Abstract
Background
There are no studies evaluating the epidemiology of pediatric acute lung injury (ALI) in the emergency department (ED), where early identification and interventions are most likely to be helpful. The purpose of this study was to describe the epidemiology of the ALI precursor acute hypoxemic respiratory failure (AHRF) in the ED.
Methods
We analyzed 11,664 pediatric patient records from 16 EDs. Records were selected if oxygen saturation (SpO2) was recorded during the visit. Virtual partial pressure of oxygen (pO2) was calculated from SpO2, thus allowing calculation of ratios of pO2 to fraction of inspired oxygen (FiO2) (PFRs). Patients with a PFR < 300 were classified as having AHRF. Univariate analyses and logistic regression were used to test the association of clinical factors with the presence of AHRF and intubation.
Results
AHRF criteria (ie, PFR < 300) were met in 121 (2.9%) of the 4,184 patients with an oxygenation measurement. The following variables were independently associated with ALI: higher Pediatric Risk of Admission II score (adjusted odds ratio [95% confidence interval (CI)] = 1.12 [1.08–1.16]; p < .001), higher heart rate (1.02 [1.01–1.03]; p = .009), a positive chest radiograph (2.35 [1.02–5.43]; p = .045), and lower temperature (0.49 [0.36–0.68]; p < .001). The final model had an R2 = .20.
Conclusion
We found nonintubated AHRF to be prevalent in the ED. The low R2 for the regression model for AHRF underscores the lack of criteria for early identification of patients with respiratory compromise. Our findings represent an important first step toward establishing the true incidence of ALI in the pediatric ED.
Keywords: respiratory distress syndrome (adult), severity of illness index, oximetry
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), account for up to 4% of all pediatric intensive care unit admissions and between 8 to 10% of children requiring mechanical ventilation.1 The 1994 American-European Consensus Conference (AECC) on ARDS defined diagnostic criteria for ALI as (1) a partial pressure of oxygen (pO2) to fraction of inspired oxygen (FiO2) ratio of less than 300, (2) diffuse bilateral infiltrates on a chest radiograph, and (3) the absence of left atrial hypertension.2–4 The criteria for ARDS are similar except that a pO2 to FiO2 ratio (PFR) of less than 200 is required.
Despite their general use in research and clinical medicine, it is widely acknowledged that the AECC criteria for ALI and ARDS suffer from a lack of sensitivity and specificity,5 especially in pediatrics.6 In fact, the AECC criteria carry a sensitivity of only 75% and a specificity of 84% for ARDS.7 However, alternative respiratory indices, such as the alveolar-arterial oxygen tension gradient (torr),8 the Oxygenation Index,9 and others, have been shown not to correlate well with ALI severity either.
The eventual impact of any diagnostic criteria on clinical care is tied to early disease recognition and treatment. For many patients, the earliest opportunity for this is in the emergency department (ED). For example, ED-based early therapies have been shown to reduce morbidity and mortality from several critical illnesses, such as asthma,10 traumatic injuries,11 and severe sepsis.12 However, the relative success of ED-based early goal-directed therapy for severe sepsis12 is hampered by poorly sensitive and specific diagnostic criteria for severe sepsis.13
Notwithstanding, ALI is still widely considered an illness diagnosed and treated in critical care units. However, the AECC acknowledged that mechanical ventilation is not required to diagnose ALI.2 It is therefore reasonable to hypothesize that ALI is prevalent among nonintubated ED patients in whom early diagnosis and intervention could be beneficial. Partly for these reasons, studies of noninvasive ventilation in pediatrics identify patients with early ALI using higher sensitivity criteria than those from the AECC.14,15 Therefore, we used criteria for the ALI precursor acute hypoxemic respiratory failure (AHRF)16 to retrospectively identify pediatric ED patients at risk for ALI in a secondary analysis of the Pediatric Risk of Admission (PRISA II) score data set.17 The purpose of this study was to identify the incidence of AHRF in the pediatric ED population and determine historical, clinical, and laboratory factors associated with an ED presentation of AHRF and short-term intubation.
