Abstract
In the present report we compared the insulin secretory responses of freshly isolated perifused rat and mouse islets to glucose. Prestimulatory glucose levels were changed to assess its influence on the subsequent secretory responses. Additional studies included experiments with the incretin factor GLP-1, the cholinergic agonist carbachol and the α2 agonist epinephrine. Our findings demonstrate that under conditions where glucose (8.5–11.1 mM) evokes a dramatic biphasic insulin secretory response from perifused rat islets, mouse islets exhibit little response. Increasing the prestimulatory glucose level to 8.5 mM dramatically distorts subsequently measured glucose-induced insulin secretion from rat islets but allows the evocation of a modest but clear biphasic response from mouse islets in response to 30 mM, but not 11.1 or 16.7 mM glucose. In the presence of a minimally effective glucose level (10 mM), mouse islets remain exquisitely sensitive to the combined stimulatory effects of GLP-1 (2.5 nM) plus carbachol (0.5 μM) and to the inhibitory influence of epinephrine (10 nM). Short term culture of rat islets in CMRL-1066 containing 5.6 mM glucose resulted in a significant decrease in the secretory response to 11.1 mM glucose while the same manipulation improved mouse islet responses. It is concluded that the process of collagenase isolating islets does not alter mouse islet sensitivity in any adverse way and that increasing the prestimulatory glucose level can indeed alter the pattern of insulin secretion in either a positive or negative manner depending upon the species being investigated. Prior short term culture of rodent islets differentially affects secretion from these two species.
Keywords: islets, secretion, GLP, 1, carbachol, epinephrine
Introduction
Glucose-induced secretion from rat pancreatic β-cells, studied using a number of different preparations including the perfused pancreas 1–7 and perifused islets 8–10, is biphasic in nature. It is characterized by an initial burst of insulin, lasting several minutes, followed by a brief nadir and a slowly rising and sustained second phase response. The magnitude of the peak second phase response to sustained maximal hyperglycemic stimulation is on average about 20–40 fold greater than prestimulatory rates of secretion measured in the presence of substimulatory glucose concentrations. It is not yet clear which of the many metabolic, cationic or second messenger events underlie biphasicity, although a number of different studies 11–16 suggest that information flow in the phospholipase C (PLC)/protein kinase C (PKC) pathway may be particularly important.
The biphasic response of rat islets to hyperglycemic stimulation is well established, having been reported on and confirmed by numerous groups. However, the pattern of insulin release evoked from mouse β-cells during sustained stimulation with high glucose differs significantly from that usually noted from rat β-cells. It is characterized by a flat sustained second phase response that is only modestly elevated above prestimulatory rates 5, 6, 12, 13, 17–20. Recently, two separate studies have attempted to explain and/or reconcile the raison d’etre for this species dichotomy in glucose-induced insulin release using a number of different approaches. The first report by Nunemaker and colleagues 21 suggested that part of this difference from rat islets may be a consequence of the isolation procedure. In the second report on this topic, Henquin and coworkers 22 suggested that the prestimulatory glucose level played a previously unrecognized but important role in determining the subsequent pattern of secretion in response to glucose. In neither of these reports, however, comparative studies employing rat islets were not performed using identical protocols, thus excluding a direct comparison of rodent islet responses under similar experimental conditions from the same laboratory. In the present report, we compared glucose-induced insulin secretion from freshly isolated, noncultured, perifused rodent islets. Our findings emphasize the complexity of stimulus-response coupling patterns in rodent islets and the contribution of both the antecedent glucose concentration and culturing on their subsequent secretory responsiveness. Under identical experimental conditions however, profound species differences to glucose stimulation were still evident.
Material and Methods
The detailed methodologies employed to assess insulin output from collagenase-isolated islets have been previously described 23, 24. Male Sprague-Dawley rats (weighing 325–425 g at the time of study) and male CD-1 mice (weighing 28–40 g at the time of study) were purchased from Charles River and used in all studies. All animals were treated in a manner that complied with the NIH Guidelines for the Care and Use of Laboratory Animals. The animals were fed ad lib. After Nembutal (pentobarbital sodium, 50 mg/kg; Abbott, North Chicago, IL) -induced anesthesia, 20–30 ml of cold Hanks’ solution (without any added glucose) was used to distend the pancreas via the biliary system. Islets were isolated by collagenase digestion and handpicked, using a glass loop pipette, under a stereomicroscope into Krebs-Ringer Bicarbonate (KRB) supplemented with 3mM glucose. They were free of exocrine contamination.
Perifusion studies
Islets were loaded onto nylon filters (Sefar America Inc., Kansas City, MO) immediately after the isolation, a process that took no longer than 90 minutes from the initiation of the pancreatectomy. The islets were perifused in a KRB buffer at a flow rate of 1ml (±0.1 ml)/minute for 30 minutes in the presence of various glucose concentrations to establish stable insulin secretory rates. After this 30 min stabilization period they were then perifused with the appropriate agonist or agonist combinations as indicated in the figure legends and Results section. In some experiments, GLP-1 and carbachol were included during the initial 30-minute period. The first phase response was that observed during the initial 10 minutes of stimulation (not corrected for the dead space in the perifusion apparatus) and the second phase response was that occurring during the final 30 minutes of stimulation. Perifusate solutions were gassed with 95% O2/5% CO2 and maintained at 37°C. Insulin released into the medium was measured by radioimmunoassay 25. At the termination of the experiment, total islet insulin content was measured and in some figures secretion is expressed as the fractional insulin secretion rate. This was calculated as the percent of islet insulin content that was secreted/minute.
