Abstract
The metabolic syndrome (MS), a clustering of metabolic disturbances, is associated with increased cardiovascular risk. Limited information is available about the relations between MS, insulin resistance and vascular function. We measured brachial artery flow-mediated dilation (n=2123), and reactive hyperemia (n=1521) in Framingham Offspring participants without diabetes or clinical cardiovascular disease (mean age 59±9 years, 57% women). MS, determined by National Cholesterol Education Program criteria, was present in 36% of participants. Insulin resistance was determined using Homeostatic Model Assessment (HOMA-IR). In age- and sex-adjusted models, MS was associated with lower flow-mediated dilation and reactive hyperemia. There was progressively lower vasodilator function with increasing number of MS components (p for trend <0.0001). In multivariable models adjusting for the 5 MS components as continuous variables, MS (presence vs. absence) remained associated with lower flow-mediated dilation (2.84±0.12% vs. 3.17±0.08%, p=0.0496) and reactive hyperemia (50.8±1.0 cm/sec vs. 54.4±0.7cm/sec, p=0.009). Insulin resistance was inversely associated with flow-mediated dilation and reactive hyperemia in age- and sex-adjusted models, but these relations were no longer significant in models adjusting for the MS components. In conclusion, our observations are consistent with the hypothesis that MS and insulin resistance impair vascular function predominantly through the influence of the component metabolic abnormalities that comprise MS.
Keywords: Metabolic Syndrome X, endothelium, epidemiology, risk factors
The metabolic syndrome (MS), a clustering of atherogenic metabolic abnormalities, has emerged as an important determinant of cardiovascular risk.1,2 Major components of the MS include central obesity, hypertriglyceridemia, low high-density lipoprotein levels, elevated blood pressure, and fasting hyperglycemia.3 Insulin resistance may be a common feature that links these physical and biochemical changes.4 Recent estimates indicate that over one third of the adult US population fulfills the criteria for the MS.5 Endothelial dysfunction, characterized by decreased nitric oxide bioavailability, is a key component of atherogenesis and promotes cardiovascular events.6 Prior studies have associated individual MS components with impaired endothelial vasomotor function.7–9 Furthermore, several lines of evidence connect the underlying pathophysiological state of insulin resistance to lower nitric oxide bioactivity and decreased endothelium-dependent vasodilation.10 We hypothesized that the MS and insulin resistance would be accompanied by perturbation of endothelial vasomotor function before the development of diabetes or clinical cardiovascular disease. Accordingly, we evaluated the relation between MS, insulin resistance and endothelial vasodilator function of the brachial artery among participants without diabetes mellitus or clinically manifest cardiovascular disease in the community-based Framingham Heart Study.
Methods
Participants
The design for the Framingham Offspring Study has been described elsewhere.11 Of the 3539 participants in the 7th examination cycle (1998–2001), 2883 participants had adequate flow-mediated dilation measurements as described previously.7 Individuals with prior clinical cardiovascular disease (n=263), diabetes mellitus defined as fasting glucose ≥ 126 mg/dl (7.0 mmol/l) or use of hypoglycemic medication (n=382), or missing covariate data (n=115) were excluded, leaving a total of 2123 subjects for investigation of flow-mediated dilation. Prior clinical cardiovascular disease (angina, coronary insufficiency, myocardial infarction, stroke, transient ischemic attack, intermittent claudication, heart failure) was determined by a panel of 3 investigators using previously published criteria.12 The acquisition of Doppler data began part way through the 7th examination; consequently, 1521 eligible subjects had Doppler flow data available.
All participants underwent routine medical history, physical examination, and laboratory assessment. Individuals were instructed not to eat or drink after 8 PM the previous evening, except water or decaffeinated black coffee or tea. Current cigarette smoking (within the past year), or within 6 hours of the test were determined by self-report. Heart rate and blood pressure were measured by automatic device (Dinamap, Critikon, Inc.). The examination included a 6-minute treadmill test (Bruce protocol Stages I and II; referred to as the ‘walk test’) in participants without contraindications (known coronary heart disease, chest pain on test day, or inability to perform test). The Boston Medical Center Institutional Review Board approved the study; all participants provided written informed consent.
