Abstract
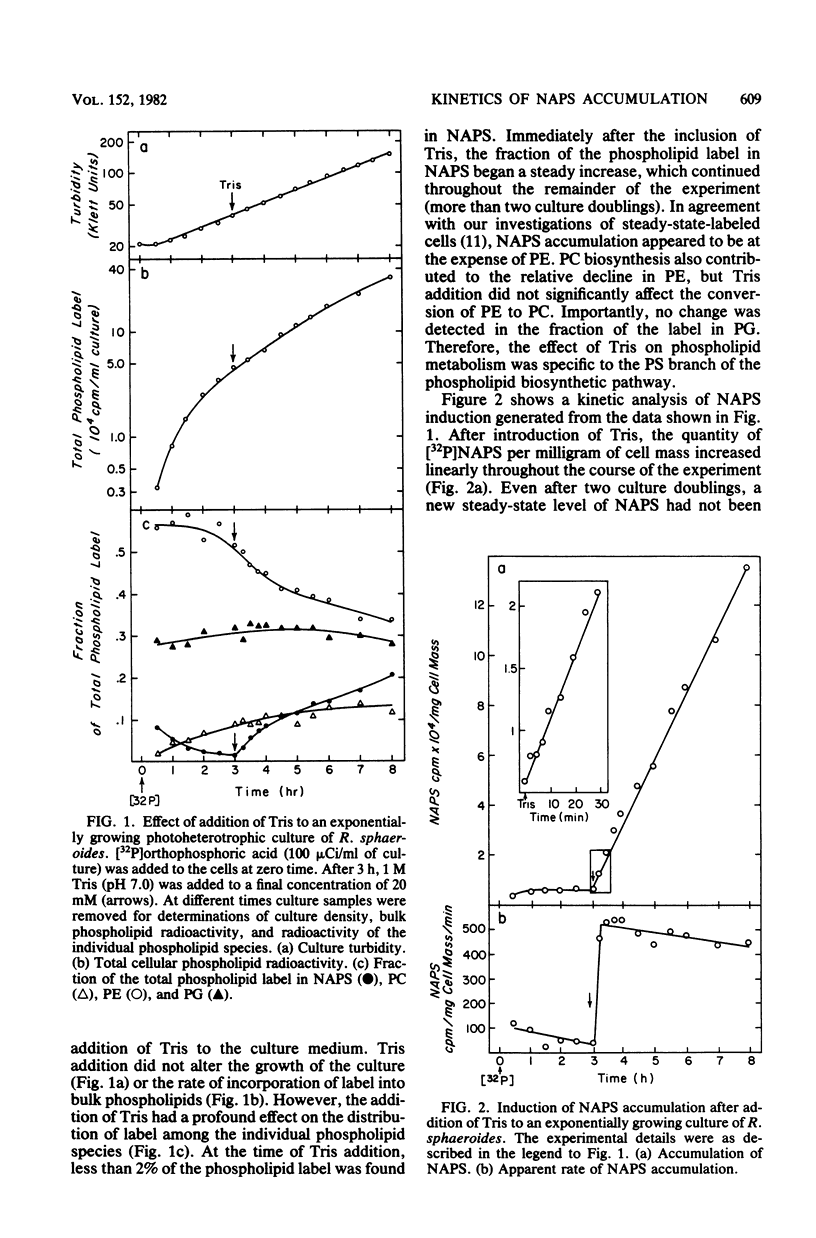
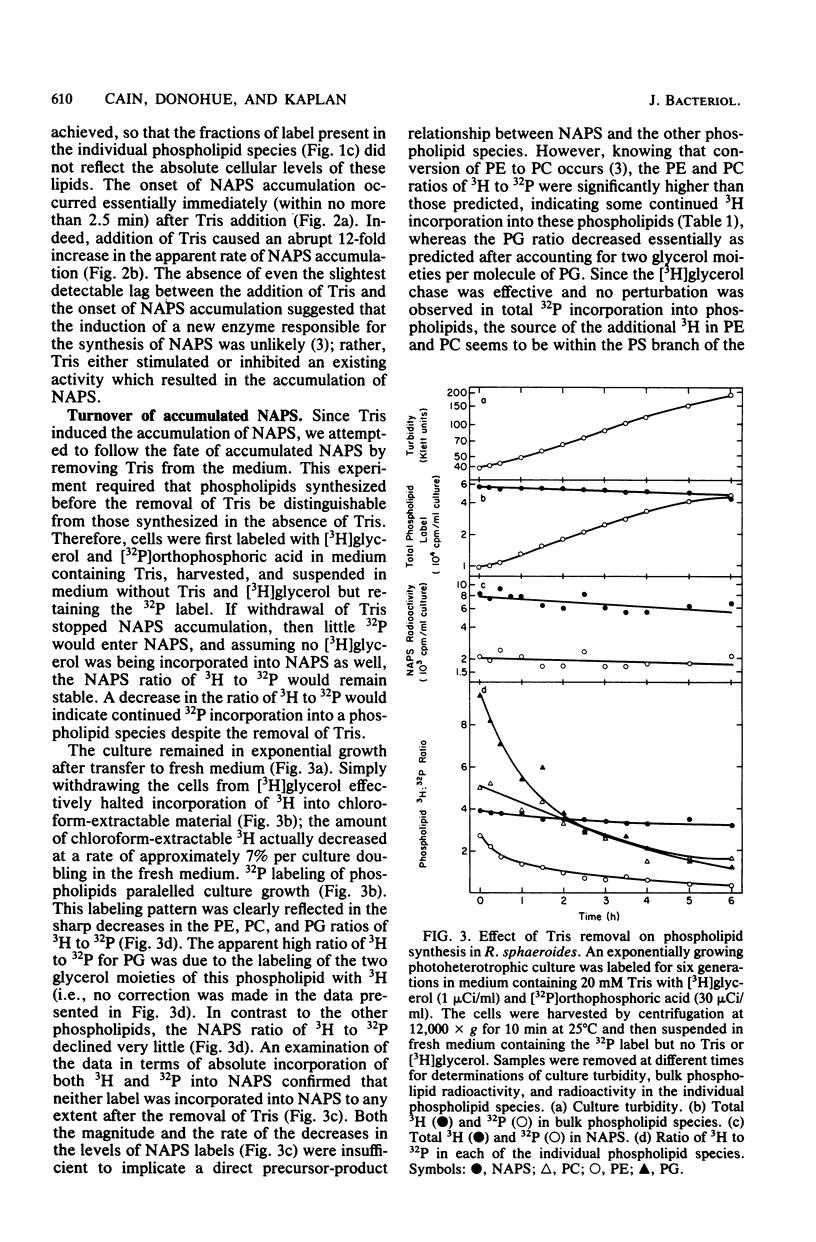
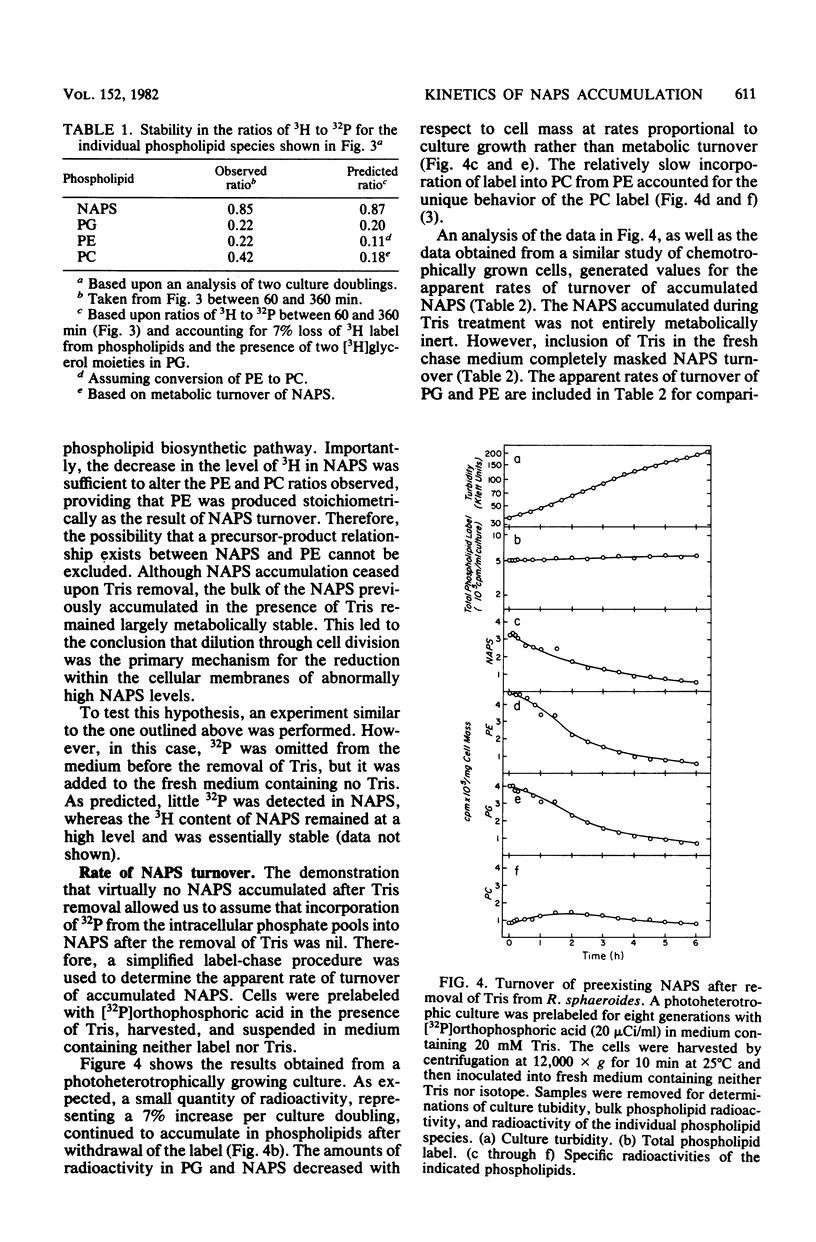
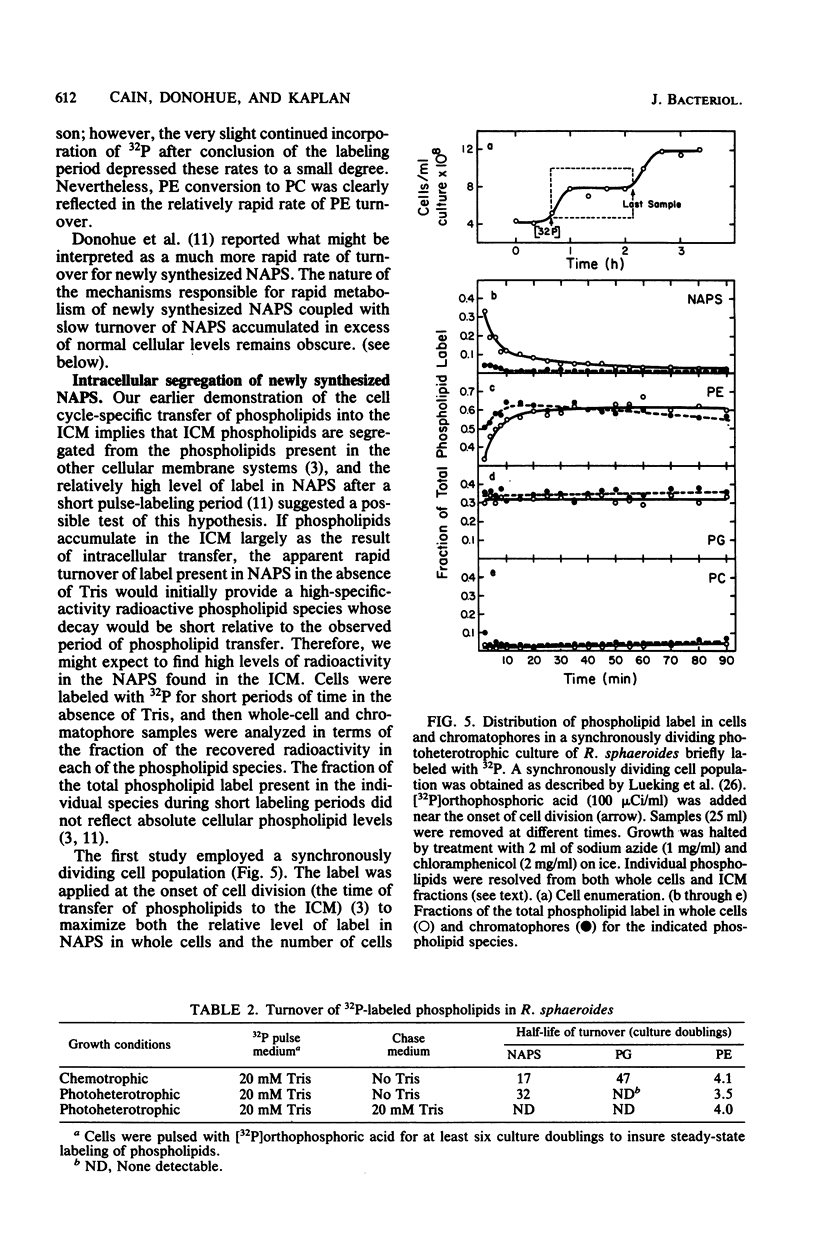
The accumulation of N-acylphosphatidylserine (NAPS) in response to the inclusion of Tris in the growth medium of Rhodopseudomonas sphaeroides strain M29-5 has been examined. In the accompanying paper (Donohue et al., J. Bacteriol. 152:000--000, 1982), we show that in response to Tris, NAPS accumulated to as much as 40% of the total cellular phospholipid content. NAPS accumulation began immediately upon addition of Tris and was reflected as an abrupt 12-fold increase in the apparent rate of NAPS accumulation. We suggest that Tris altered the flow of metabolites through a preexisting and previously unknown metabolic pathway. NAPS accumulation ceased immediately upon the removal of Tris; however, accumulated NAPS remained largely metabolically stable. Importantly, under conditions in which NAPS was not accumulated, the intracytoplasmic membrane was shown to be virtually devoid of newly synthesized NAPS. The significance of this observation is discussed in terms of its physiological implications on phospholipid transfer and membrane biogenesis in R. sphaeroides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bayatti K. K., Takemoto J. Y. Phospholipid topography of the photosynthetic membrane of Rhodopseudomonas sphaeroides. Biochemistry. 