Total Islet Insulin
After the perifusion, the islets still on filters were retrieved and placed in small glass vials. Hanks’ solution (240 μL) was gently added and the samples were sonicated for 20 seconds. Aliquots were then frozen for the subsequent analysis of insulin content.
Cultured Islet Studies
In a number of experiments, isolated rodent islets were cultured for 3 hours in CMRL 1066 containing 5.6 mM glucose. After this they were perifused as described above and their secretory responses to 11.1 mM glucose determined.
Reagents
Ice-cold Hanks’ solution without any added glucose was used for the islet isolation. The perifusion medium consisted of 115 mM NaCl, 5 mM KCl, 2.2 mM CaCl2, 1 mM MgCl2, 24 mM NaHCO3 and 0.17 g/dl bovine serum albumin. The 125I-labeled insulin for the insulin assay was purchased from PerkinElmer Life Sciences (Boston, MA). Bovine serum albumin (RIA grade), glucose, GLP-1, carbachol, epinephrine bitartrate salt and the salts used to make the Hanks’ solution and perifusion medium were purchased from Sigma (St. Louis, MO). CMRL 1066 (Gibco #11530) was purchased from Invitrogen. Rat insulin standard (lot #615-ZS-157) was the generous gift of Dr. Gerald Gold, Eli Lilly Co. (Indianapolis, IN). Collagenase (Type P) was obtained from Roche Applied Science (Indianapolis, IN).
Statistics
Statistical significance was determined using the Student's t test for unpaired data or analysis of variance. A p value ≤0.05 was taken as significant. Values presented in the figures and results represent means ± SEMs of at least three observations.
Results
Total insulin contents
The total insulin contents of the freshly isolated islets used in these studies, calculated as the amount contained in the islet at the end of the experiment and the amount secreted during the perifusion period, averaged 182±8 (n=44) and 190± 9 (n=35) ng/islet for rat and mouse islets respectively.
Stimulation of rodent islets with 8.5 mM glucose
Most investigators, including ourselves, generally perifuse islets with substimulatory glucose concentrations for 20–30 minutes prior to stimulating them with higher glucose levels. This is done to establish basal, stable insulin secretory rates. The subsequent response to hyperglycemia can then be conveniently assessed, compared to prestimulatory rates of insulin secretion and the magnitude of the response ascertained. Since the threshold for glucose-induced secretion is approximately 5.0–5.5 mM 4, 26, 27 a level below this is most often used. In many previous studies with rodent islets we have used 3 mM (54 mg%) glucose. According to Henquin et al 22, the prestimulatory glucose level “can secondarily affect the magnitude and pattern of subsequent glucose-induced insulin secretion”22. As a first step to validate this concept with both rat and mouse islets we initially established how islets from both species respond to 8.5 mM (153 mg%) glucose stimulation when 3 mM glucose was used during the 30 minute prestimulatory stabilization period. We chose 8.5 mM glucose since Henquin and coworkers 22 cultured mouse islets with this glucose level for 18 hrs prior to being studied. The results are given in Figure 1, left panel. The response of rat islets was most robust and release rates increased about 20-fold from 20±2 (n=6) pg/islet/min in the presence of 3 mM glucose to 417±33 pg/islet/min after 40 minutes of stimulation with 8.5 mM glucose (n=6). A sharp dichotomy was noted when mouse islets, isolated and perifused using the exact same experimental protocols, were stimulated with 8.5 mM glucose. Mouse islet responses were notable for their virtual lack of a response under this experimental condition. The most dramatic difference was the failure of mouse islets, in sharp contrast to rat islets, to mount a second phase insulin secretory response. In order to allow a direct comparison between our data and that contained within the report of Henquin and coworkers 22, we also calculated the fractional insulin secretory rates of these islets and this data is presented in Figure 1, right panel. Two points should be made. First, the secretory profile that emerges is identical to that where the actual secretion rates are presented (Figure 1, top and bottom panels). Second, the amount of insulin secreted represents a very small fraction of the total insulin content. For example, even during the robust secretory response to 8.5 mM glucose less than 0.2%/min of the total insulin content is being mobilized.
Figure 1. Effects of prior 3.0 mM glucose on insulin secretion from freshly isolated, perifused rodent islets stimulated with 8.5 mM glucose.

Groups of islets were isolated from rats or mice and perifused immediately after the isolation. For the initial 30 minutes of the perifusion, the glucose level was maintained at 3 mM (G3). For the next 40 minutes, onset indicated by the vertical line, both groups of islets were stimulated with 8.5 mM glucose (G8.5). The actual insulin secretion rates expressed as pg/islet/min (top panel) were measured and the amount of insulin secreted was also calculated as the % of the total islet insulin content (bottom panel). The data point above the 30 min mark on this and all subsequent graphs was collected from minutes 25–30 of the perifusion and reflects the secretory rate prior to switching to a higher glucose concentration. This and subsequent perifusion figures have not been corrected for the dead space, about 2.5 ml or 2.5 minutes with a flow rate of 1 ml/min. Mean values ±SEM of at least 3 experiments were performed under each condition.
Changing the stabilization and stimulatory glucose levels
In the comprehensive report by Henquin and coworkers 22 studying glucose-induced insulin secretion from cultured perifused mouse islets, the glucose level used during the prestimulatory, stabilization period was varied from 3 mM-8.5 mM. In the next series of experiments comparable studies were conducted with both rat and mouse islets. Freshly isolated islets were perifused with either 5 or 8.5 mM glucose for 30 minutes prior to a 40 minute stimulatory period with 11.1, 16.7 or 30 mM glucose, the exact same levels employed in the previous study 22. The results are given in Figures 2–4.