Determination of Flow-Mediated Dilation and Reactive Hyperemia
The methodology and reproducibility for measuring brachial artery flow-mediated dilation and reactive hyperemia have been previously described.7,13 Briefly, images of the brachial artery at baseline and 1 minute after induction of reactive hyperemia by a 5-minute cuff occlusion of the forearm were recorded by high resolution ultrasound (Toshiba #SSH-140A, 7.5 MHz linear array transducer). Arterial images were measured off-line using commercially available software (Brachial Analyzer, Medical Imaging Applications, Iowa City, Iowa, version3.2.3.sp2) by sonographers blinded to participants’ status. Doppler flow was assessed at baseline and for 15 seconds following cuff release using a carrier frequency of 3.75 MHz. Mean flow velocity at baseline and during reactive hyperemia was analyzed with correction for insonation angle using a semi-automated signal-averaging method, blinded to clinical information.13
Determination of Metabolic Syndrome
MS was defined as the presence of 3 or more of the following 5 components, based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel-III guidelines: 1) abdominal obesity (waist circumference: men ≥ 40 inches, women ≥ 35 inches), 2) elevated triglycerides (≥ 150 mg/dl), 3) low HDL cholesterol (<40mg/dl in men, <50mg/dl in women), 4) elevated blood pressure (systolic or diastolic blood pressure ≥130/85 mmHg or use of antihypertensive medication), and 5) elevated fasting glucose.3 We used a glucose threshold of ≥ 100mg/dl and <126mg/dl, rather than the NCEP threshold of ≥ 110 mg/dl, based on a revision of the blood glucose criterion for the MS.14
Assessment of Insulin Resistance
Fasting blood samples were obtained from participants at the 7th examination visit and were stored at −80°C. Plasma glucose was measured in fresh specimens with a hexokinase reagent kit (A-Gent Glucose Test, Abbott). Glucose assays were run in duplicate; the intra-assay coefficient of variation was <3%. Fasting plasma insulin was measured with a specific insulin level having essentially no cross-reactivity to insulin split-products (Linco Research, Inc., St. Charles, MO); assay CVs <6.8%.15 Insulin resistance was estimated using the homeostasis model assessment-insulin resistance (HOMA-IR) from fasting glucose and insulin concentrations using the following formula: HOMA-IR=(fasting plasma insulin[microU/ml])×(fasting plasma glucose[mmol/l])/22.5.16 The HOMA-IR method has been validated by comparison with results of glucose clamp studies and frequently sampled intravenous glucose tolerance tests.16 We defined insulin resistance as HOMA-IR>4.6 representing the upper quartile of HOMA-IR in this study sample.17
Statistical Analysis
Descriptive characteristics were tabulated by the presence or absence of MS. Age- and sex-adjusted means of brachial artery measures were compared between participants with and without MS and insulin resistance. Sequential multivariable linear regression models were constructed to assess the relations of MS and insulin resistance with brachial artery measures adjusting for clinical covariates and the components of MS. Model 1 included clinical covariates previously reported to be associated with flow-mediated dilation in this cohort, excluding those directly associated with the MS components; hence, we included age, heart rate, total cholesterol as continuous variables and sex, hormone replacement therapy, lipid lowering therapy, smoking 6 hours prior to test, and walk test (before/after) vascular examination as categorical variables.7 In multivariable Model 2, the Model 1 covariates were entered together with the 5 individual components of the MS as continuous variables (waist circumference, HDL, systolic blood pressure, diastolic blood pressure, triglycerides, and fasting glucose, and dichotomous hypertensive treatment). Model 3 included the Model 1 covariates and the Model 2 MS components as continuous variables plus MS (Model 3A) or insulin resistance (Model 3B) entered as dichotomous variables. The R2 is presented for each sequential model; and the p values in Table 3 are derived from the F-tests of the increment in R2 across sequential models. Regression diagnostics, specifically variance inflation factor, were examined for MS and for insulin resistance in the multivariable models. A priori we specified two secondary analyses to examine effect modification by age (≥60 vs. <60) and sex. Analyses were performed using SAS 8.1.18 Two-sided p values <0.05 were considered statistically significant.