Materials and Methods
Data Collection
We conducted a secondary analysis of data comprising the PRISA II data set. Full details of the methodology used to form this data set are available in the original publication by Chamberlain and colleagues.17,18 Briefly, the PRISA data set contains data from 11,664 pediatric patients presenting to the EDs at 16 US hospitals. The participating EDs were selected attempting to broadly represent variable volumes and the presence of specialists and residents. Participating sites had pediatric intensive care unit capabilities. For 375 consecutive days, the hospital records of two patients per day from each site were randomly selected, regardless of admission or discharge status, for historical, laboratory, radiologic, and clinical data abstraction. The PRISA score was designed to describe the probability of hospital admission among pediatric ED patients as an index of illness severity. There is a direct correlation between the PRISA score and severity of illness or risk of admission.17
All patients between the ages of 0 months and 18 years with either oxygen saturation (SpO2) or partial pressure of oxygen (pO2) values available in their record were included in our analysis. This secondary analysis was approved by our Institutional Review Board.
Oximetry and pO2 Calculations
It is unusual, except in critically ill children, to have an arterial pO2 obtained in a pediatric ED patient. However, SpO2 is obtained regularly for children with respiratory symptoms. Therefore, the methods of Ellis and Sevringhaus19 were used to convert the SpO2 for each patient in the data set to a “virtual” pO2 (VRpO2). VRpO2 represents the pO2 corresponding to a given SpO2 under normalized hemoglobin-oxygen dissociation conditions. Equation 1 shows the mathematical relationship between SpO2 and VRpO2, where SpO2 is represented by S. Of note, this equation does not function for an SpO2 of 100%. Therefore, all recorded SpO2 values of 100% were adjusted to 99.9%:
| (1) |
Equation 2 from Kelman20 was then used to calculate a pO2 from the VRpO2 in a way that accounts for variations in the hemoglobin-oxygen dissociation curve owing to body temperature, blood pH, and carbon dioxide partial pressure (pCO2) values:
| (2) |
These newly derived pO2 values were recalibrated to sea level values for the two sites significantly above sea level using the alveolar gas equation incorporating the average barometric pressure at those sites. FiO2 data were extracted from measurements recorded in the medical record at the time of pulse oximetry. Room air oxygen was assumed where FiO2 was not recorded. It should be noted that because SpO2 values less than 70% are considered unreliable, we excluded the two patients in our data set with less than 70% saturation.
Because patients with respiratory distress often exhibit respiratory alkalosis until exhausted, we performed a sensitivity analysis to determine the potential effects on our results of overestimating pH and pCO2 (ie, assuming a normal pH of 7.4 rather than 7.3 and a normal pCO2 of 40 rather than 30, as would be expected in an ill child). The results are depicted in Figure 1 and indicate that underrecognizing acidosis and hypercarbia would bias our results toward the null hypothesis. It is important to note that these modestly overestimated imputed values were used only for conversion of SpO2 to pO2 and not used for any other analyses.
Figure 1.
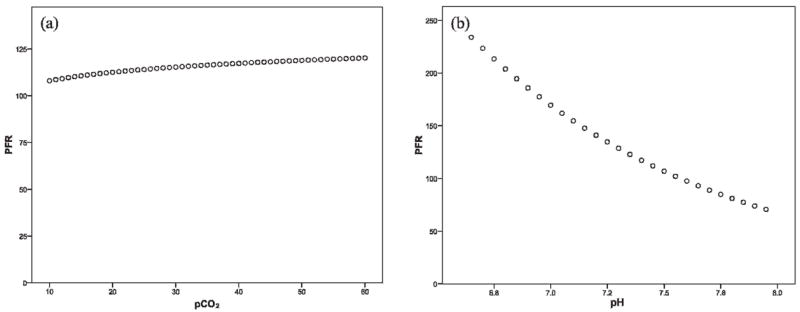
Scatterplots were used to test the validity of the assumption that underrecognizing acidosis and hypercarbia would bias our results toward the null hypothesis. We generated scatterplots for a range of carbon dioxide partial pressure (pCO2) values and their calculated partial pressure of oxygen (pO2) to fraction of inspired oxygen (FiO2) ratios (PFRs) as well as for a range of pH values and their corresponding PFRs while holding all other values constant (ie, FiO2 = 0.5, temperature = 37°C, and either pCO2 = 40 or pH = 7.4). The effect of pH on PFR was much larger than that of pCO2 and the data trends confirmed that overestimating pCO2 and underestimating pH would result in higher than true PFRs, thus leading to an underestimation of acute hypoxemic respiratory failure.