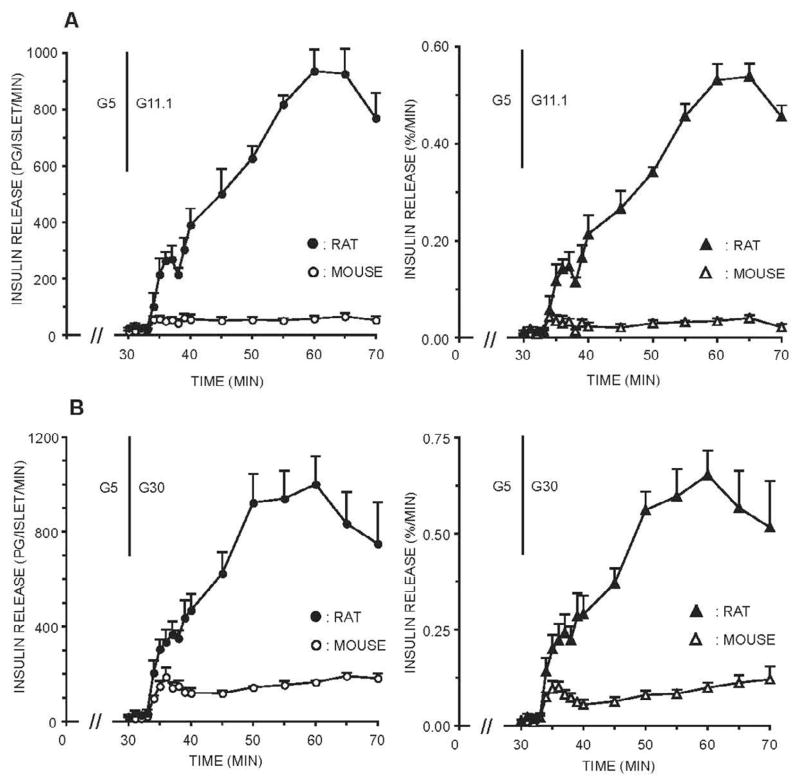
Figure 2. Effects of prior 5.0 mM glucose on insulin secretion from freshly isolated, perifused rodent islets stimulated with 11.1 and 30 mM glucose.
Groups of islets were isolated from rats or mice and perifused immediately after the isolation. For the initial 30 minutes of the perifusion, the glucose level was maintained at 5 mM (G5). For the next 40 minutes (onset indicated by the vertical line), islets were stimulated with 11.1 mM or 30 mM glucose (G8.5; G30). The actual insulin secretion rates expressed as pg/islet/min (left panels, top and bottom) were measured and the amount of insulin secreted was also calculated as the % of the total islet insulin content (right panels, top and bottom).
Figure 4. Effects of prior 5.0 or 8.5 mM glucose on insulin secretion from freshly isolated, perifused rat islets stimulated with 16.7 mM glucose.
Groups of rat islets were isolated and perifused immediately after the isolation. For the initial 30 minutes of the perifusion, the glucose level was maintained at either 5 mM (G5) or 8.5 mM (G8.5). For the next 40 minutes (onset indicated by the vertical line), islets were stimulated with 16.7 mM glucose (G16.7). The actual insulin secretion rates expressed as pg/islet/min (left panel) were measured and the amount of insulin secreted was also calculated as the % of the total islet insulin content (right panel).
When the glucose level was increased from 5 mM to 11.1 mM, perifused rat islets responded with a dramatic biphasic insulin secretory response (Figure 2, top left panel). Prestimulatory release rates of approximately 25 pg/islet/min increased to 269±47 (n=4) pg/islet/min during the first phase response and increased to 700–900 pg/islet/min during the final 20 minutes of stimulation. When compared to prestimulatory rates, a 25–30-fold response was evident. Mouse islet responses differed significantly from rat islet responses. A small first phase response was noted, increasing from prestimulatory rates of approximately 25 pg/islet/min to 69±8 pg/islet/min. In contrast to the large 25-fold increase in sustained second phase release observed from rat islets, a small 3–4 fold response was noted from mouse islets stimulated with 11.1 mM glucose (Figure 2, top left panel). These second phase responses were sustained but flat. Larger first phase and second phase responses were observed during stimulation of rat islets with 30 mM glucose (Figure 2, bottom left panel). At 30 mM glucose, the emergence of a biphasic response from mouse islets was apparent although the magnitude of the second phase response was only about 20% of the rat islet response. When the amount of insulin released was expressed as % total insulin content/min, profiles similar to the actual secretion rates emerged (Figure 2, bottom right panel).
