Abstract
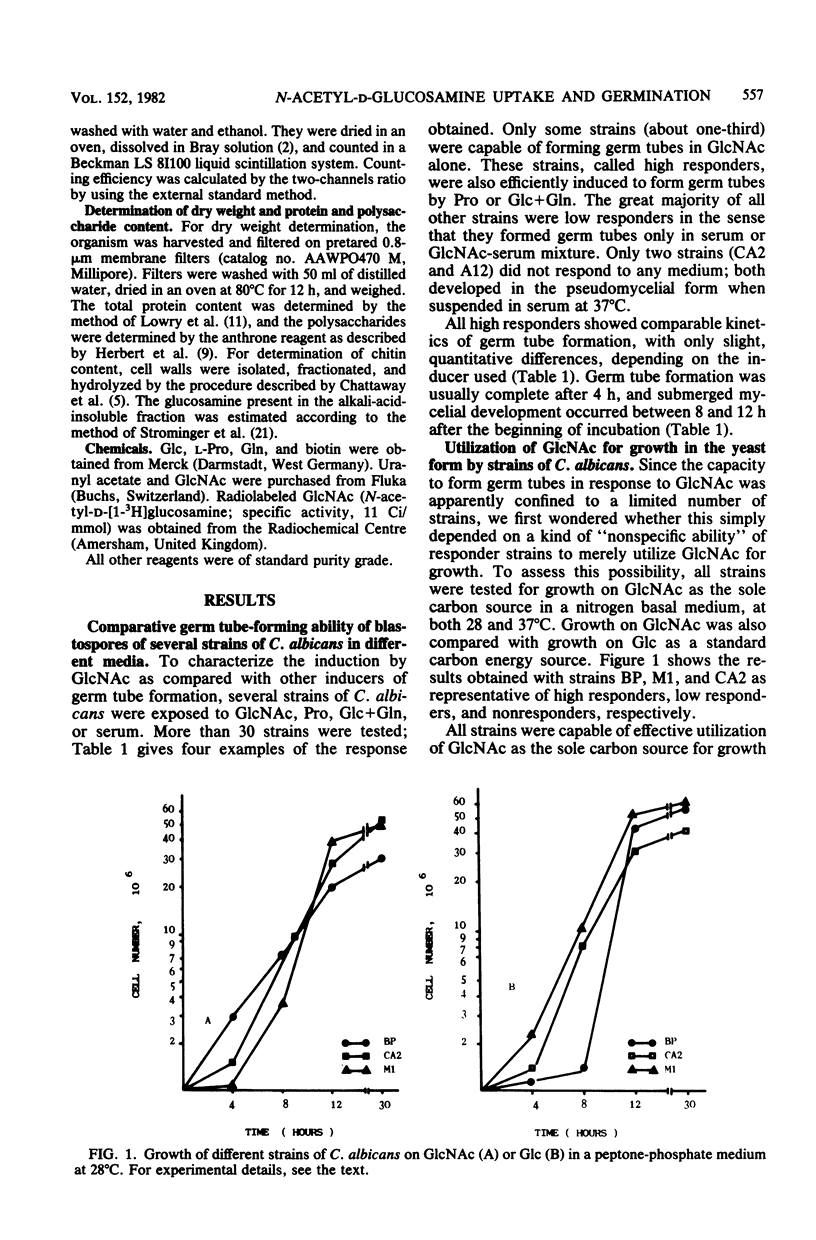
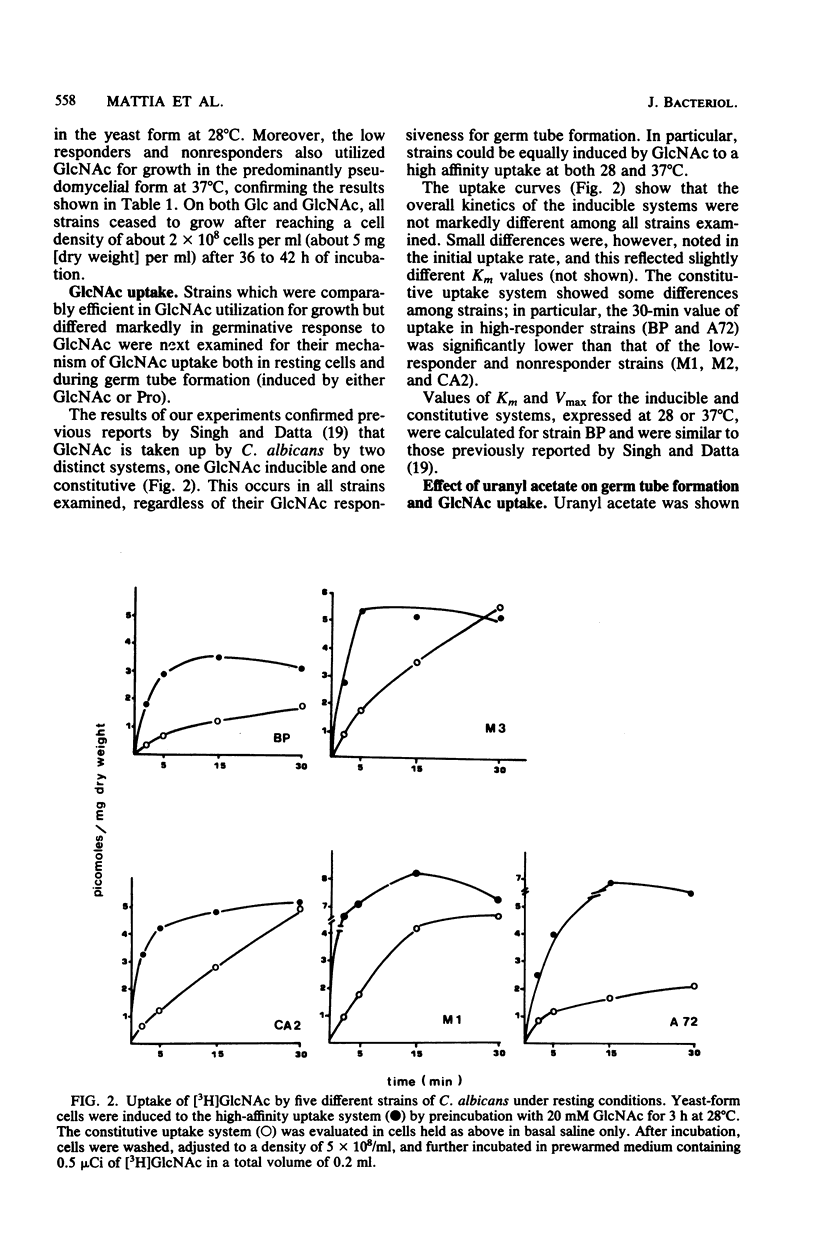
A number of strains of Candida albicans were tested for germ tube formation after induction by N-acetyl-D-glucosamine (GlcNAc) and other simple (proline, glucose plus glutamine) or complex (serum) compounds. A proportion of strains (high responders) were induced to form germ tubes evolving to true hyphae by GlcNAc alone or by proline or glucose plus glutamine mixture. The majority of strains were low responders because they could be induced only by serum or GlcNAc-serum medium. Two strains were found to be nonresponders: they grew as pseudohyphae in serum. Despite minor quantitative differences, all strains efficiently utilized GlcNAc for growth under the yeast form at 28 degrees C. They also had comparable active, inducible, and constitutive uptake systems for GlcNAc. During germ tube formation in GlcNAc, the inducible uptake system was modulated, as expected from induction and decay of GlcNAc kinase. Uranyl acetate, at a concentration of 0.01 mM, inhibited both GlcNAc uptake and germ tube formation and was reversed by phosphates. Germinating and nongerminating cells differed in the rapidity and extent of GlcNAc incorporation into acid-insoluble and alkali-acid-insoluble cell fractions. During germ tube formation induced by proline, GlcNAc was almost totally incorporated into the acid-insoluble fraction after 60 min. Moreover, hyphal development on induction by either GlcNAc or proline was characterized by an apparent "uncoupling" between protein and polysaccharide metabolism, the ratio between the two main cellular constituents falling from more than 1 to less than 0.5 after 270 min of development. The data suggest that utilization of the inducer for wall synthesis is a determinant of germ tube formation C. albicans but that the nature and extent of inducer uptake is not a key event for this phenomenon to occur.