182. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- Mozell MM. The spatiotemporal analysis of odorants at the level of the olfactory receptor sheet. J Gen Physiol. 1966;50:25–41. doi: 10.1085/jgp.50.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell MM. Evidence for a chromatographic model of olfaction. J Gen Physiol. 1970;56:46–63. doi: 10.1085/jgp.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science. 1973;181:1247–1249. doi: 10.1126/science.181.4106.1247. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Sheehe PR, Hornung DE, Kent PF, Youngentob SL, Murphy SJ. “Imposed” and “inherent” mucosal activity patterns. Their composite representation of olfactory stimuli. J Gen Physiol. 1987;90:625–650. doi: 10.1085/jgp.90.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak EH. Multiple profile-multiple receptor site model for vertebrate olfaction. J Theor Biol. 1973;40:469–484. doi: 10.1016/0022-5193(73)90005-2. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci. 1999;11:3970–3982. doi: 10.1046/j.1460-9568.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Sanz G, Schlegel C, Pernollet JC, Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem Senses. 2005;30:69–80. doi: 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TA, Knott TK. NADPH diaphorase activity in olfactory receptor neurons and their axons conforms to a rhinotopically-distinct dorsal zone of the hamster nasal cavity and main olfactory bulb. J Chem Neuroanat. 2002;24:269–285. doi: 10.1016/s0891-0618(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Knott TK. Evidence for the disproportionate mapping of olfactory airspace onto the main olfactory bulb of the hamster. J Comp Neurol. 2004;476:186–201. doi: 10.1002/cne.20218. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Clancy AN, Forbes WB, Macrides F. The spatial organization of the peripheral olfactory system of the hamster. Part I: Receptor neuron projections to the main olfactory bulb. Brain Res Bull. 1994;34:183–210. doi: 10.1016/0361-9230(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Scott JW, Davis LM, Shannon D, Kaplan C. Relation of chemical structure to spatial distribution of sensory responses in rat olfactory epithelium. J Neurophysiol. 1996;75:2036–2049. doi: 10.1152/jn.1996.75.5.2036. [DOI] [PubMed] [Google Scholar]
- Scott JW, Brierley T, Schmidt FH. Chemical determinants of the rat electro-olfactogram. J Neurosci. 2000;20:4721–4731. doi: 10.1523/JNEUROSCI.20-12-04721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Johnson PE, Blakley D, Scott JW. Effects of air flow on rat electroolfactogram. Chem Senses. 2000;25:761–768. doi: 10.1093/chemse/25.6.761. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 2000;84:3036–3042. doi: 10.1152/jn.2000.84.6.3036. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:1241–1233. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rogers M, Tian H, Zhang X, Zou DJ, Liu J, Ma M, Shepherd GM, Firestein SJ. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proc Natl Acad Sci U S A. 2004;101:14168–14173. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]