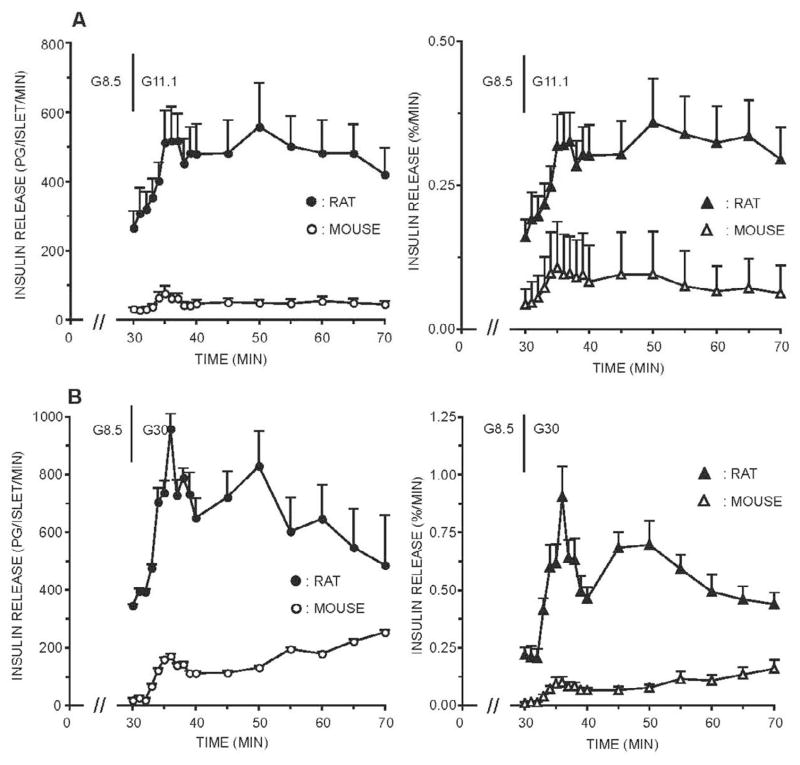
If the glucose level used during the 30 minute stabilization period prior to stimulation with 11.1 or 30 mM glucose was increased to 8.5 mM glucose, an entirely different picture emerged when rat islets were examined (Figures 3 and 4). The intensely stimulatory effect of 8.5 mM glucose on rat islets during the initial 30 minute stabilization period was most obvious and distorted the subsequent secretory response to even higher glucose levels. For example, while stimulation with 11.1 mM glucose after 5 mM glucose resulted in an approximately 30-fold increment in sustained second phase release rates, perifusion with 8.5 mM glucose prior to 11.1 mM glucose resulted in a maximal 2-fold response to 11.1 mM glucose, from 300 to 600 pg/islet/min (Figure 3, left panel). Unlike the response where the prestimulatory glucose level was 5 mM, there was no rising second phase of insulin secretion and the maximal secretory rates were reduced as well. This altered response pattern, a consequence of raising the prestimulatory glucose concentration to 8.5 mM, was also noted if rat islets were subsequently stimulated with 30 mM glucose (Figure 3) or 16.7 mM glucose (Figure 4).
Figure 3. Effects of prior 8.5 mM glucose on insulin secretion from freshly isolated, perifused rodent islets stimulated with 11.1 and 30 mM glucose.
Groups of islets were isolated from rats or mice and perifused immediately after the isolation. For the initial 30 minutes of the perifusion, the glucose level was maintained at 8.5 mM (G8.5). For the next 40 minutes (onset indicated by the vertical line), islets were stimulated with 11.1 mM or 30 mM glucose (G8.5; G30). The actual insulin secretion rates expressed as pg/islet/min (left panels, top and bottom) were measured and the amount of insulin secreted was also calculated as the % of the total islet insulin content (right panels, top and bottom).
Mouse islet responses after an increase in glucose level during the stabilization period to 8.5 mM are also given in Figure 3. After 30 minutes in 8.5 mM glucose, perifusion with 11.1 mM glucose resulted in a small initial first phase and a minimal sustained second phase response (Figure 3). A more vigorous response from mouse islets was observed during stimulation with 30 mM glucose, a response that was somewhat biphasic with a slowly rising second phase response.
After a 30 minute stabilization period with 5 mM glucose, the secretory profile observed from rat islets in response to 16.7 mM glucose was similar to that noted to 30 mM glucose stimulation (Figure 4). The sustained second phase response averaged about 1,000 pg/islet/min (n=5), a more than 30-fold increase from prestimulatory rates of insulin release. Again, the presence of 8.5 mM glucose during the initial 30 minute stabilization period distorted the response of rat islets to 16.7 mM glucose stimulation, as it did to the 11 or 30 mM glucose responses.
Effects of carbachol on 11.1 mM glucose-induced secretion from mouse islets
It might be reasonably argued that mouse islets are indeed more vulnerable to the possible deleterious effects of the collagenase used to isolate them and that at least part of the disparity noted between these two species’ response to glucose is a result of this. An additional experimental manipulation was designed to address this issue. Groups of mouse islets were stimulated with 50 μM carbachol after first being stimulated with 11.1 mM glucose. As shown in Figure 5, although these islets were minimally responsive to glucose alone, a dramatic response to the cholinergic agonist in the presence of this weakly effective glucose concentration was seen. Thus these islets clearly retain their sensitivity to stimulation and peak response rates comparable to those evoked from rat islets stimulated by a maximally effective glucose stimulus were evoked (compare Figures 2 and 5).
Figure 5. Effects of carbachol on insulin secretion rates from mouse islets.

Groups of mouse islets were perifused with 5 mM glucose for 30 minutes and for an additional 70 minutes with 11.1 mM glucose. After 40 minutes with 11.1 mM glucose alone, the islets were subjected to an additional 30 minutes stimulation period with 50 μM carbachol.
Rodent islet responses to GLP-1, carbachol and epinephrine
In the final experiments, we explored the potential contribution of combined GLP-1 and cholinergic stimulation to evocation of biphasic secretion and the impact of the inhibitor epinephrine on these responses. In the report by Nunemaker 21, it proved possible to elicit biphasic secretion in vivo with hyperglycemic stimulation. However, the inclusion of additional agonists for secretion present in vivo but not in vitro may have contributed to this response. In an attempt to address this issue, we perifused mouse islets with 3 mM glucose alone or with 3 mM glucose plus the combination of 0.5 μM carbachol and 2.5 nM GLP-1. The presence of these 2 compounds had no impact on basal release rates at this glucose level (Figure 6). However, upon increasing the glucose level to 10 mM, their presence profoundly altered the subsequent secretory response to 10 mM glucose. For example, mouse islets exposed to 10 mM glucose alone exhibited an immediate first phase and a small, flat second phase secretory response (Figure 6). The presence of GLP-1 and carbachol markedly amplified the first phase response to 10 mM glucose and also resulted in a rising second phase response as well. Further attesting to the retention of the physiologic integrity of the collagenase-isolated islet preparation, the addition of epinephrine (10 nM) totally abolished the amplified secretory response to this combination of agonists.