1981 Sep 15;20(19):5489–5495. doi: 10.1021/bi00522a022. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain B. D., Deal C. D., Fraley R. T., Kaplan S. In vivo intermembrane transfer of phospholipids in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1981 Mar;145(3):1154–1166. doi: 10.1128/jb.145.3.1154-1166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty C. E., Ingram L. O. Lipid synthesis during the Escherichia coli cell cycle. J Bacteriol. 1981 Jan;145(1):472–478. doi: 10.1128/jb.145.1.472-478.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Cohen L. K., Lueking D. R., Kaplan S. Intermembrane phospholipid transfer mediated by cell-free extracts of Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Feb 10;254(3):721–728. [PubMed] [Google Scholar]
- Cottrell S. F., Getz G. S., Rabinowitz M. Phospholipid accumulation during the cell cycle in synchronous cultures of the yeast, Saccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10973–10978. [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Fleming B. D., Coolbear K. P., Keough K. M. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines. Biochemistry. 1981 Jun 9;20(12):3633–3636. doi: 10.1021/bi00515a051. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Alterations in the phospholipid composition of Rhodopseudomonas sphaeroides and other bacteria induced by Tris. J Bacteriol. 1982 Nov;152(2):595–606. doi: 10.1128/jb.152.2.595-606.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Purification and characterization of an N-acylphosphatidylserine from Rhodopseudomonas sphaeroides. Biochemistry. 1982 May 25;21(11):2765–2773. doi: 10.1021/bi00540a029. [DOI] [PubMed] [Google Scholar]