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P. C., Calderone R. A. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978 Mar;133(3):1472–1477. doi: 10.1128/jb.133.3.1472-1477.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Mason R. E., Kerridge D. Lysis of growing yeast-form cells of Candida albicans by echinocandin: a cytological study. Sabouraudia. 1981 Jun;19(2):97–110. [PubMed] [Google Scholar]
- Chattaway F. W., Bishop R., Holmes M. R., Odds F. C., Barlow A. J. Enzyme activities associated with carbohydrate synthesis and breakdown in the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1973 Mar;75(1):97–109. doi: 10.1099/00221287-75-1-97. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Wheeler P. R., O'Reilly J. Involvement of adenosine 3':5'-cyclic monophosphate in the germination of blastospores of Candida albicans. J Gen Microbiol. 1981 Apr;123(2):233–240. doi: 10.1099/00221287-123-2-233. [DOI] [PubMed] [Google Scholar]
- Chiew Y. Y., Shepherd M. G., Sullivan P. A. Regulation of chitin synthesis during germ-tube formation in Candida albicans. Arch Microbiol. 1980 Mar;125(1-2):97–104. doi: 10.1007/BF00403204. [DOI] [PubMed] [Google Scholar]
- Dabrowa N., Taxer S. S., Howard D. H. Germination of Candida albicans induced by proline. Infect Immun. 1976 Mar;13(3):830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattia E., Cassone A. Inducibility of germ-tube formation in Candida albicans at different phases of yeast growth. J Gen Microbiol. 1979 Aug;113(2):439–442. doi: 10.1099/00221287-113-2-439. [DOI] [PubMed] [Google Scholar]