Table 3.
Relations of Metabolic Syndrome and Insulin Resistance with Vascular Function Measures: Multivariable Models
| Baseline brachial diameter | Flow-mediated dilation mm | Flow-mediated dilation % | Baseline mean flow velocity | Hyperemic mean flow velocity | ||
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Age, sex, heart rate, lipid lowering treatment, hormone replacement therapy, smoking 6 hours prior to test, total cholesterol, and walk test | R2 * | 0.551 | 0.084 | 0.116 | 0.096 | 0.208 |
| Model 2 | R2 | 0.582 | 0.123 | 0.158 | 0.139 | 0.270 |
| Model 1 + MS components (continuous: waist circumference, triglycerides, HDL, systolic blood pressure, diastolic blood pressure, fasting glucose; dichotomous: hypertensive treatment) | p value† | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Model 3A | R2 | 0.584 | 0.124 | 0.160 | 0.140 | 0.273 |
| Model 2 + MS | p value‡ | 0.001 | 0.17 | 0.0496 | 0.49 | 0.009 |
| Yes | Least Square Mean (SE) | 4.28mm (0.03) | 0.115mm (0.005) | 2.84% (0.12) | 7.9cm/sec (0.3) | 50.8cm/sec (1.0) |
| No | Least Square Mean (SE) | 4.16mm (0.02) | 0.124mm (0.003) | 3.17% (0.08) | 8.2cm/sec (0.2) | 54.4cm/sec (0.7) |
| Model 3B | R2 | 0.582 | 0.124 | 0.158 | 0.142 | 0.271 |
| Model 2 + Insulin Resistance | p value‡ | 0.61 | 0.55 | 0.55 | 0.02 | 0.08 |
| Yes | Least Square Mean (SE) | 4.22mm (0.03) | 0.118mm (0.005) | 2.97% (0.13) | 8.7cm/sec (0.3) | 51.4cm/sec (1.1) |
| No | Least Square Mean (SE) | 4.20mm (0.01) | 0.122mm (0.003) | 3.07% (0.07) | 7.9cm/sec (0.1) | 53.7cm/sec (0.5) |
Brachial artery diameter and flow-mediated dilation n=2123; Doppler flow response n=1521.
R2 is the proportion of the variability for each vascular measure that is explained by regression on specified covariates
These p values test for an incremental change in R2 between Model 1 and Model 2
These p values test for an incremental change in R2 between Model 2 and Model 3
Results
Participant Characteristics and Metabolic Syndrome
The clinical characteristics of the study participants (mean age 59±9 years, 57% women) classified by MS are shown in Table 1. MS was present in over one third of the individuals, similar to a recent estimate for the prevalence of MS in the adult US population.5 The measure of insulin resistance, HOMA-IR, was higher in participants with versus without the MS (see Table 1, p<0.0001). Similarly, the prevalence of insulin resistance (defined as upper quartile of HOMA-IR) was higher in the participants with versus without MS (52% vs. 10%).
Table 1.