- Dutt A., Dowhan W. Intracellular distribution of enzymes of phospholipid metabolism in several gram-negative bacteria. J Bacteriol. 1977 Oct;132(1):159–165. doi: 10.1128/jb.132.1.159-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. The relationship of intracytoplasmic membrane assembly to the cell division cycle in Rhodopseudomonas sphaeroides. J Biol Chem. 1979 Mar 25;254(6):1980–1986. [PubMed] [Google Scholar]
- Fraley R. T., Yen G. S., Lueking D. R., Kaplan S. The physical state of the intracytoplasmic membrane of Rhodopseudomonas sphaeroides and its relationship to the cell division cycle. J Biol Chem. 1979 Mar 25;254(6):1987–1991. [PubMed] [Google Scholar]
- Freire E., Snyder B. Estimation of the lateral distribution of molecules in two-component lipid bilayers. Biochemistry. 1980 Jan 8;19(1):88–94. doi: 10.1021/bi00542a014. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Leonard J. M., Raetz C. R. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980 Feb 25;255(4):1623–1629. [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kovatchev S., Vaz W. L., Eibl H. Lipid dependence of the membrane-bound D-lactate dehydrogenase of Escherichia coli. J Biol Chem. 1981 Oct 25;256(20):10369–10374. [PubMed] [Google Scholar]
