Abstract
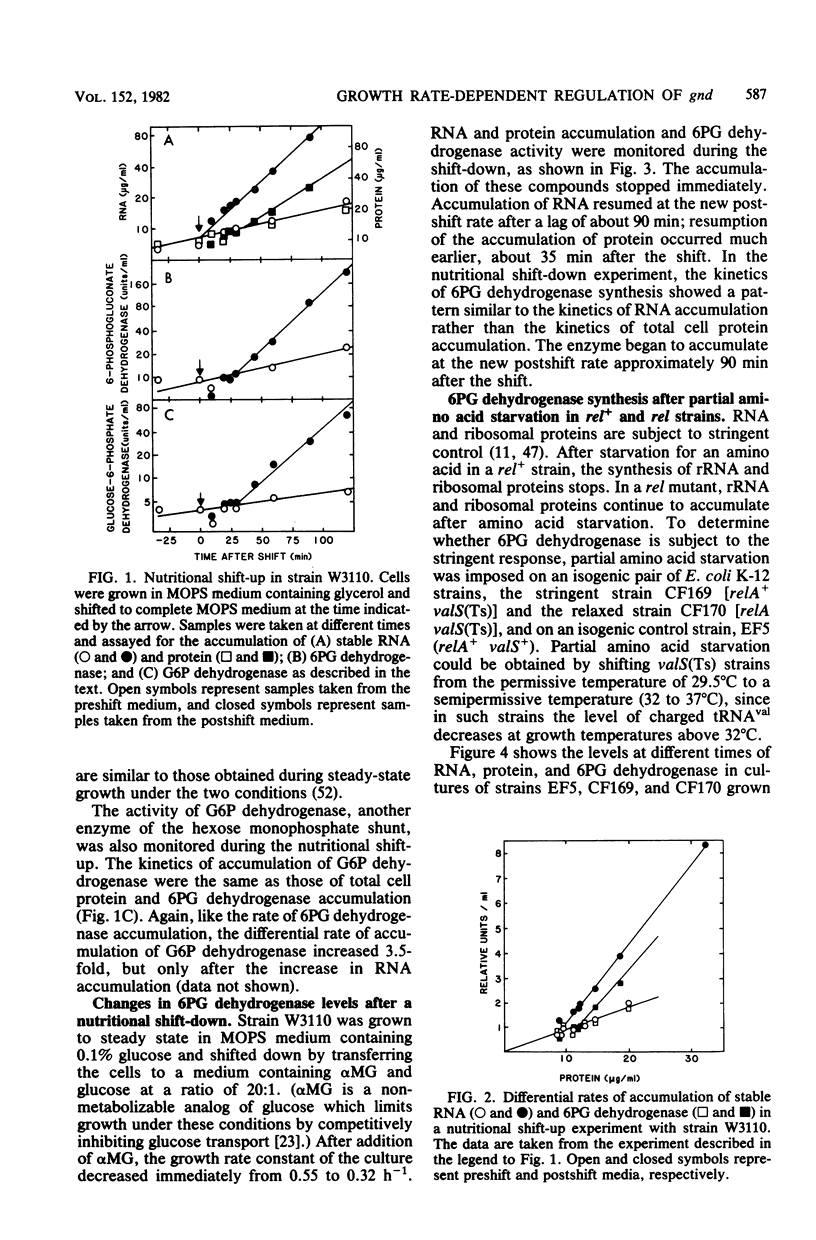
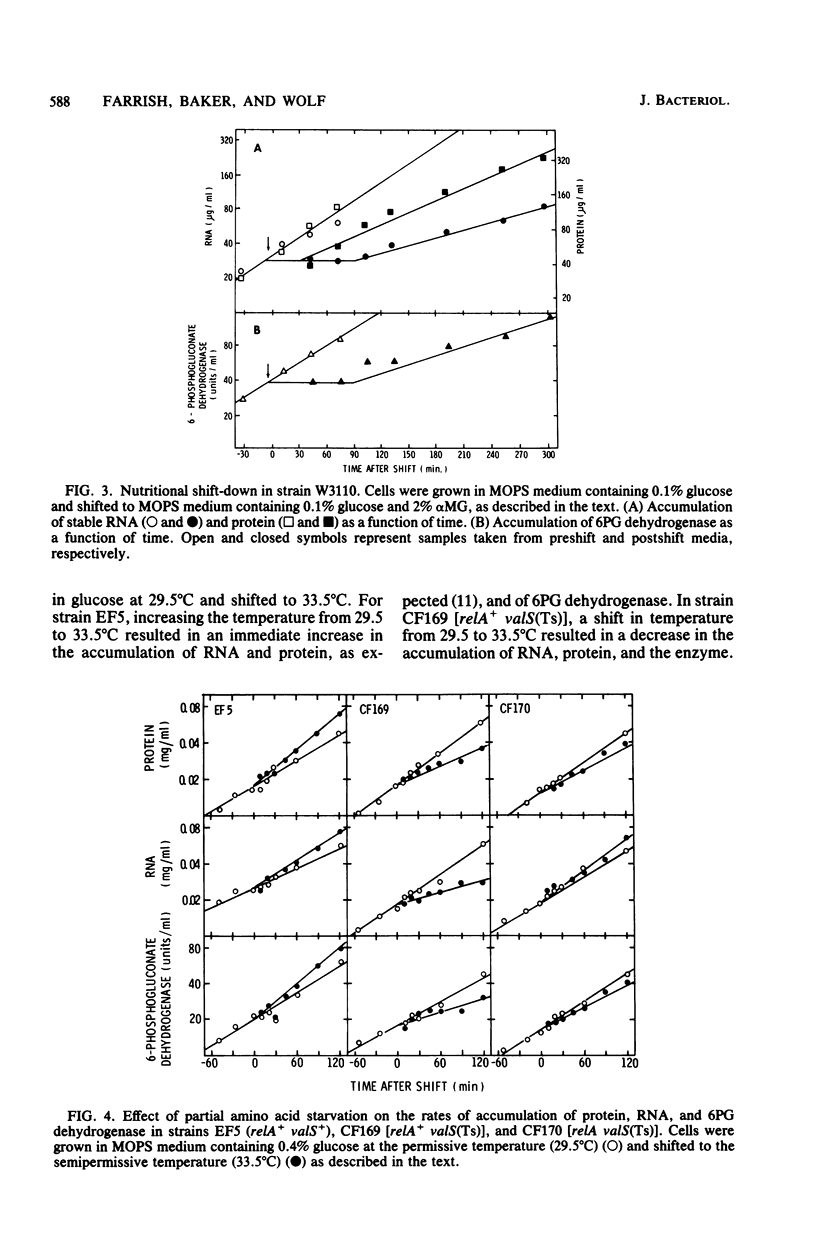
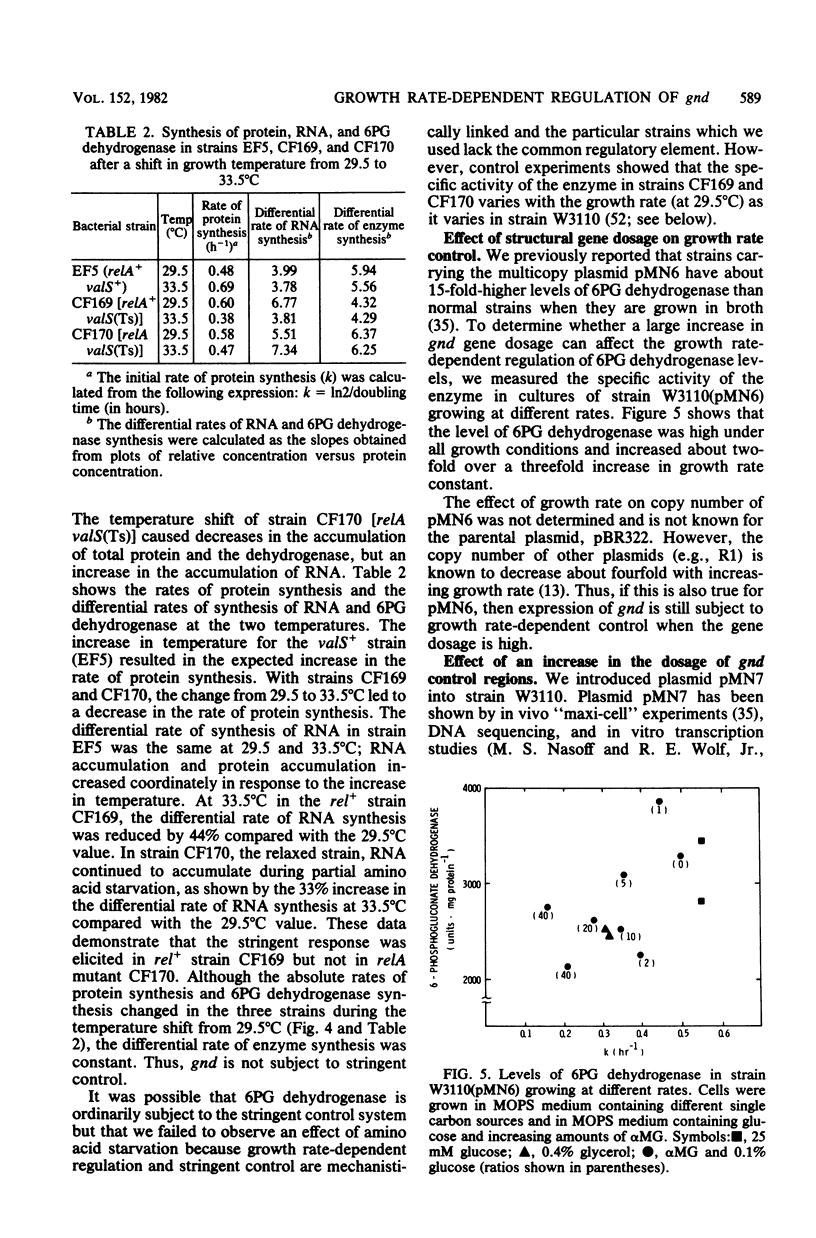
Previous studies showed that the level of 6-phosphogluconate (6PG) dehydrogenase increases about fourfold with increasing growth rate when the growth rate is varied by varying the carbon source. When the growth rate was reduced by anaerobic growth or by using mutations to divert metabolism to less efficient pathways, the level of 6PG dehydrogenase was the same as in a wild-type strain growing aerobically on other carbon sources that yielded the same growth rate. Thus, expression of gnd, which encodes 6PG dehydrogenase, is regulated by the cellular growth rate and not by specific nutrients in the medium. Growth rate-dependent regulation was independent of temperature. After a nutritional shift-up, 6PG dehydrogenase and total protein did not attain the postshift rate of accumulation for 30 min, whereas RNA accumulation increased immediately. The kinetics of accumulation of 6PG dehydrogenase and RNA were coincident after a nutritional shift-down. Partial amino acid starvation of a strain that controls RNA synthesis stringently (rel+) had no effect on the differential rate of accumulation of the enzyme. The level of 6PG dehydrogenase in cells harboring a gnd+ multicopy plasmid was in approximate proportion to gene dosage and somewhat higher at faster growth rates. Growth rate control of chromosomal gnd was normal in strains carrying multiple copies of the promoter-proximal and promoter-distal portions of gnd. These results show that gnd is not part of the same regulatory network as components of the translational apparatus since gnd shows a delayed response to a nutritional shift-up, is not autoregulated, and is not subject to stringent control. Models to account for growth rate-dependent regulation of gnd are discussed.