Participant Characteristics
| Metabolic Syndrome | ||
|---|---|---|
| Characteristic (units) | Yes (n=762) | No (n=1361) |
| Age (years)* | 61±9 | 58±9 |
| Women* | 51% | 60% |
| Waist circumference (cm)* | 107±11 | 93±12 |
| Systolic blood pressure (mm Hg)* | 133±18 | 121±17 |
| Diastolic blood pressure (mmHg)* | 77±10 | 73±9 |
| Total cholesterol/High-density lipoprotein (ratio)* | 4.7±1.3 | 3.5±1.0 |
| Triglycerides (mg/dL)* | 177±89 | 101±52 |
| Fasting glucose (mg/dL)* | 103±9 | 94±8 |
| Homeostatic Model Assessment of Insulin Resistance* | 5.3±3.0 | 2.9±1.5 |
| Insulin resistance*† | 52% | 10% |
| Body mass index (kg/m2)* | 30.5±4.8 | 26.1±4.3 |
| Hypertension* | 64% | 23% |
| Heart rate (beats per minute)* | 66±10 | 63±10 |
| Antihypertensive therapy* | 43% | 15% |
| Current Smoking* | 9% | 15% |
| Smoking 6 hrs prior to test* | 6% | 10% |
| Hormone replacement therapy (women)* | 31% | 34% |
| Lipid lowering medication* | 21% | 10% |
| Walk test: | ||
| Before brachial testing* | 36% | 46% |
| After brachial testing | 42% | 42% |
Continuous variables, mean±SD;
p value<0.05 Yes vs. No Metabolic Syndrome;
Insulin resistance defined as upper quartile of HOMA-IR in this study sample
Metabolic syndrome and vascular function
Age- and sex-adjusted values for baseline brachial artery diameter, flow-mediated dilation (mm), flow-mediated dilation %, baseline flow velocity and hyperemic flow velocity by MS status are presented in Table 2. MS was associated with lower flow mediated-dilation (mm), flow-mediated dilation %, and hyperemic flow velocity. As we have reported previously that hyperemic flow is a major determinant of flow-mediated dilation,13 we also analyzed flow-mediated dilation % adjusted for hyperemic flow velocity, which remained lower in participants with MS than in those without MS (2.74±0.11% vs 3.22±0.08 respectively, p=0.0005). Finally, MS was associated with higher resting brachial artery diameter and baseline flow velocity.
Table 2.
Measures of Vascular Function in Metabolic Syndrome and Insulin Resistance: Age- and Sex-Adjusted Model
| Metabolic Syndrome | Insulin Resistance | |||||
|---|---|---|---|---|---|---|
| Characteristic (units) | Yes | No | p value | Yes | No | p value |
| Baseline brachial diameter (mm) | 4.36±0.02 | 4.12±0.02 | <0.0001 | 4.35±0.03 | 4.16±0.01 | <0.0001 |
| Flow-mediated dilation (mm) | 0.107±0.004 | 0.128±0.003 | <0.0001 | 0.112±0.005 | 0.123±0.003 | 0.038 |
| Flow-mediated dilation (%) | 2.61±0.10 | 3.29±0.07 | <0.0001 | 2.75±0.12 | 3.15±0.07 | 0.004 |
| Baseline mean flow velocity (cm/sec) | 9.0±0.2 | 7.6±0.2 | <0.0001 | 9.6±0.3 | 7.6±0.1 | <0.0001 |
| Hyperemic mean flow velocity (cm/sec) | 49.9±0.8 | 54.9±0.6 | <0.0001 | 50.3±1.0 | 54.0±0.5 | 0.002 |
Values expressed as mean±standard error
Brachial artery diameter and flow-mediated dilation n=2123
Doppler flow response n=1521
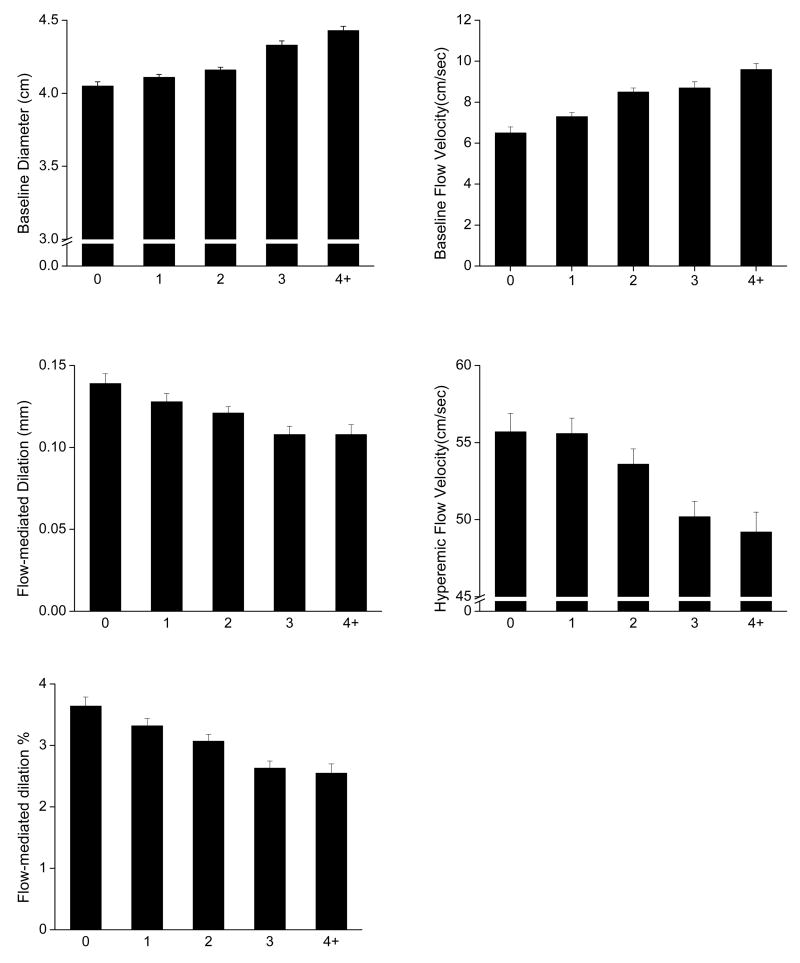
The relation between the MS components and vasodilator function is displayed in Figure 1. Flow-mediated dilation (mm), flow-mediated dilation % and hyperemic flow velocity were lower in the presence of increasing number of MS components (p<0.0001 for trend for each measure). Conversely, baseline brachial diameter and baseline flow velocity were progressively higher with increasing number of MS components, Figure 1 (p<0.0001 for trend for both).