- Lightner V. A., Larson T. J., Tailleur P., Kantor G. D., Raetz C. R., Bell R. M., Modrich P. Membrane phospholipid synthesis in Escherichia coli. Cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyl/transferase. J Biol Chem. 1980 Oct 10;255(19):9413–9420. [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Meissner G., Young R. C. Proton permeability of sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Jul 25;255(14):6814–6819. [PubMed] [Google Scholar]
- Nagle J. F., Scott H. L., Jr Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta. 1978 Nov 2;513(2):236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Characterization of two membrane-associated glycolipids from an Escherichia coli mutant deficient in phosphatidylglycerol. J Biol Chem. 1981 Oct 25;256(20):10690–10696. [PubMed] [Google Scholar]
- Nordlund J. R., Schmidt C. F., Thompson T. E. Transbilayer distribution in small unilamellar phosphatidylglycerol-phosphatidylcholine vesicles. Biochemistry. 1981 Oct 27;20(22):6415–6420. doi: 10.1021/bi00525a020. [DOI] [PubMed] [Google Scholar]
- Ong R. L., Marchesi V. T., Prestegard J. H. Small unilamellar vesicles containing glycophorin A. Chemical characterization and proton nuclear magnetic resonance studies. Biochemistry. 1981 Jul 21;20(15):4283–4292. doi: 10.1021/bi00518a008. [DOI] [PubMed] [Google Scholar]