Figure 6. Effects of GLP-1, carbachol and epinephrine on 10 mM glucose induced insulin secretion from mouse islets.

Three groups of islets were studied. The first group (n=8, open circles) was perifused with 3 mM glucose (G3) for 30 minutes prior to being stimulated for 40 minutes with 10 mM glucose (G10, onset of stimulation indicated by the vertical line). The second group (closed circles, n=9) was perifused for 30 minutes with 3 mM glucose plus the combination of 2.5 nM GLP-1 and 0.5 μM carbachol. At this time and in the continued presence of GLP-1 and carbachol, the glucose level was increased to 10 mM. The final group (open triangles, n=3) was treated in a similar fashion to the second group except that epinephrine (10 nM) was also included in the perifusate during the 40 minute stimulatory period with 10 mM glucose, GLP-1 and carbachol,
Additional studies using rat islets were also performed. After 30 minutes with 3 mM glucose alone or with 3 mM glucose plus the combination of 0.5 μM carbachol and 2.5 nM GLP-1, these islets were then stimulated for 40 minutes. In this case, however, islets were stimulated with 6.5 mM glucose alone or in the continued presence of 0.5 μM carbachol and 2.5 nM GLP-1. A biphasic insulin secretory response occurred in response to this combination of agonists but not to 6.5 mM glucose alone. Similar to mouse islets, these responses were abolished by 10 nM epinephrine (results not shown).
Impact of short term culture on rodent islet responses to 11.1 mM glucose
We considered the possibility that prior culture of islets may be a contributory factor to the disparate secretory response patterns observed from these two species. To address this issue islets were cultured in CMRL 1066 for 3 hours after their isolation. They were then perifused for 30 minutes with 5 mM glucose prior to stimulation with 11.1 mM glucose. No significant impact on prestimulatory secretory rates in the presence of 5 mM glucose was observed in either species. However, as shown in Figure 7, a differential effect of culturing was noted when islets were stimulated with 11.1 mM glucose. For example, when compared to the responses of freshly studied islets, a significant decrease was observed from rat islets to 11.1 mM glucose stimulation. In contrast, an improvement in mouse islets to this glucose level was observed after the same experimental manipulation. Closer scrutiny of the data in Figure 7 also show a convergence of the glucose’s stimulatory effect in these two species under this condition; the secretory profiles from cultured rodent islets, unlike the clear and significant deviation noted from freshly studied islets, are almost superimposable.
Figure 7. Effects of short term culture on glucose induced insulin secretion from rodent islets.

Groups of rat (left panel) or mouse (right panel) islets were isolated and perifused immediately (open circles and open triangles) or after a 3 hour incubation period in CMRL-1066 (closed circles, closed triangles). All islets were perifused for 30 minutes with 5 mM glucose (G5) to establish stable insulin secretory rates and for an additional 40 minutes with 11.1 mM glucose (G11.1). In mouse islets, an additional 20 minute stimulatory period was included where 50 μM carbachol was included together with 11.1 mM glucose.
Discussion
A remarkable disparity exists in the glucose responsiveness of the many preparations utilized to study insulin secretion. At the two extremes are tumoral cell lines, many of which are minimally responsive to glucose, and the perfused rat pancreas preparation, a model that displays a robust biphasic insulin secretory response. Profound species differences also exist between the two most commonly employed species used to experimentally dissect out the factors that control insulin exocytosis. This topic has been the subject of two recent studies that have attempted to provide the possible reasons for this species dichotomy. Nunemaker et al 21 suggested two possibilities for these species differences. First, the collagenase isolation procedure somehow altered vulnerable response elements in mouse islets. The potential nature of the lesion was not addressed nor any reason provided as to why this occurs uniquely with mouse islets but not with rat islets. Second, based on their studies with the intact animal, they suggested the possibility that the absence of in vivo input to the isolated islet preparation may account for the lack of biphasicity. These inputs might include acetylcholine released from the vagus nerve or circulating incretin factors such as GLP-1. Experimental attempts to duplicate the in vivo observation of biphasicity with in vitro perifusion of mouse islets were not performed in this report, so the potential nature of the added stimulants remains to be clarified.
On the other hand, Henquin and coworkers 22 argued that the prestimulatory glucose level may be a key component responsible for the anomalous behavior of mouse islet, when compared to rat islet, responses to glucose. Unfortunately, both studies utilized only mouse islets to address this issue of species disparity and comparable studies with rat islets, pretreated and studied under the identical conditions, were not provided. In the present study, we have attempted to fill this void. While our studies demonstrate, in agreement with many previous reports 5, 6, 10, 12, 17, 20, 28, that profound species difference do exist, they also demonstrate that it is indeed possible to evoke a biphasic pattern of secretion from mouse islets under selected conditions as proposed by Henquin 22. Moreover, our results also suggest that culturing may also significantly influence the secretory responses observed from rodent islets.
When stimulated with 8.5 mM glucose, after a stabilization period of 30 minutes with 3 mM glucose, freshly isolated rat islets exhibit a brisk biphasic secretory response. When compared to prestimulatory secretion rates in the presence of 3 mM glucose, about a 20-fold increase in secretion is evoked by 8.5 mM glucose. Even greater secretory responses were recorded with higher glucose levels (11.1–30 mM) that are more pharmacological than physiologic. However these findings with collagenase-isolated, perifused rat islets agree well with comparable studies using the perfused rat pancreas and attest to the retention of the glucose sensitivity of these islets after the isolation procedure.