Definitions and Patient Classification
The pO2 and FiO2 data were then used to calculate the PFR for each patient using the following equation:
| (3) |
AHRF criteria were modified from AECC ALI criteria2–4 to accommodate our study’s retrospective and ED-based design. Patients were classified as having AHRF if their calculated PFR was less than 300. However, bilateral infiltrates on chest radiograph were used only as supporting evidence for AHRF since the frequency of this finding in early ALI is uncertain. Additionally, echocardiography data were very rare in our data set, but the incidence of heart failure as a significant contributor to AHRF in a pediatric ED was presumed to be nominal. Records for patients with an explicit heart failure–related diagnosis were excluded from the analysis.
Patients with chest radiograph reports identifying lung infiltrates of any kind were categorized as having a positive chest radiograph. This included all patients with radiographs positive for pneumonia, effusion, empyema or abscess, or other pulmonary abnormalities.
A chronic disease variable was defined as positive if patients had a history of congenital, infectious, metabolic, or autoimmune diseases. In this data set, this most commonly included patients with chronic lung diseases such as bronchopulmonary dysplasia, asthma, and human immunodeficiency virus (HIV).
Among the patients meeting AHRF criteria, all patients intubated within the first 24 hours of ED presentation were identified.
Statistical Analyses
All calculations were performed using Microsoft Excel version 2003 SP2 (Microsoft Corporation, Redmond, WA). SPSS version 13 (SPSS, Chicago, IL) was used for statistical testing. Univariate associations by the Student t-test or chi-square with p values less than .15 were entered into a binomial multiple logistic regression model with AHRF or intubation as the dependent variable. Regression was backwards conditional stepwise with variable removal for p ≥ .10.
Results
Of the 11,664 patients entered into the original 16-center study, 11,356 patients met the pediatric age requirement. The demographics are described in Table 1. SpO2 was recorded during the ED stay in 4,184 (37%) patients with a mean ± SE of 97.7 ± 0.04% and a range of 60 to 100%. Arterial pO2 values were recorded in the ED for 27 (0.6%) patients with a range of 33 to 484 mm Hg and mean ± SE of 156 ± 26 mm Hg. The mean ± SE SpO2-derived pO2 distribution was similar with a mean of 119 ± 0.6 and range from 33 to 331 mm Hg. The results of a Bland-Altman analysis can be seen in Figure 2 and validate the derived pO2 values using patients with simultaneously measured pO2 and SpO2.
Table 1.
Demographic Description of Acute Hypoxemic Respiratory Failure Patients in the PRISA II Data Set
| Demographic Variables | AHRF n (%) | Non-AHRF n (%) | p Value |
|---|---|---|---|
| Age, mo (mean ± SE) | 62 ± 5 | 70 ± 1 | .17 |
| Male gender | 70 (58) | 2138 (54) | .51 |
| Race* | |||
| Asian | 3 (3) | 33 (1) | .02 |
| Black | 20 (20) | 1058 (32) | |
| Caucasian | 44 (44) | 1071 (33) | |
| Hispanic | 33 (33) | 1060 (32) | |
| Other | 1 (1) | 54 (2) | |
| Insurance† | |||
| Medicaid Managed care | 14 (18) | 447 (20) | .02 |
| Private managed care | 22 (28) | 386 (18) | |
| Medicaid | 13 (16) | 313 (14) | |
| Private | 11 (14) | 171 (8) | |
| Self | 5 (6) | 368 (17) | |
| Other | 14 (18) | 491 (23) | |
AHRF = acute hypoxemic respiratory failure; PRISA = Pediatric Risk of Admission.
Based on n = 101 for AHRF owing to unavailable race data.
Based on n = 79 for AHRF owing to unavailable insurance data.
Figure 2.
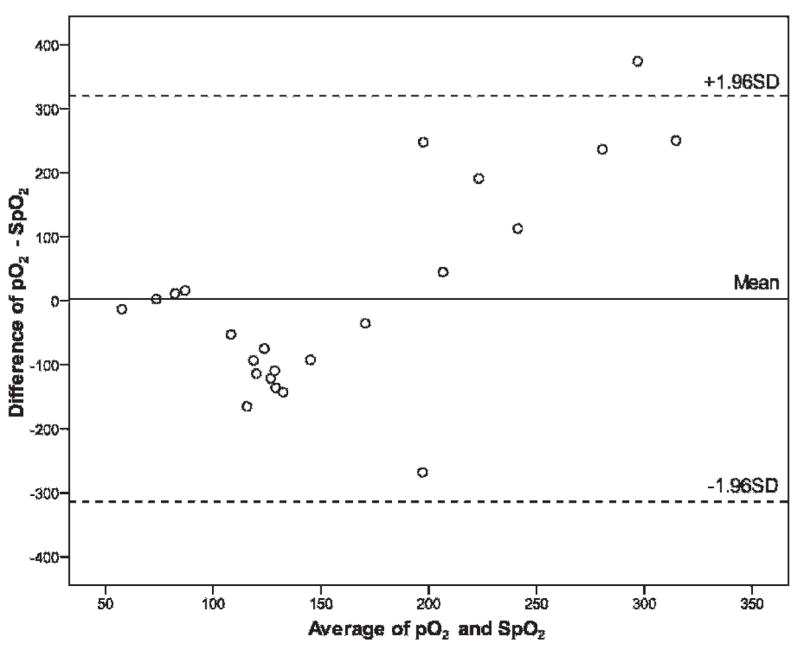
Bland-Altman plot of pO2 versus SpO2 in patients with simultaneously measured values.