- Onishi J. C., Niederman R. A. Rhodopseudomonas sphaeroides membranes: alterations in phospholipid composition in aerobically and phototrophically grown cells. J Bacteriol. 1982 Mar;149(3):831–839. doi: 10.1128/jb.149.3.831-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Pal R., Barenholz Y., Wagner R. R. Fluorescence studies of dipalmitoylphosphatidylcholine vesicles reconstituted with the glycoprotein of vesicular stomatitis virus. Biochemistry. 1981 May 12;20(10):2796–2800. doi: 10.1021/bi00513a014. [DOI] [PubMed] [Google Scholar]
- Pierucci O. Phospholipid synthesis during the cell division cycle of Escherichia coli. J Bacteriol. 1979 May;138(2):453–460. doi: 10.1128/jb.138.2.453-460.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Russell N. J., Harwood J. L. Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J. 1979 Aug 1;181(2):339–345. doi: 10.1042/bj1810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S., Park J. T. Penicillin-binding proteins of Rhodopseudomonas sphaeroides and their membrane localization. J Bacteriol. 1981 Aug;147(2):354–361. doi: 10.1128/jb.147.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Kouyama T., Kinosita K., Jr, Ikegami A. Effect of double bonds on the dynamic properties of the hydrocarbon region of lecithin bilayers. Biochemistry. 1981 Jul 21;20(15):4257–4262. doi: 10.1021/bi00518a004. [DOI] [PubMed] [Google Scholar]
- Wohlrab H. Purification of a reconstitutively active mitochondrial phosphate transport protein. J Biol Chem. 1980 Sep 10;255(17):8170–8173. [PubMed] [Google Scholar]