Figure 1.
Age- and sex-adjusted means and standard errors for vascular function measures according to number of MS components. For brachial artery diameter and flow-mediated dilation n=337, 479, 545, 462, 300 and for baseline flow velocity and hyperemic flow velocity n=257, 372, 374, 322, 196 for 0, 1, 2, 3, and 4+ components respectively. Error bars represent one standard error. P value for trend <0.0001 for each measure.
The associations between the MS components and MS with the brachial artery measures in multivariable regression models are presented in Table 3. Model 1 adjusted for clinical covariates previously reported to be associated with flow-mediated dilation in this cohort,7 excluding those covariates used to define the MS. In Model 2, the addition of the 5 MS components as continuous measures improved the model R2 (a representation of the proportion of the variability for each measure that is explained by regression on the model covariates). For baseline diameter, flow-mediated dilation (mm), flow-mediated dilation %, baseline flow velocity and hyperemic flow velocity; the change in model R2 from model 1 to model 2 was 0.031, 0.039, 0.042, 0.043 and 0.062, respectively. In Model 3A, after adjusting for the MS components, additional adjustment for the MS, per se, resulted in a statistically significant, but minimal improvement in the model R2 for baseline diameter, flow-mediated dilation %, and hyperemic flow velocity (change in model R2 from model 2 to model 3A, 0.002, 0.002 and 0.003, respectively) reflecting modestly larger baseline diameter and lower flow-mediated dilation and hyperemic flow velocity associated with MS. Collinearity was not a problem between MetS and its component variables (variance inflation factor for MetS was 2.0 in Model 3A). For Model 3A, there were no significant age or sex interactions with MS in relation to the vasodilator measures.
Insulin Resistance and Vascular Function
In age- and sex-adjusted models, insulin resistance was associated with lower flow-mediated dilation (mm), flow-mediated dilation %, and hyperemic flow velocity (Table 2). After adjusting for hyperemic flow velocity, there was no significant difference in flow-mediated dilation % between those with (2.89±0.14) and those without (3.11±0.07, p=0.15) insulin resistance. Insulin resistance also was associated with larger baseline brachial artery diameter and higher baseline flow. After adjusting for clinical covariates including the MS components, insulin resistance was no longer associated with any of the vasodilation measures, although insulin resistance remained associated with higher baseline flow (Table 3, Model 3B). Collinearity was not a problem between insulin resistance and MetS component variables (variance inflation factor for insulin resistance was 1.5 in Model 3B). In Model 3B, there were no significant age or sex interactions with insulin resistance in relation to the vascular function measures examined. Given the possibility that examining HOMA-IR as a dichotomous variable may underestimate an association with the vascular measures, we performed a secondary analysis entering log HOMA-IR as a continuous variable in Model 3B. As with HOMA-IR as categorical variable, in this analysis log HOMA-IR was associated only with higher baseline flow (model R2 0.149, p<0.0001).
