Abstract
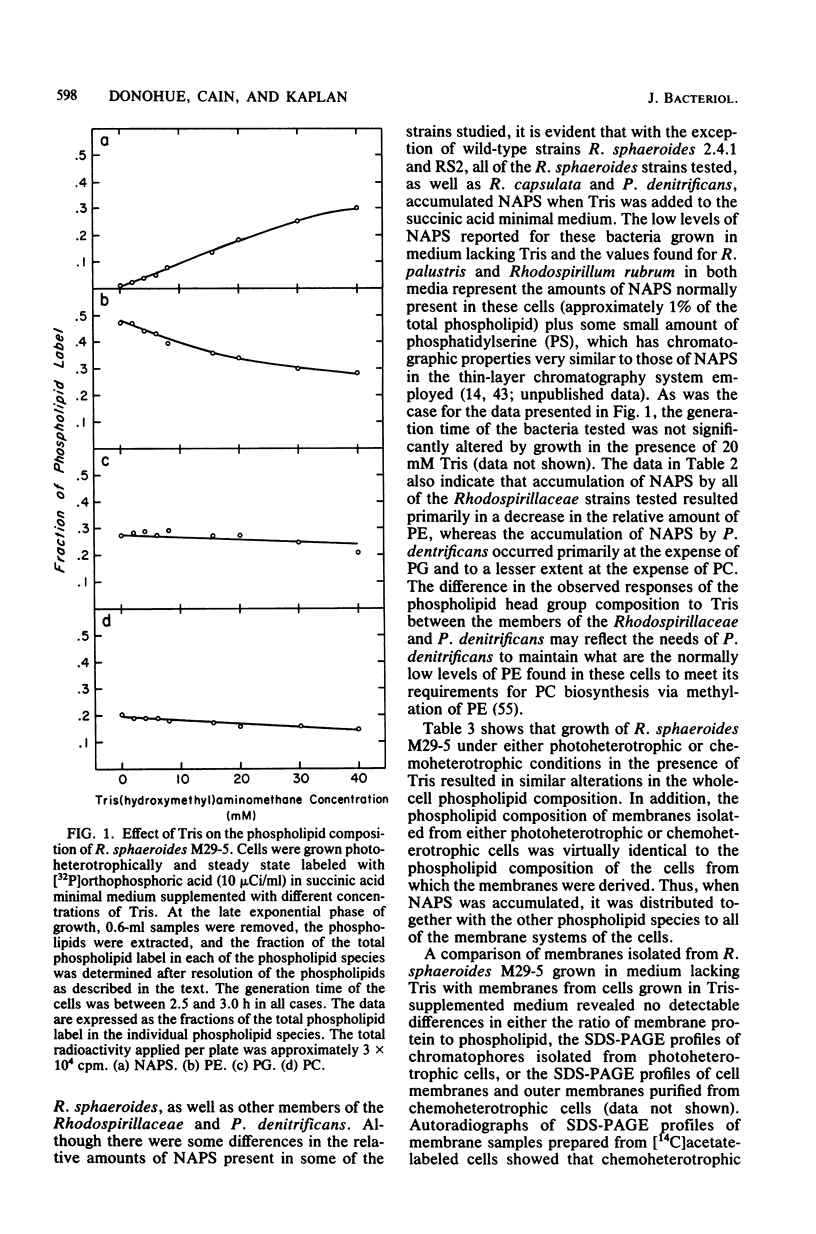
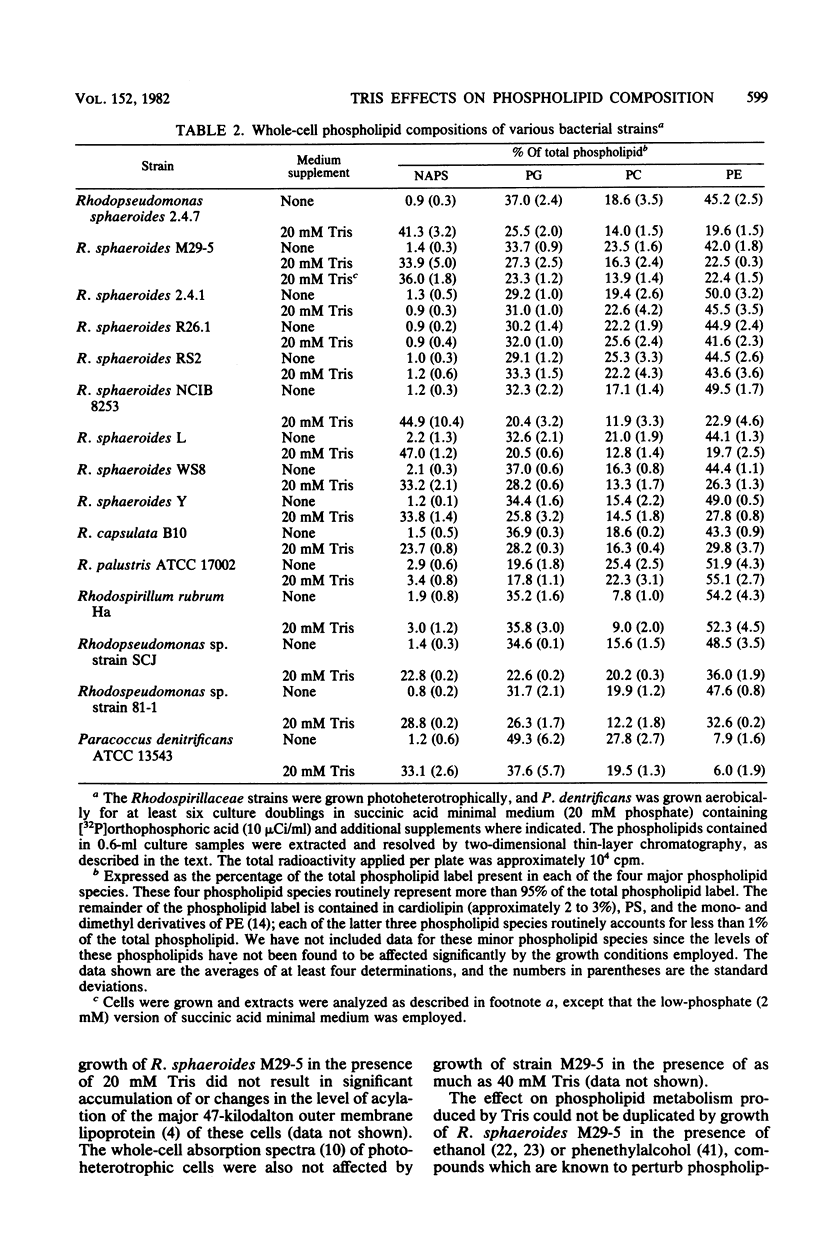
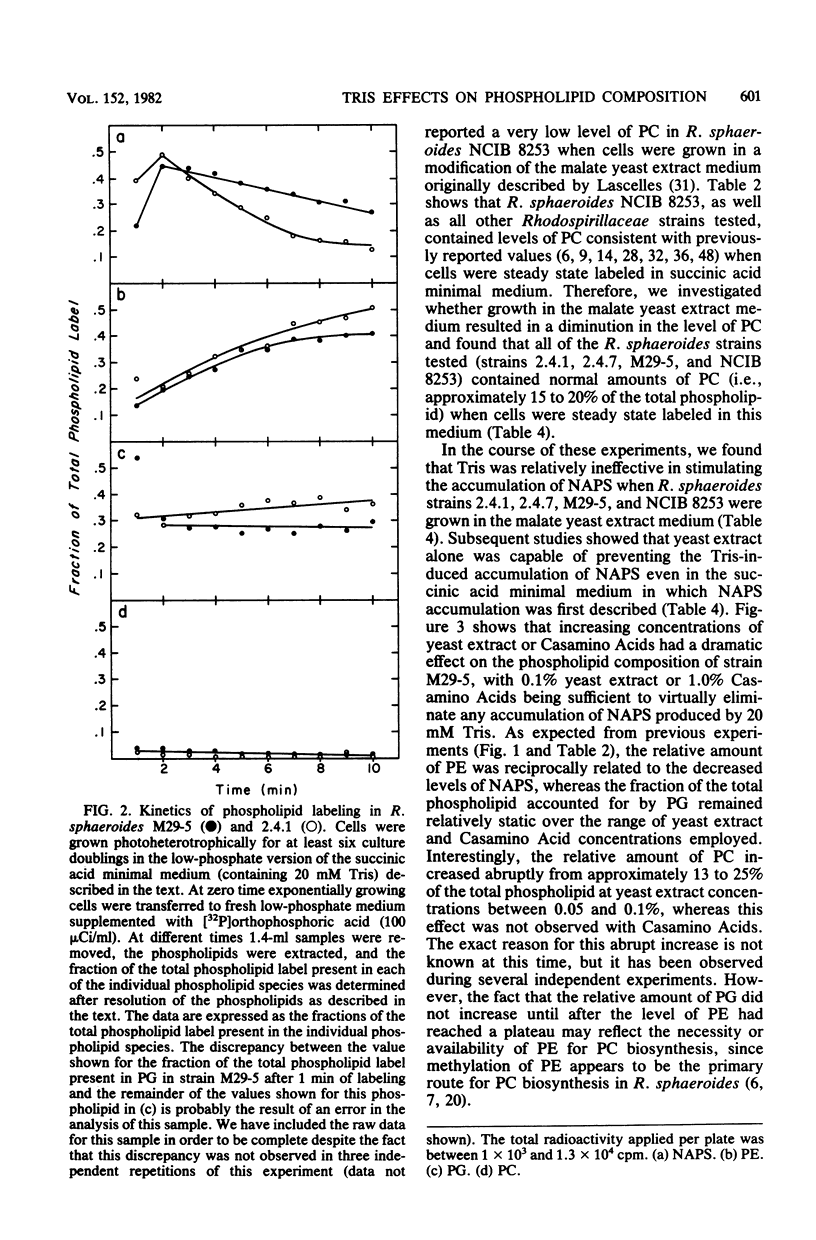
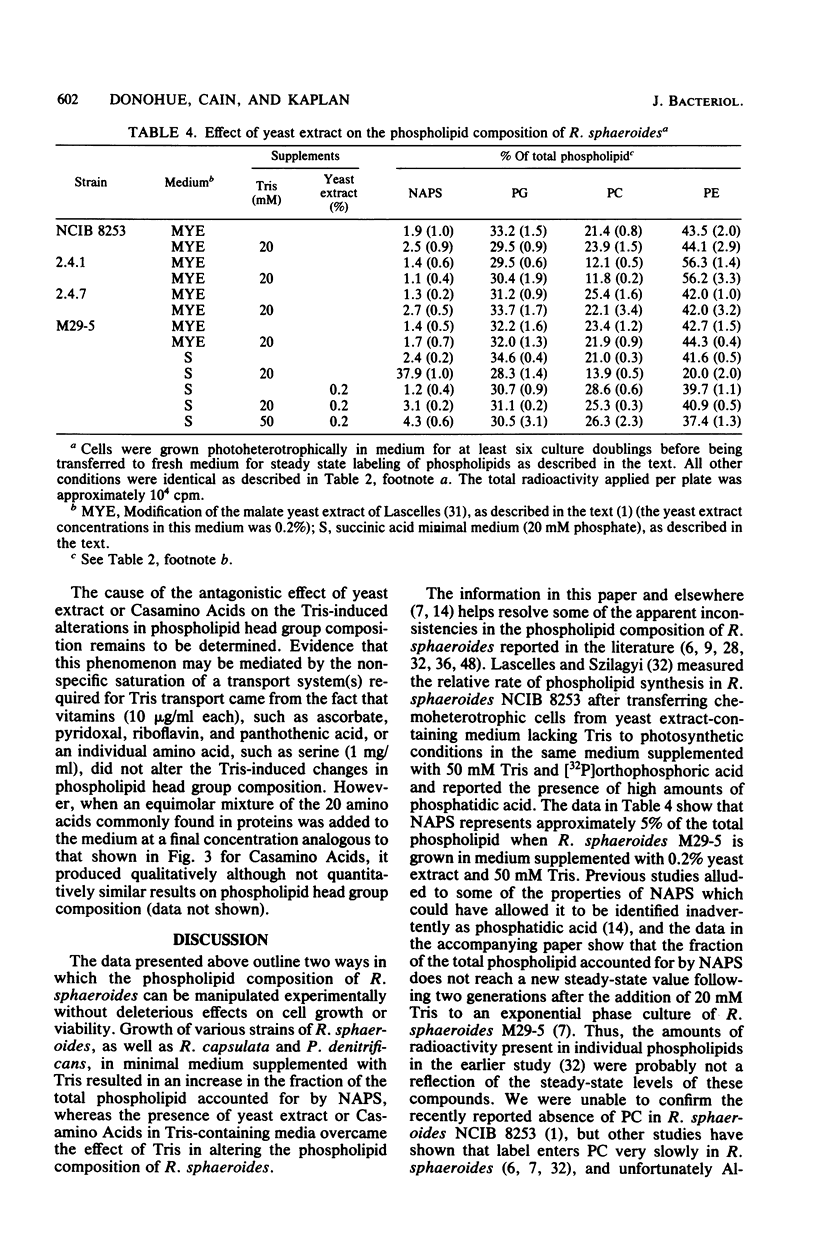
Alterations in the phospholipid head group composition of most strains of Rhodopseudomonas sphaeroides, as well as Rhodopseudomonas capsulata and Paracoccus denitrificans, occurred when cells were grown in medium supplemented with Tris. Growth of R. sphaeroides M29-5 in Tris-supplemented medium resulted in the accumulation of N-acylphosphatidylserine (NAPS) to as much as 40% of the total whole-cell phospholipid, whereas NAPS represented approximately 28 an 33% of the total phospholipid when R. capsulata and P. denitrificans respectively, were grown in medium containing 20 mM Tris. The accumulation of NAPS occurred primarily at the expense of phosphatidylethanolamine in both whole cells and isolated membranes of R. sphaeroides and had no detectable effect on cell growth under either chemoheterotrophic or photoheterotrophic conditions. Yeast extract (0.1%) and Casamino Acids (1.0%) were found to be antagonistic to the Tris-induced (20 mM) alteration in the phospholipid composition of R. sphaeroides. The wild-type strains R. sphaeroides 2.4.1 and RS2 showed no alteration in their phospholipid composition when they were grown in medium supplemented with Tris. In all strains of Rhodospirillaceae tested, as well as in P. denitrificans, NAPS represented between 1.0 and 2.0% of the total phospholipid when cells were grown in the absence of Tris. [32P]orthophosphoric acid entered NAPS rapidly in strains of R. sphaeroides that do (strain M29-5) and do not (strain 2.4.1) accumulate this phospholipid in response