AHRF criteria (ie, PFR < 300) were met in 121 (2.9%) of the 4,184 patients with an oxygenation measurement. Patients meeting the AHRF criteria had similar ED diagnoses (infectious causes: 49.6%; traumatic causes: 28.8%) when compared with non-AHRF patients (infectious causes: 49.8%; traumatic causes: 29.0%). Among the nontraumatic AHRF patients, the majority (73%) were diagnosed with bacterial infections. The remainder were sparsely scattered among various noninfectious, nontraumatic diagnoses.
Univariate analyses were used to compare patients who met AHRF criteria with those who did not and intubated AHRF patients with those who were not. Variables significantly and positively associated with AHRF (p ≤ .015) included PRISA II score (p < .001), triage respiratory rate (p < .001), triage heart rate (p = .002), presence of a chronic disease (p = .019), and a positive chest radiograph (p < .001) (Table 2). Negative associations included triage temperature (p < .001), diastolic blood pressure (p = .024), and triage Glasgow Coma Scale (p < .001). No association was noted for age, gender, systolic blood pressure, bicarbonate level, hemoglobin level, or white blood cell count and differential.
Table 2.
Predictors of Acute Hypoxemic Respiratory Failure in Pediatric Emergency Department Patients
| Clinical Predictors | AHRF (mean ± SE) | Non-AHRF (mean ± SE) | Unadjusted Odds Ratio for AHRF (95% CI) | Adjusted Odds Ratio for AHRF (95% CI) |
|---|---|---|---|---|
| PRISA II | 7.55 ± 0.8 | 2.8 ± 0.7 | 1.11 (1.08–1.13) | 1.12 (1.08–1.16) |
| Triage temperature | 37.1 ± 0.8 | 37.5 ± 0.0 | 0.7 (0.6–0.9) | 0.49 (0.36–0.68) |
| Triage heart rate | 131 ± 3.0 | 122.0 ± 0.5 | 1.1 (1.0–1.2) | 1.02 (1.01–1.03) |
| Triage respiratory rate | 31.1 ± 1.3 | 27.1 ± 0.02 | 1.03 (1.02–1.04) | NS |
| Triage systolic blood pressure | 111 ± 3.0 | 115 ± 0.4 | NS | — |
| Triage diastolic blood pressure | 63.0 ± 2.0 | 67.0 ± 0.3 | 0.97 (0.95–0.99) | 0.98 (0.96–1.00) |
| Triage Glasgow Coma Scale | 12.8 ± 1.0 | 14.9 ± 0.0 | 0.7 (0.6–0.8) | NS |
| Bicarbonate level (mmol/L) | 21.4 ± 1.1 | 22.6 ± 0.2 | NS | — |
| Hemoglobin level (g/dL) | 12.5 ± 0.5 | 12.3 ± 0.1 | NS | — |
| White blood cell count (× 103/μL) | 13.5 ± 1.3 | 12.3 ± 0.3 | NS | — |
| Positive chronic disease | 54 (45.8%) | 1,380 (34.9%) | 1.6 (1.1–2.3) | NS |
| Positive chest radiograph | 24 (20%) | 328 (8.1%) | 2.8 (1.8–4.5) | 2.35 (1.02–5.43) |
AHRF = acute hypoxemic respiratory failure; CI = confidence interval; NS = not significant; PRISA = Pediatric Risk of Admission. Percentages for AHRF were calculated for 121 patients and for non-AHRF patients using 4,184 patients.