Discussion
In our large community-based cohort, we observed an inverse relation between the MS and flow-mediated dilation, a measure of conduit artery vasodilator function, and reactive hyperemia, a measure of microvascular function, in individuals without diabetes or clinically apparent cardiovascular disease. Our data suggest that the relation between the MS and vasodilator dysfunction is largely attributable to the cumulative contribution of the component risk factors that comprise MS, because only a minimal relation between MS and the vasodilator function measures remained after adjustment for the component risk factors. We also observed lower vasodilator function in participants with insulin resistance, as reflected by higher levels of HOMA-IR, in age- and sex-adjusted models; however, these relations were no longer significant after adjusting for risk factors including the MS components.
It is well established that the individual components of the MS are related to endothelial dysfunction. Previous studies have shown that obesity, low HDL, impaired glucose tolerance, hypertriglyceridemia, and hypertension are all associated with decreased endothelium-dependent vasodilation.7–9,19 Furthermore treatments targeted at these conditions improve endothelial function.20,21 In select samples, MS itself has been associated with impaired endothelial function; yet, these studies had limited statistical power to adjust for the component conditions of MS.22 Recent epidemiological evidence has raised the possibility that the grouping of the MS components in an individual may confer heightened cardiovascular risk.1,2 Conversely, it has been argued that MS may confer little or no additional risk above and beyond that associated with the component risk factors.23,24 Our findings suggest that the association of MS with endothelial dysfunction is predominantly mediated through the component conditions with only minimal incremental contribution of the clustering in MS.
Prior studies suggest that insulin resistance also is associated with vascular dysfunction.10,25 An association between impaired endothelial function and insulin resistance has been shown in select samples of subjects. For example, in young obese individuals, microvascular vasodilatory function has been correlated with insulin sensitivity.26 In addition, Balletshofer and colleagues demonstrated decreased brachial artery flow-mediated dilation in insulin resistant offspring of individuals with diabetes.27 Lteif and colleagues showed an association of insulin resistance with leg microvessel vasodilation in young subjects including a group with MS.22 Finally, endothelial dysfunction predicts the development of diabetes suggesting a link between endothelial abnormalities and insulin resistant states.28
In contrast to these prior studies, we observed that the association between insulin resistance and vascular dysfunction was no longer apparent after adjusting for concurrent risk factors, including the MS components. These apparently discrepant results have several possible explanations. Population differences between previous reports and our study, including older age and relatively low flow-mediated dilation in our cohort, may have made it more difficult to detect a relation between insulin resistance and vascular dysfunction. We consider this possibility unlikely as we observed a strong relation in the age- and sex-adjusted models and a similar pattern was observed for reactive hyperemia. Our study also differs from prior studies because of the larger sample size, which permitted more complete adjustment for confounders. Our findings may reflect the strong links between the components of MS and insulin resistance. In support of this possibility, we observed significantly higher HOMA-IR in patients with the MS (Table 1). Whereas it is not possible to draw conclusions about causality in our cross-sectional study, we speculate that the MetS components may represent intermediate mechanisms linking insulin resistance and vascular dysfunction. Alternatively, insulin resistance may represent a common pathophysiological mechanism that underlies vascular dysfunction and the MetS component conditions.
Our study had several interesting findings regarding resting arterial characteristics, MS, and insulin resistance. MS was associated with increased brachial diameter suggesting the presence of arterial remodeling. The larger arterial diameter may reflect a response to sustained elevations in flow; we observed an association of the MS components with higher basal flow rate.30 Alternatively, the increased arterial diameter may reflect flow-independent changes in arterial wall structure or relate to the vasodilator properties of insulin.29 Interestingly, insulin resistance was associated with increased basal flow without an increase in arterial diameter in adjusted models. This finding raises the possibility that insulin resistance might be associated with a defect in flow-induced remodeling.