to Tris. Our data indicate that the phospholipid head group composition of many Rhodospirillaceae strains, as well as P. denitrificans, is easily manipulated; thus, these bacteria may provide good model systems for studying the effects of these modifications on membrane structure and function in a relatively unperturbed physiological system.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bayatti K. K., Takemoto J. Y. Phospholipid topography of the photosynthetic membrane of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Sep 15;20(19):5489–5495. doi: 10.1021/bi00522a022. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baumgardner D., Deal C., Kaplan S. Protein composition of Rhodopseudomonas sphaeroides outer membrane. J Bacteriol. 1980 Jul;143(1):265–273. doi: 10.1128/jb.143.1.265-273.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cain B. D., Deal C. D., Fraley R. T., Kaplan S. In vivo intermembrane transfer of phospholipids in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1981 Mar;145(3):1154–1166. doi: 10.1128/jb.145.3.1154-1166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain B. D., Donohue T. J., Kaplan S. Kinetic analysis of N-acylphosphatidylserine accumulation and implications for membrane assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Nov;152(2):607–615. doi: 10.1128/jb.152.2.607-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. K., Kaplan S. The non-detergent solubilization and isolation of intracytoplasmic membrane polypeptides from Rhodopseudomonas sphaeroides. J Biol Chem. 1981 Jun 10;256(11):5901–5908. [PubMed] [Google Scholar]