Very different responses were observed from mouse islets when studied under conditions that culminated in robust secretory responses from rat islets. No rising second phase of secretion using stimulatory glucose levels of 8.5–16.7 mM, levels that evoke 20–30 fold increases in insulin release from rat islets, could be elicited from mouse islets.
In agreement with the report of Henquin and coworkers 22, it was possible, by manipulating the antecedent glucose concentration, to improve mouse islet responses. In their report, increasing the glucose level prior to stimulation to 8.5 mM resulted in a biphasic response to 30 mM glucose. We confirmed this effect here and also show that a less salient biphasic response could also be evoked by 30 mM glucose after a prestimulatory period with 5 mM glucose. In the previous report by Henquin et al 22 they employed 8.5 mM glucose during the stabilization period because the in vivo glucose level at the start of their hyperglycemic clamp studies was 8.5 mM. In addition, their islets were also cultured for 18 hrs with 8.5 mM glucose as well. It was not determined in these studies how culturing influenced the subsequently measured secretory responses but, as discussed below, this has to be taken into consideration. It is also unlikely that mouse islets in vivo in their natural state would be subjected to such a sustained hyperglycemic stimulus. Not withstanding these caveats, it was indeed possible to evoke a clear biphasic insulin secretory response from freshly isolated mouse islets.
While the in vivo and in vitro studies of Henquin and colleagues 22 established the significance of antecedent hyperglycemia on the elicitation of biphasic secretion, they did not address its potential effect on rat islet responses. However, this type of manipulation when performed on rat islets totally distorted their response to glucose, again leading to a pronounced species dichotomy. The intensely stimulatory effect of 8.5 mM glucose on rat β-cells, shown in Figures 1–4, resulted in secretory responses to 11.1, 16.7 or 30 mM glucose that were altered in a major way. In particular the robustness, as well as the kinetics, of the responses to 11, 16.7 or 30 mM glucose were profoundly reduced by prior exposure to 8.5 mM glucose. The adverse impact of prior hyperglycemia on rat islet responses has been observed previously and potential mechanisms discussed in other reports 29–34.
Despite the recognition that the patterns of glucose-induced insulin secretion from rats and mice deviate in such a major way, there is as yet no general agreement as to the underlying cause for this difference. Previous studies have demonstrated that glucose utilization rates of rat and mouse islets are similar 13, 35 thus excluding any gross derangement in metabolism as the underlying cause. Moreover, this dichotomy cannot be explained by differences in insulin content but most likely reflects differences in signal transduction events. Based on a large number of observations using a variety of agonists, we have suggested that differences in information flow in the phospholipase C (PLC)/protein kinase C (PKC) may in part explain these species differences. For example, glucose alone induces a 400–500% increase in 3H-inositol phosphate (IP) accumulation in rat islets, a barometer of PLC activation 10, 13, 36. On the other hand, mouse islet responses to the same manipulation are minimal, about a 25–50 % increase in IP levels to glucose stimulation 13, 37. The under expression of a nutrient-activated PLC isozyme may be responsible for this lack of responsiveness to glucose 12, 13.
If the difference in these species’ responses to glucose is indeed the result of this biochemical anomaly, how does stimulation with 30 mM glucose result in the evocation of biphasic secretion, especially after prior prolonged exposure to 8.5 mM glucose? There are at least several possibilities. First, it should be recalled that mouse islet PLC is activated, albeit significantly less than in rat islets, by glucose. It may well be that the intensely stimulatory effects of 30 mM glucose allows for the accumulation of sufficient PLC-derived second messengers molecules to support biphasic secretion. Alternatively, this maneuver may culminate in an increase in intracellular calcium that facilitates increased activation of PLC itself or down stream signaling proteins such as calcium-sensitive protein kinase C isozymes. In this context it should be noted that the PKC activator phorbol 12-myristate 13-acetate is a most potent inducer of second phase secretion in both rat and mouse islets 38, 39. The translocation of this calcium sensitive enzyme, motivated by a sustained small increase in PLC activation by glucose in mouse islets, may provide an added impetus for biphasic secretion in response to sustained stimulation with 30 mM glucose.
Because many investigators culture islets prior to assessing secretory integrity, we also explored whether this manipulation influences rodent islet sensitivity to glucose stimulation. We did not attempt to optimize culture conditions, by using various media or altering the glucose level, to improve the maintenance of secretory responsiveness. Disparate species responses to short term culture in CMRL 1066 media supplemented with 5.6 mM glucose were observed. Mouse islet responses to glucose increased but rat islet responses were reduced. Enzyme induction, their altered activation or the more complete elimination of any prior in vivo augmenting or inhibitory factors should be considered as possible candidates to explain these findings. For example, it is known that within the same time frame used in these culture studies alterations in either protein synthesis by cycloheximide or enzyme induction by glucocorticoids can exert profound effects on islet responses 40–43. Other biochemical or cationic alterations should be considered as well. In support of these findings with normal mouse islets, it has been reported 44 most recently that culturing dramatically enhances glucose-induced secretion from Sur1KO islets as well.
In conclusion, several salient points emerge from these studies. First, rat and mouse islets contain similar amounts of insulin. Second, the amount of insulin secreted during intense stimulation with glucose in either species is at most only a small fraction of the total insulin content. Third, irrelevant of the glucose levels used during the initial stabilization phase of the perifusion, clear species differences are still evident between freshly studied rat and mouse islet responses. Fourth, it is possible to convert the flat second phase secretory response of mouse islets to glucose to a rising one. Fifth, freshly studied rat and mouse islets retain their exquisite sensitivity to membrane effective agonists including GLP-1, carbachol and epinephrine. Sixth, via mechanisms that remain to be explored in much greater detail, short term culture influences subsequently measured responses to glucose stimulation in both species. Finally, as suggested by our group in previous reports 10, 24, 45, comparative studies using these species may help us identify those pathways that are quantitatively most important in the regulation of glucose-induced secretion.