The present study has a number of limitations. The cross-sectional design of our study precludes the establishment of causal or temporal relations among MS, insulin resistance and endothelial dysfunction. We estimated insulin resistance using HOMA-IR; more direct measurement of insulin resistance might have revealed a stronger independent effect of insulin resistance on vascular function. We did not include lipid lowering treatment (niacin and fibrates) in our determination of MetS;14 however, the percentage of participants using these medications was low (1.4%). Due to the community-based design of our study, we were unable to administer nitroglycerin to the participants; therefore, we do not have a measure of endothelium-independent vasodilation. Although we adjusted for antihypertensive medications, we acknowledge different classes of such medications vary in their relation to endothelial function. Our study cohort was predominantly white, and middle-aged to elderly; therefore, our findings may not be generalizable to other ethnic/racial groups, nor to younger individuals. In balance to these limitations, our study has several strengths including a large sample size and routine ascertainment of coexistent cardiovascular risk factors that facilitate adjustment for multiple covariates and provides excellent power. In addition, the community-based design reduces selection and referral biases. In summary, our findings are consistent with the hypothesis that MS and insulin resistance impair vascular function predominantly through the influence of the metabolic risk factors that constitute MS.
Acknowledgments
This study was supported by the grants N01-HC-25195, HL060040, HL70100, 2K24-HL-04334 from the NHLBI, Bethesda, Maryland and the Donald W. Reynolds Foundation, Las Vegas, Nevada. Dr. Meigs is supported by a Career Development Award from the American Diabetes Association, Alexandria, Virginia. Dr. Hamburg is supported by an ACCF/Merck Fellowship Award, Washington, DC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Girman CJ, Rhodes T, Mercuri M, Pyorala K, Kjekshus J, Pedersen TR, Beere PA, Gotto AM, Clearfield M. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93:136–141. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 6.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez CJ, Miyake Y, Grahame-Clarke C, Di Tullio MR, Sciacca RR, Boden-Albala B, Sacca RL, Homma S. Relation of plasma glucose and endothelial function in a population-based multiethnic sample of subjects without diabetes mellitus. Am J Cardiol. 2005;96:1273–1277. doi: 10.1016/j.amjcard.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 9.Kuvin JT, Patel AR, Sidhu M, Rand WM, Sliney KA, Pandian NG, Karas RH. Relation between high-density lipoprotein cholesterol and peripheral vasomotor function. Am J Cardiol. 2003;92:275–279. doi: 10.1016/s0002-9149(03)00623-4. [DOI] [PubMed] [Google Scholar]
- 10.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2004;20:255–268. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.Abbott RD, McGee DL. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 37: The Probability of Developing Certain Cardiovascular Diseases in Eight Years at Specified Values of Some Characteristics. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 13.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Dupuis J, Herbert AG, Liu C, Wilson PW, Cupples LA. The insulin gene variable number tandem repeat and risk of type 2 diabetes in a population-based sample of families and unrelated men and women. J Clin Endocrinol Metab. 2005;90:1137–1143. doi: 10.1210/jc.2004-1212. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 18.SAS Institute Inc. SAS Procedures Guide, Version 8. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 19.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT. Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2004;93:362–365. doi: 10.1016/j.amjcard.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 22.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 23.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 24.Sundstrom J, Vallhagen E, Riserus U, Byberg L, Zethelius B, Berne C, Lind L, Ingelsson I. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29:1673–1674. doi: 10.2337/dc06-0664. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 27.Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Haring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101:1780–1784. doi: 10.1161/01.cir.101.15.1780. [DOI] [PubMed] [Google Scholar]
- 28.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45:623–634. doi: 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- 30.Tulis DA, Unthank JL, Prewitt RL. Flow-induced arterial remodeling in rat mesenteric vasculature. Am J Physiol. 1998;274:H874–H882. doi: 10.1152/ajpheart.1998.274.3.H874. [DOI] [PMC free article] [PubMed] [Google Scholar]