- Cohen L. K., Lueking D. R., Kaplan S. Intermembrane phospholipid transfer mediated by cell-free extracts of Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Feb 10;254(3):721–728. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Birge C. H., Vagelos P. R. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1969 Nov;100(2):601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Ding D. H., Kaplan S. Separation of inner and outer membranes of Rhodopseudomonas spheroides. Prep Biochem. 1976;6(1):61–79. doi: 10.1080/00327487608061599. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Purification and characterization of an N-acylphosphatidylserine from Rhodopseudomonas sphaeroides. Biochemistry. 1982 May 25;21(11):2765–2773. doi: 10.1021/bi00540a029. [DOI] [PubMed] [Google Scholar]
- Federiuk C. S., Shafer J. A. Inactivation of D-serine dehydratase by alkylamines via a transimination of enzyme-linked cofactor. J Biol Chem. 1981 Jul 25;256(14):7416–7423. [PubMed] [Google Scholar]
- Fornari C. S., Kaplan S. Genetic transformation of Rhodopseudomonas sphaeroides by plasmid DNA. J Bacteriol. 1982 Oct;152(1):89–97. doi: 10.1128/jb.152.1.89-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. The relationship of intracytoplasmic membrane assembly to the cell division cycle in Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Mar 25;254(6):1980–1986. [PubMed] [Google Scholar]
- Gorchein A., Neuberger A., Tait G. H. Incorporation of radioactivity from [me-14C]methionine and [2-14C]glycine into the lipids of Rhodopseudomonas spheroides. Proc R Soc Lond B Biol Sci. 1968 Jul 2;170(1020):299–310. doi: 10.1098/rspb.1968.0040. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Preferential inhibition of phosphatidyl ethanolamine synthesis in E. coli by alcohols. Can J Microbiol. 