A backwards conditional stepwise logistic regression model incorporating all of these variables showed the following variables independently and positively associated with AHRF: PRISA II score (adjusted odds ratio [OR] [95% confidence interval (CI)] = 1.12[1.08–1.16]; p < .001), triage heart rate (1.02 [1.01–1.03]; p = .009), and a positive chest radiograph (2.35 [1.02–5.43]; p = .045). Elevated triage temperature (0.49 [0.36–0.68]; p < .001) was negatively associated with AHRF (see Table 2). Triage diastolic blood pressure (0.98 [0.96–1.00]; p = .06) was not significantly independently associated with AHRF but contributed to the final model. The final model including these five variables had an R2 = .20 and Hosmer and Lemeshow test p = .39.
Among the 121 AHRF patients, 6 (5%) patients were intubated within 24 hours of presentation. The OR for endotracheal intubation within 24 hours of presentation among patients meeting AHRF criteria in the ED compared with those who did not was 13.2 (5.1–34.3). Intubation was associated significantly with higher PRISA II score (unadjusted OR = 1.17 [1.08–1.27]; p < .001) and lower triage Glasgow Coma Scale (1.42 [1.08–1.86]; p = .011). No multicollinearity was found between these two variables. Multivariable logistic regression for these data was not performed because of the small number of intubated patients.
Discussion
ED-based early therapies, such as goal-directed therapy in severe sepsis,12 demonstrate the potential benefits of early recognition and treatment of critical illness in the ED. An important part of forming criteria for disease identification is to understand the prevalence and presentation of the illness. Therefore, as a first step toward early identification of ALI, our study aimed to determine the incidence of AHRF among pediatric ED patients.
This study resulted in three major findings. First, AHRF is a fairly prevalent illness (2.9%) among pediatric ED patients with a triage measurement of pulse oximetry (including 5% intubated by 24 hours). For comparison, occult bacteremia is estimated to occur in 1 to 3%21–24 and urinary tract infections in 3 to 10%25–28 of febrile pediatric ED patients without a clinically apparent source of infection. Second, several easily detected clinical factors are independently associated with the presence of AHRF in the ED: (1) higher PRISA II score, (2) higher heart rate, (3) positive chest radiograph, and (4) lower temperature. Third, most of the patients with AHRF did not require intubation and mechanical ventilation during the first 24 hours of their hospital stay.
The high percentage of AHRF patients who did not immediately require mechanical ventilation suggests that a broad spectrum of AHRF severity exists in the ED, including a large proportion of patients with mild to moderate disease. Many of these patients likely suffer from acute respiratory illnesses that may be fairly easily reversible (eg, hypoxic asthma). Even so, this is an important finding because the AECC criteria, although recognizing that these milder patients do exist, tend to identify patients with more severe disease. In fact, Flori and colleagues showed that 50% of spontaneously ventilating patients in their ALI cohort eventually required mechanical ventilation.29 Prospective studies of the evolving AHRF patient group are warranted to determine which interventions these patients ultimately need and which treatments can be instituted in the ED to prevent progression to ALI.
The value of these findings as a first attempt to identify the incidence of early ALI in the pediatric ED is not outweighed by study limitations inherent to the retrospective design. The major one of these limitations is missing data. Many patients did not have SpO2 recorded; therefore, it is possible that some AHRF patients were among them. Also, we were forced to impute missing data for some variables (ie, pH and pCO2), but, as stated earlier, the values we entered would bias our results toward the null and lower our measured incidence. Additionally, owing to the controversy over ALI definitions, we used highly sensitive AHRF criteria as a screening variable to classify patients. By choosing AHRF criteria (ie, PFR < 300) instead of the more specific AECC ALI criteria, we may have a degree of misclassification bias as some AHRF patients could have a different hypoxemic pathology. The higher risk among the AHRF patients for an abnormal chest radiograph and intubation is somewhat reassuring, but only a prospective evaluation of this question would be able to alleviate this potential bias entirely.
In conclusion, we found a moderate incidence of nonintubated AHRF in the pediatric ED. The low R2 for the regression model for AHRF underscores the lack of criteria for early identification of patients with significant respiratory compromise. There were, however, several variables associated with short-term prognosis. Our findings represent an important first step toward establishing the true prevalence of ALI in the pediatric ED and support the need for additional studies aimed at early identification of ALI and other critical illnesses.
Acknowledgments
Supported by National Institutes of Health grant K23-RR-020069 (to R.J.F.).
Footnotes
Presented in part at the Pediatric Academic Societies’ Annual Meeting in San Francisco, CA, May 1, 2006.