- Medoff J., Jacobson E., Medoff G. Regulation of dimorphism in Histoplasma capsulatum by cyclic adenosine 3',5'-monophosphate. J Bacteriol. 1981 Mar;145(3):1452–1455. doi: 10.1128/jb.145.3.1452-1455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi M., Niimi K., Tokunaga J., Nakayama H. Changes in cyclic nucleotide levels and dimorphic transition in Candida albicans. J Bacteriol. 1980 Jun;142(3):1010–1014. doi: 10.1128/jb.142.3.1010-1014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Sacco M., Maresca B., Kumar B. V., Kobayashi G. S., Medoff G. Temperature- and cyclic nucleotide-induced phase transitions of Histoplasma capsulatum. J Bacteriol. 1981 Apr;146(1):117–120. doi: 10.1128/jb.146.1.117-120.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G., Yin C. Y., Ram S. P., Sullivan P. A. Germ tube induction in Candida albicans. Can J Microbiol. 1980 Jan;26(1):21–26. doi: 10.1139/m80-004. [DOI] [PubMed] [Google Scholar]
- Simonetti N., Strippoli V., Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974 Jul 26;250(464):344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- Singh B., Datta A. Regulation of N-acetylglucosamine uptake in yeast. Biochim Biophys Acta. 1979 Oct 19;557(1):248–258. doi: 10.1016/0005-2736(79)90107-x. [DOI] [PubMed] [Google Scholar]
- Singh B., Guptaroy B., Hasan G., Datta A. Inhibitory effect of glucose and adenosine 3',5'-monophosphate on the synthesis of inducible N-acetylglucosamine catabolic enzymes in yeast. Biochim Biophys Acta. 1980 Oct 15;632(3):345–353. doi: 10.1016/0304-4165(80)90230-5. [DOI] [PubMed] [Google Scholar]
- Wain W. H., Brayton A. R., Cawson R. A. Variations in the response to N-acetyl-D-glucosamine by isolates of Candida albicans. Mycopathologia. 1976 Jun 4;58(1):27–29. doi: 10.1007/BF00493590. [DOI] [PubMed] [Google Scholar]