1977 Jun;23(6):779–789. doi: 10.1139/m77-115. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Chan R., Costerton J. W. Citrate-tris(hydroxymethyl)aminomethane-mediated release of outer membrane sections from the cell envelope of a deep-rough (heptose-deficient lipopolysaccharide) strain of Escherichia coli O8. J Bacteriol. 1981 Mar;145(3):1386–1396. doi: 10.1128/jb.145.3.1386-1396.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Baty D., Pagès J. M. Procaine, a local anesthetic interacting with the cell membrane, inhibits the processing of precursor forms of periplasmic proteins in Escherichia coli. Eur J Biochem. 1979 May 2;96(1):49–57. doi: 10.1111/j.1432-1033.1979.tb13012.x. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Tris (hydroxmethyl) aminomethane permits the expression of insulin-induced receptor loss in isolated rat adipocytes. Biochem Biophys Res Commun. 1981 Sep 30;102(2):646–653. doi: 10.1016/s0006-291x(81)80181-7. [DOI] [PubMed] [Google Scholar]
- Nunn W. D. The inhibition of phospholipid synthesis in escherichia coli by phenethyl alcohol. Biochim Biophys Acta. 1975 Mar 24;380(3):403–413. doi: 10.1016/0005-2760(75)90108-3. [DOI] [PubMed] [Google Scholar]
- Onishi J. C., Niederman R. A. Rhodopseudomonas sphaeroides membranes: alterations in phospholipid composition in aerobically and phototrophically grown cells. J Bacteriol. 1982 Mar;149(3):831–839. doi: 10.1128/jb.149.3.831-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kantor G. D., Nishijima M., Newman K. F. Cardiolipin accumulation in the inner and outer membranes of Escherichia coli mutants defective in phosphatidylserine synthetase. J Bacteriol. 