References
- 1.Costil J, Cloup M, Leclerc F, et al. Acute respiratory distress syndrome (ARDS) in children: Multicenter Collaborative Study of the French Group of Pediatric Intensive Care. Pediatr Pulmonol Suppl. 1995;11:106–7. doi: 10.1002/ppul.1950191152. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee Intensive Care Med. 1994;20:225–32. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 5.Huang DT, Angus DC. Designing clinical trials in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2006;12:32–6. doi: 10.1097/01.ccx.0000198997.29695.29. [DOI] [PubMed] [Google Scholar]
- 6.Flori HR. Toward a more accurate definition of acute lung injury and the acute respiratory distress syndrome in pediatric patients. Pediatr Crit Care Med. 2006;7:393–4. doi: 10.1097/01.PCC.0000225007.91201.09. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A, Fernandez-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med. 2004;141:440–5. doi: 10.7326/0003-4819-141-6-200409210-00009. [DOI] [PubMed] [Google Scholar]
- 8.Peters MJ, Tasker RC, Kiff KM, et al. Acute hypoxemic respiratory failure in children: case mix and the utility of respiratory severity indices. Intensive Care Med. 1998;24:699–705. doi: 10.1007/s001340050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–11. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 10.Rowe BH, Camargo CA., Jr Emergency department treatment of severe acute asthma. Ann Emerg Med. 2006;47:564–6. doi: 10.1016/j.annemergmed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Blow O, Magliore L, Claridge JA, et al. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 1999;47:964–9. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HB, Rivers EP, Abrahamian FM, et al. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48:28–54. doi: 10.1016/j.annemergmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Bernet V, Hug MI, Frey B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med. 2005;6:660–4. doi: 10.1097/01.pcc.0000170612.16938.f6. [DOI] [PubMed] [Google Scholar]
- 15.Shah PS, Ohlsson A, Shah JP. Continuous negative extrathoracic pressure or continuous positive airway pressure for acute hypoxemic respiratory failure in children. Cochrane Database Syst Rev. 2005;(3):CD003699. doi: 10.1002/14651858.CD003699.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Randolph AG, Meert KL, O’Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–40. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain JM, Patel KM, Pollack MM. The Pediatric Risk of Hospital Admission score: a second-generation severity-of-illness score for pediatric emergency patients. Pediatrics. 2005;115:388–95. doi: 10.1542/peds.2004-0586. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain JM, Patel KM, Pollack MM, et al. Recalibration of the Pediatric Risk of Admission score using a multi-institutional sample. Ann Emerg Med. 2004;43:461–8. doi: 10.1016/j.annemergmed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 20.Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–6. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- 21.Kuppermann N. Occult bacteremia in young febrile children. Pediatr Clin North Am. 1999;46:1073–109. doi: 10.1016/s0031-3955(05)70176-0. [DOI] [PubMed] [Google Scholar]
- 22.Kuppermann N, Fleisher GR, Jaffe DM. Predictors of occult pneumococcal bacteremia in young febrile children. Ann Emerg Med. 1998;31:679–87. doi: 10.1016/s0196-0644(98)70225-2. [DOI] [PubMed] [Google Scholar]
- 23.Sard B, Bailey MC, Vinci R. An analysis of pediatric blood cultures in the postpneumococcal conjugate vaccine era in a community hospital emergency department. Pediatr Emerg Care. 2006;22:295–300. doi: 10.1097/01.pec.0000215137.51909.16. [DOI] [PubMed] [Google Scholar]
- 24.Teach SJ, Fleisher GR. Efficacy of an observation scale in detecting bacteremia in febrile children three to thirty-six months of age, treated as outpatients. Occult Bacteremia Study Group. J Pediatr. 1995;126:877–81. doi: 10.1016/s0022-3476(95)70200-8. [DOI] [PubMed] [Google Scholar]
- 25.Gorelick MH, Hoberman A, Kearney D, et al. Validation of a decision rule identifying febrile young girls at high risk for urinary tract infection. Pediatr Emerg Care. 2003;19:162–4. doi: 10.1097/01.pec.0000081238.98249.40. [DOI] [PubMed] [Google Scholar]
- 26.Hoberman A, Wald ER, Reynolds EA, et al. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15:304–9. doi: 10.1097/00006454-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102(2):e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 28.Teach SJ, Geil PA. Incidence of bacteremia, urinary tract infections, and unsuspected bacterial meningitis in children with febrile seizures. Pediatr Emerg Care. 1999;15:9–12. doi: 10.1097/00006565-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]