Full text
PDF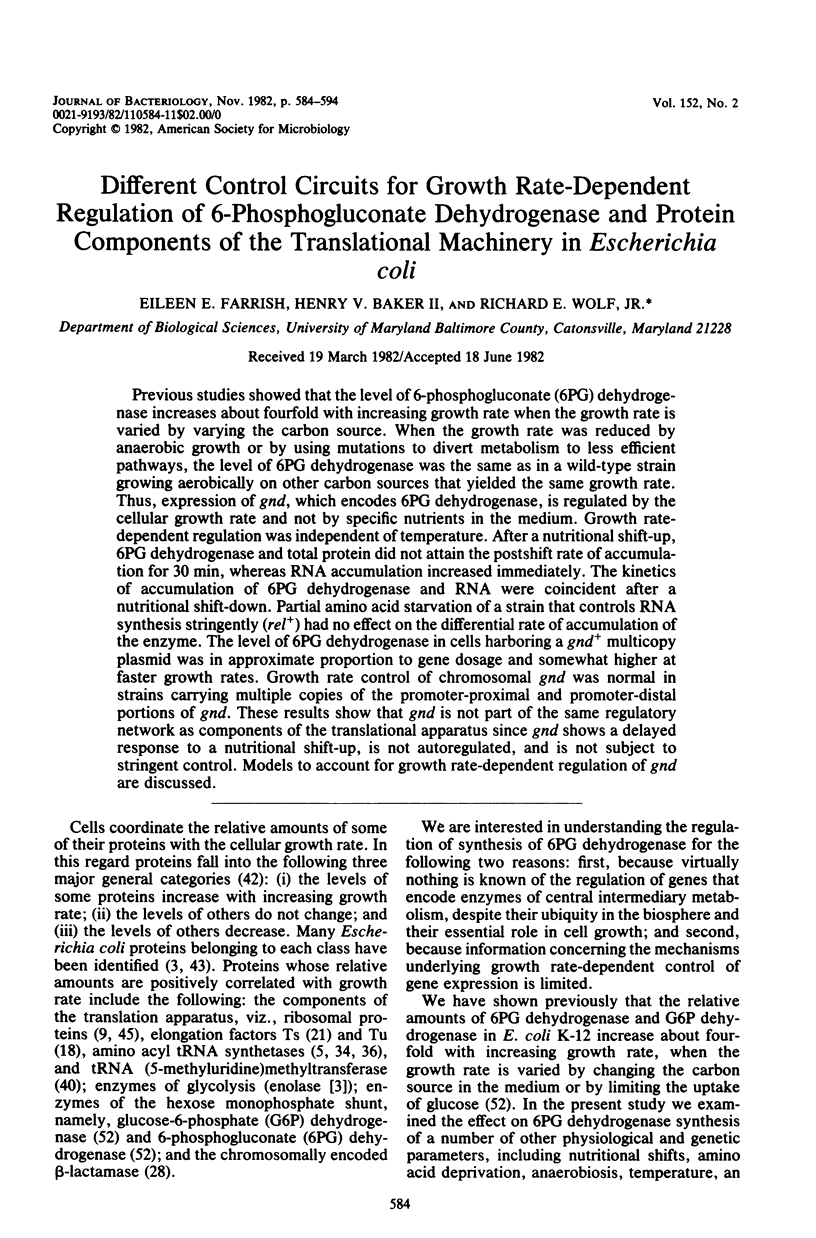






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebricher C. K., Druminski M. Inhibition of RNA polymerase activity by the Escherichia coli protein biosynthesis elongation factor Ts. Proc Natl Acad Sci U S A. 1980 Feb;77(2):866–869. doi: 10.1073/pnas.77.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch P. L., Phillips T. A., Neidhardt F. C. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J Bacteriol. 1980 Mar;141(3):1409–1420. doi: 10.1128/jb.141.3.1409-1420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debenham P. G., Pongs O., Travers A. A. Formylmethionyl-tRNA alters RNA polymerase specificity. Proc Natl Acad Sci U S A. 1980 Feb;77(2):870–874. doi: 10.1073/pnas.77.2.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J Mol Biol. 1974 Apr 15;84(3):407–422. doi: 10.1016/0022-2836(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Fill N. P. Transcriptional and post-transcriptional control of RNA polymerase and ribosomal protein genes cloned on composite ColE1 plasmids in the bacterium Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):7540–7547. [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Regulation of ribosomal and transfer ribonucleic acid synthesis in Escherichia coli B-r. J Biol Chem. 1972 May 10;247(9):2842–2845. [PubMed] [Google Scholar]
- Engberg B., Nordström K. Replication of R-factor R1 in Scherichia coli K-12 at different growth rates. J Bacteriol. 1975 Jul;123(1):179–186. doi: 10.1128/jb.123.1.179-186.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon A. M., Jinks C. S., Strycharz G. D., Nomura M. Regulation of ribosomal protein synthesis in Escherichia coli by selective mRNA inactivation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3411–3415. doi: 10.1073/pnas.76.7.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Levisohn S. R. Glucose and gluconate metabolism in an Escherichia coli mutant lacking phosphoglucose isomerase. J Bacteriol. 1967 May;93(5):1571–1578. doi: 10.1128/jb.93.5.1571-1578.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyl D., Böck A. Synthesis of ribosomal proteins in merodiploid strains and in minicells of Escherichia coli. Mol Gen Genet. 1977 Sep 9;154(3):327–334. doi: 10.1007/BF00571290. [DOI] [PubMed] [Google Scholar]
- Gitelman D. R., Apirion D. The synthesis of some proteins is affected in RNA processing mutants of Escherichia coli. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1063–1070. doi: 10.1016/0006-291x(80)90060-1. [DOI] [PubMed] [Google Scholar]
- Gordon J. Regulation of the in vivo synthesis of the polypeptide chain elongation factors in Escherichia coli. Biochemistry. 1970 Feb 17;9(4):912–917. doi: 10.1021/bi00806a028. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., McLimont M., Hanly S. Termination of transcription by nusA gene protein of Escherichia coli. Nature. 1981 Jul 16;292(5820):215–220. doi: 10.1038/292215a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Isturiz T., Wolf R. E., Jr In vitro synthesis of a constitutive enzyme of Escherichia coli, 6-phosphogluconate dehydrogenase. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4381–4384. doi: 10.1073/pnas.72.11.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T., Edlund T., Normark S. The E. coli beta-lactamase attenuator mediates growth rate-dependent regulation. Nature. 1981 Mar 19;290(5803):221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Normark S. In vivo regulation of chromosomal beta-lactamase in Escherichia coli. J Bacteriol. 1979 Jun;138(3):896–902. doi: 10.1128/jb.138.3.896-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- McKeever W. G., Neidhardt F. C. Growth rate modulation of four aminoacyl-transfer ribonucleic acid synthetases in enteric bacteria. J Bacteriol. 1976 May;126(2):634–645. doi: 10.1128/jb.126.2.634-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Nasoff M. S., Wolf R. E., Jr Molecular cloning, correlation of genetic and restriction maps, and determination of the direction of transcription of gnd of Escherichia coli. J Bacteriol. 1980 Aug;143(2):731–741. doi: 10.1128/jb.143.2.731-741.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Growth rate-dependent regulation of transfer ribonucleic acid (5-methyluridine) methyltransferase in Escherichia coli B/r. J Bacteriol. 1980 Jan;141(1):67–73. doi: 10.1128/jb.141.1.67-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Phillips T. A., Bloch P. L., Neidhardt F. C. Protein identifications on O'Farrell two-dimensional gels: locations of 55 additional Escherichia coli proteins. J Bacteriol. 1980 Dec;144(3):1024–1033. doi: 10.1128/jb.144.3.1024-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Reusser F., Nomura M. DNA sequences of promoter regions for the str and spc ribosomal protein operons in E. coli. Cell. 1978 Sep;15(1):215–229. doi: 10.1016/0092-8674(78)90096-x. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Debenham P. G., Pongs O. Translation initiation factor 2 alters transcriptional selectivity of Escherichia coli ribonucleic acid polymerase. Biochemistry. 1980 Apr 15;19(8):1651–1656. doi: 10.1021/bi00549a020. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Fraenkel D. G. Isolation of specialized transducing bacteriophages for gluconate 6-phosphate dehydrogenase (gnd) of Escherichia coli. J Bacteriol. 1974 Feb;117(2):468–476. doi: 10.1128/jb.117.2.468-476.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Prather D. M., Shea F. M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J Bacteriol. 1979 Sep;139(3):1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Shea F. M. Combined use of strain construction and affinity chromatography in the rapid, high-yield purification of 6-phosphogluconate dehydrogenase from Escherichia coli. J Bacteriol. 1979 Apr;138(1):171–175. doi: 10.1128/jb.138.1.171-175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotny R., Fraenkel D. G. Glucose and gluconate metabolism in a mutant of Escherichia coli lacking gluconate-6-phosphate dehydrase. J Bacteriol. 1967 May;93(5):1579–1581. doi: 10.1128/jb.93.5.1579-1581.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A. O., Fraenkel D. G. The 6-phosphogluconate dehydrogenase reaction in Escherichia coli. J Biol Chem. 1979 Oct 25;254(20):10237–10242. [PubMed] [Google Scholar]