1979 Aug;139(2):544–551. doi: 10.1128/jb.139.2.544-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Rothman J. E., Kennedy E. P. Asymmetrical distribution of phospholipids in the membrane of Bacillus megaterium. J Mol Biol. 1977 Mar 5;110(3):603–618. doi: 10.1016/s0022-2836(77)80114-9. [DOI] [PubMed] [Google Scholar]
- Russell N. J., Harwood J. L. Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J. 1979 Aug 1;181(2):339–345. doi: 10.1042/bj1810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre M., Kennedy E. P. Identification of bound pyruvate essential for the activity of phosphatidylserine decarboxylase of Escherichia coli. J Biol Chem. 1978 Jan 25;253(2):479–483. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Takano M., Asada K. Tris-induced cross-linking of thylakoid peptides; thiol oxidation catalyzed by Tris-Cu2+ complexes as a possible mechanism. J Biochem. 1981 Jul;90(1):87–94. doi: 10.1093/oxfordjournals.jbchem.a133472. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Tris(hydroxymethyl)aminomethane inhibits calcium uptake in vascular smooth muscle. Biochim Biophys Acta. 1979 Mar 8;551(2):459–462. doi: 10.1016/0005-2736(89)90021-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Morman M. R., White D. C. Phospholipid composition and metabolism of Micrococcus denitrificans. J Bacteriol. 1972 Dec;112(3):1288–1294. doi: 10.1128/jb.112.3.1288-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight C. A., Lueking D. R., Fraley R. T., Kaplan S. Synthesis of photopigments and electron transport components in synchronous phototrophic cultures of Rhodopseudomonas sphaeroides. J Biol Chem. 1978 Jan 25;253(2):465–471. [PubMed] [Google Scholar]