Acknowledgments
These studies were supported by NIH grant # 41230
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curry DL. Insulin content and insulinogenesis by the perfused rat pancreas: effects of long term glucose stimulation. Endocrinology. 1986;118:170–175. doi: 10.1210/endo-118-1-170. [DOI] [PubMed] [Google Scholar]
- 2.Grodsky GM. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. Journal of Clinical Investigation. 1972;51:2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Conner MDL, Landahl H, Grodsky GM. Comparison of storage- and signal-limited models of pancreatic insulin secretion. American Journal of Physiology. 1980;238:R378–R389. doi: 10.1152/ajpregu.1980.238.5.R378. [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE, Charles MA, Grodsky GM. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. Journal of Clinical Investigation. 1974;54:833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenzen S. Insulin secretion by isolated perfused rat and mouse pancreas. American Journal of Physiology. 1979;236:E391–E400. doi: 10.1152/ajpendo.1979.236.4.E391. [DOI] [PubMed] [Google Scholar]
- 6.Berglund O. Different dynamics of insulin secretion in the perfused pancreas of the mouse and rat. Acta Endocrinologica. 1980;93:54–60. doi: 10.1530/acta.0.0930054. [DOI] [PubMed] [Google Scholar]
- 7.Grill V, Adamson U, Cerasi E. Immediate and time-dependent effects of glucose on insulin release from rat pancreatic tissue. Journal of Clinical Investigation. 1978;61:1034–1043. doi: 10.1172/JCI109002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henquin J-C, Lambert A. Bicarbonate modulation of glucose-induced biphasic insulin release by rat islets. American Journal of Physiology. 1976;231:713–721. doi: 10.1152/ajplegacy.1976.231.3.713. [DOI] [PubMed] [Google Scholar]
- 9.Zawalich WS, Zawalich KC, Rasmussen H. Control of insulin secretion: a model involving Ca2+, cAMP and diacylglycerol. Molecular and Cellular Endocrinology. 1990;70:119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]
- 10.Zawalich WS, Zawalich KC. Regulation of insulin secretion by phospholipase C. American Journal of Physiology. 1996;271:E409–E416. doi: 10.1152/ajpendo.1996.271.3.E409. [DOI] [PubMed] [Google Scholar]
- 11.Ganesan S, Calle R, Zawalich K, et al. Immunocytochemical localization of α-protein kinase C in rat pancreatic β-cells during glucose-induced insulin secretion. Journal of Cell Biology. 1992;119:313–324. doi: 10.1083/jcb.119.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zawalich WS, Zawalich KC, Kelley GG. Regulation of insulin release by phospholipase C activation in mouse islets: differential effects of glucose and neurohumoral stimulation. Endocrinology. 1995;136:4903–4909. doi: 10.1210/endo.136.11.7588223. [DOI] [PubMed] [Google Scholar]
- 13.Zawalich WS, Bonnet-Eymard M, Zawalich KC. Insulin secretion, inositol phosphate levels and phospholipase C isozymes in rodent pancreatic islets. Metabolism. 2000;49:1156–1163. doi: 10.1053/meta.2000.8613. [DOI] [PubMed] [Google Scholar]
- 14.Nesher R, Anteby E, Yedovizky M, et al. β-cell protein kinases and the dynamics of the insulin secretory response to glucose. Diabetes. 2002;51(Suppl 1):S68–S73. doi: 10.2337/diabetes.51.2007.s68. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan S, Calle R, Zawalich K, et al. Glucose-induced translocation of protein kinase C in rat pancreatic islets. Proceedings of the National Academy of Sciences, USA. 1990;87:9893–9897. doi: 10.1073/pnas.87.24.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vadakekalam J, Rabaglia ME, Chen Q-H, et al. Role for GTP in glucose-induced phospholipase C activation in pancreatic islets. American Journal of Physiology. 1996;271:E85–E95. doi: 10.1152/ajpendo.1996.271.1.E85. [DOI] [PubMed] [Google Scholar]
- 17.Ma MYH, Wang J, Rodd GG, et al. Differences in insulin secretion between rat and mouse islets: role of cAMP. European Journal of Endocrinology. 1995;132:370–376. doi: 10.1530/eje.0.1320370. [DOI] [PubMed] [Google Scholar]
- 18.Maechler P, Gjinovci A, Wollheim CB. Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes. 2002;51(Suppl 1):S99–S102. doi: 10.2337/diabetes.51.2007.s99. [DOI] [PubMed] [Google Scholar]
- 19.Zawalich WS, Tesz GJ, Zawalich KC. Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. Journal of Endocrinology. 2002;174:247–258. doi: 10.1677/joe.0.1740247. [DOI] [PubMed] [Google Scholar]
- 20.Noda M, Komatsu M, Sharp GWG. The BHC-9 pancreatic B-cell line preserves the characteristics of progenitor mouse islets. Diabetes. 1996;45:1766–1773. doi: 10.2337/diab.45.12.1766. [DOI] [PubMed] [Google Scholar]
- 21.Nunemaker CS, Wasserman DH, McGuinness OP, et al. Insulin secretion in the conscious mouse is biphasic and pulsatile. American Journal of Physiology. 2006;290:E523–E529. doi: 10.1152/ajpendo.00392.2005. [DOI] [PubMed] [Google Scholar]
- 22.Henquin J-C, Nenquin M, Stiernet P, Ahren B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in β-cells. Diabetes. 2006;55:441–451. doi: 10.2337/diabetes.55.02.06.db05-1051. [DOI] [PubMed] [Google Scholar]
- 23.Zawalich WS, Zawalich KC, Kelley GG. Effects of short term culturing on islet phosphoinositide and insulin secretory responses to glucose and carbachol. Acta Diabetologia. 1995;32:158–164. doi: 10.1007/BF00838485. [DOI] [PubMed] [Google Scholar]
- 24.Zawalich WS, Zawalich KC. Species differences in the induction of time dependent potentiation of insulin secretion. Endocrinology. 1996;137:1664–1669. doi: 10.1210/endo.137.5.8612499. [DOI] [PubMed] [Google Scholar]
- 25.Albano JDM, Ekins RP, Maritz G, et al. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinologica. 1972;70:487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- 26.Zawalich WS, Matschinsky FM. Sequential analysis of the releasing and fuel function of glucose in isolated perifused pancreatic islets. Endocrinology. 1977;100:1–8. doi: 10.1210/endo-100-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Malaisse WJ, Sener A, Boschero AC, et al. The stimulus-secretion coupling of glucose-induced insulin release. Cationic and secretory effects of menadione in the endocrine pancreas. European Journal of Biochemistry. 1978;87:111–120. doi: 10.1111/j.1432-1033.1978.tb12356.x. [DOI] [PubMed] [Google Scholar]
- 28.Eto K, Yamashita T, Tsubamoto Y, et al. Phosphatidyinositol 3-kinase suppresses glucose-stimulated insulin secretion by affecting post-cytosolic [Ca2+] elevation signals. Diabetes. 2002;51:87–97. doi: 10.2337/diabetes.51.1.87. [DOI] [PubMed] [Google Scholar]
- 29.Bolaffi JL, Heldt A, Lewis LD, et al. The third phase of in vitro insulin secretion. Evidence for glucose insensitivity. Diabetes. 1986;35:370–373. doi: 10.2337/diab.35.3.370. [DOI] [PubMed] [Google Scholar]
- 30.Bolaffi JL, Bruno Z, Heldt A, et al. Characteristics of desensitization of insulin secretion in fully in vitro systems. Endocrinology. 1988;122:1801–1809. doi: 10.1210/endo-122-5-1801. [DOI] [PubMed] [Google Scholar]
- 31.Bolaffi JL, Rodd GG, Ma YH, et al. The role of Ca2+-related events in glucose-stimulated desensitization of insulin secretion. Endocrinology. 1991;129:2131–2138. doi: 10.1210/endo-129-4-2131. [DOI] [PubMed] [Google Scholar]
- 32.Grodsky GM. A new phase of insulin secretion. How will it contribute to our understanding of β-cell function? Diabetes. 1989;38:673–678. doi: 10.2337/diab.38.6.673. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki H, Philbrick W, Zawalich KC, et al. Acute and chronic effects of glucose and carbachol on insulin secretion and phospholipase C activation: studies with diazoxide and atropine. American Journal of Physiology. 2006;290:E26–E33. doi: 10.1152/ajpendo.00149.2005. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki H, Zawalich KC, Zawalich WS. Desensitization of the pancreatic β-cell: Effects of physiologic hyperglycemia and hyperkalemia. American Journal of Physiology. 2006;i291:E1381–E1387. doi: 10.1152/ajpendo.00137.2006. [DOI] [PubMed] [Google Scholar]
- 35.Ashcroft SJ, Bassett JM, Randle RJ, et al. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972;126:525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best L, Malaisse WJ. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984;115:1820–1831. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y, Henquin J-C. The K+-ATP channel-independent pathway of insulin secretion by glucose. Diabetes. 1998;47:1713–1721. doi: 10.2337/diabetes.47.11.1713. [DOI] [PubMed] [Google Scholar]
- 38.Malaisse WJ, Lebrun P, Herchuelz A, et al. Synergistic effect of tumor-promoting phorbol ester and a hypoglycemic sulfonylurea upon insulin release. Endocrinology. 1983;113:1870–1822. doi: 10.1210/endo-113-5-1870. [DOI] [PubMed] [Google Scholar]
- 39.Zawalich WS, Zawalich KC, Ganesan S, et al. Effects of the phorbol ester phorbol 12-myristate 13-acetate on islet-cell responsiveness. Biochemical Journal. 1991;278:49–56. doi: 10.1042/bj2780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambillotte C, Gilon P, Henquin J-C. Direct glucocorticoid inhibition of insulin secretion. Journal of Clinical Investigation. 1997;99:414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Barrado MJ, Ravier MA, Rolland J-F, et al. Inhibition of protein synthesis sequentially impairs distinct steps of stimulus-secretion coupling in pancreatic β cells. Endocrinology. 2001;142:299–307. doi: 10.1210/endo.142.1.7910. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich S, Berchtold S, Ranta F, et al. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes. 2005;54:1090–1099. doi: 10.2337/diabetes.54.4.1090. [DOI] [PubMed] [Google Scholar]
- 43.Zawalich WS, Tesz GJ, Yamazaki H, et al. Dexamethasone suppresses phospholipase C activation and insulin secretion from isolated rat islets. Metabolism. 2006;55:35–42. doi: 10.1016/j.metabol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Szollosi AS, Nenquin M, Henquin J-C. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. Journal of Biological Chemistry. 2007 doi: 10.1074/jbc.M701382200. in press. [DOI] [PubMed] [Google Scholar]
- 45.Zawalich WS. Regulation of insulin secretion by phosphoinositide-specific phospholipase C and protein kinase C activation. Diabetes Reviews. 1996;4:160–176. [Google Scholar]