Abstract
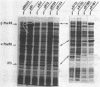
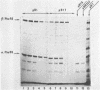
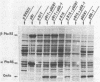
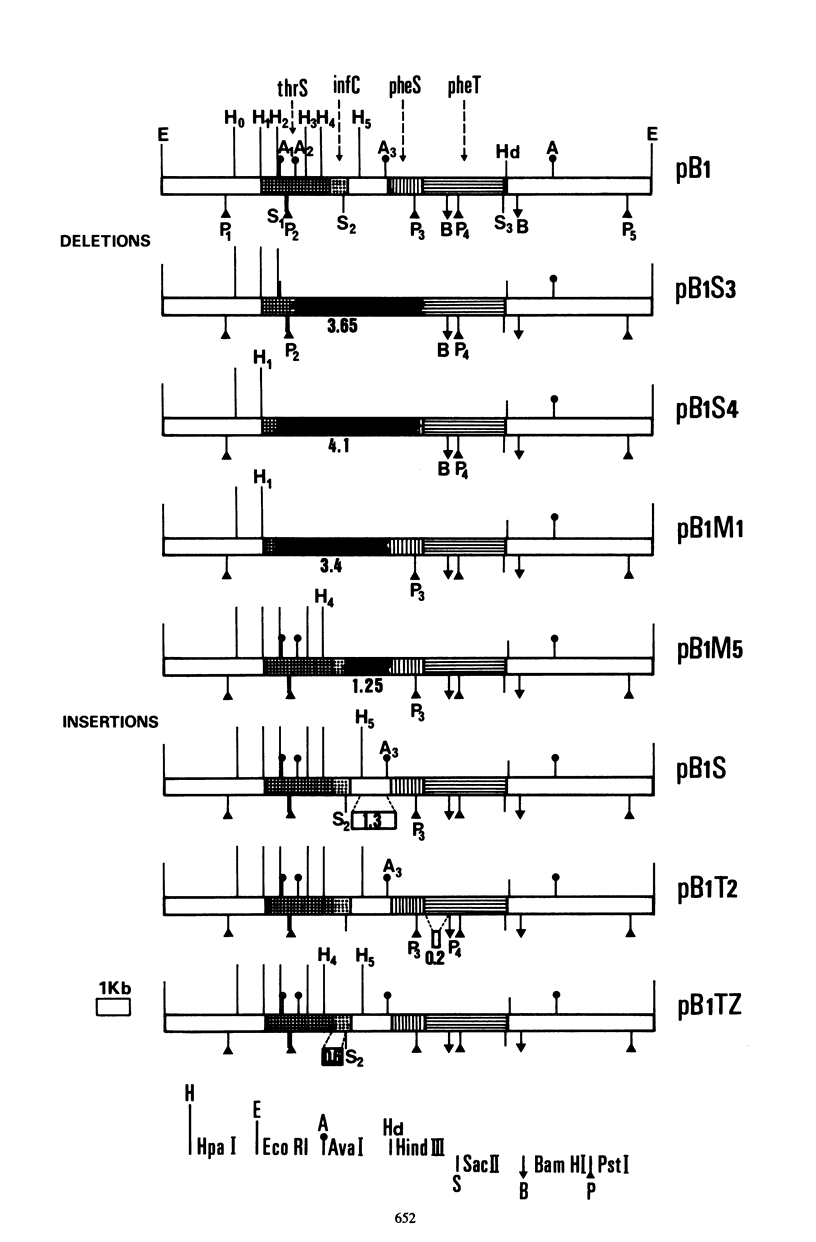
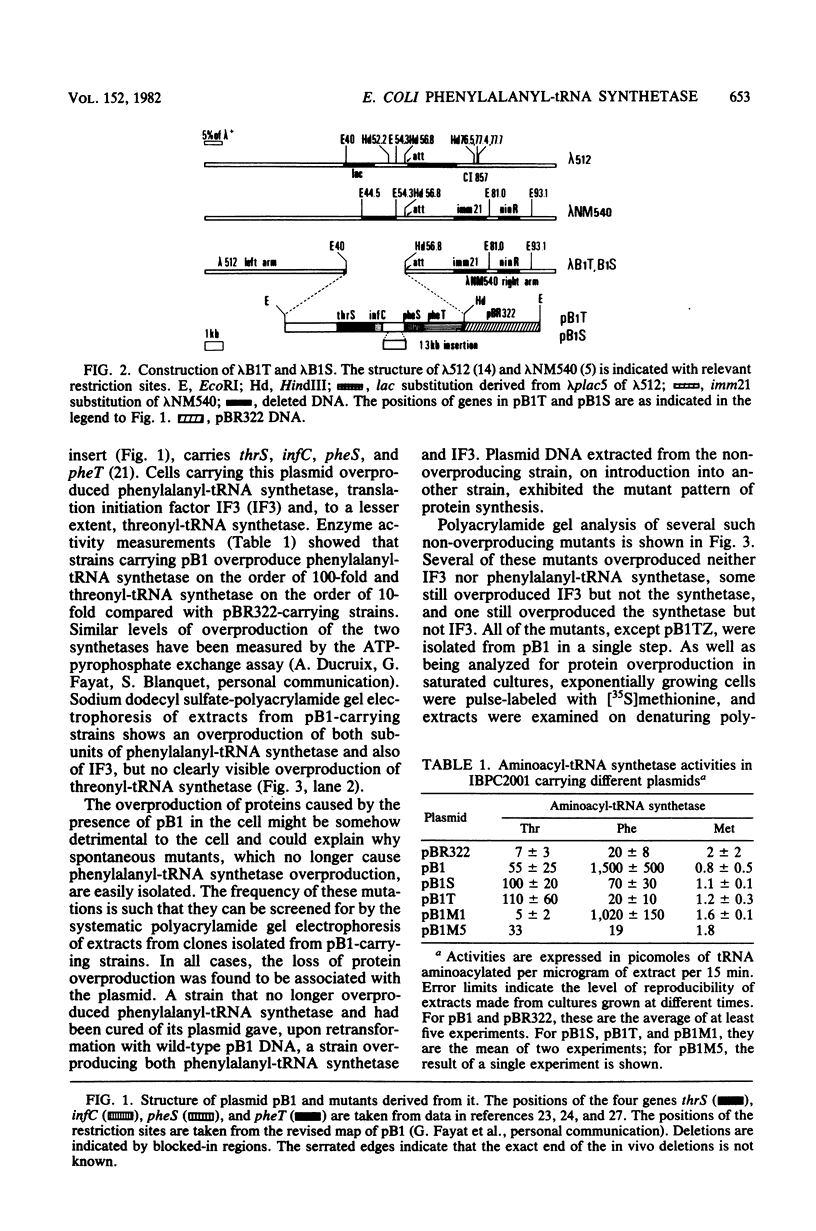
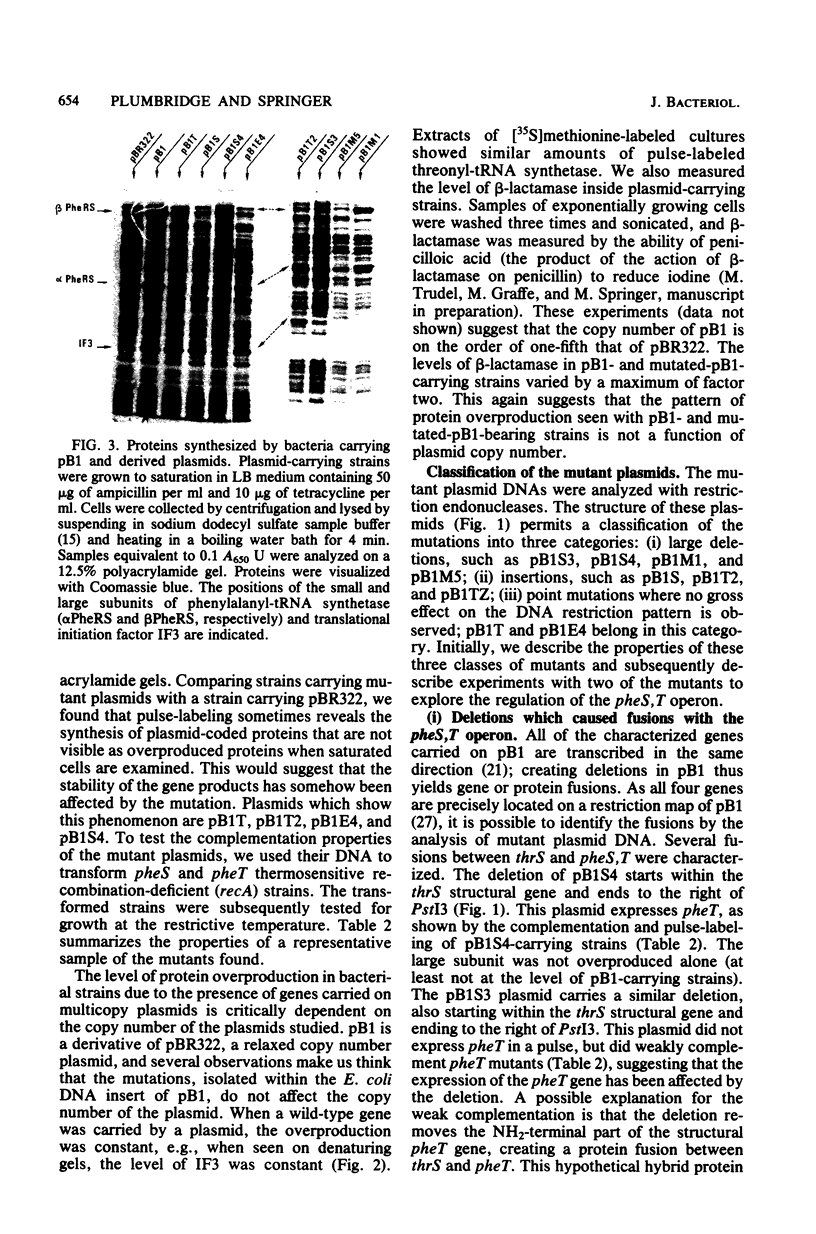
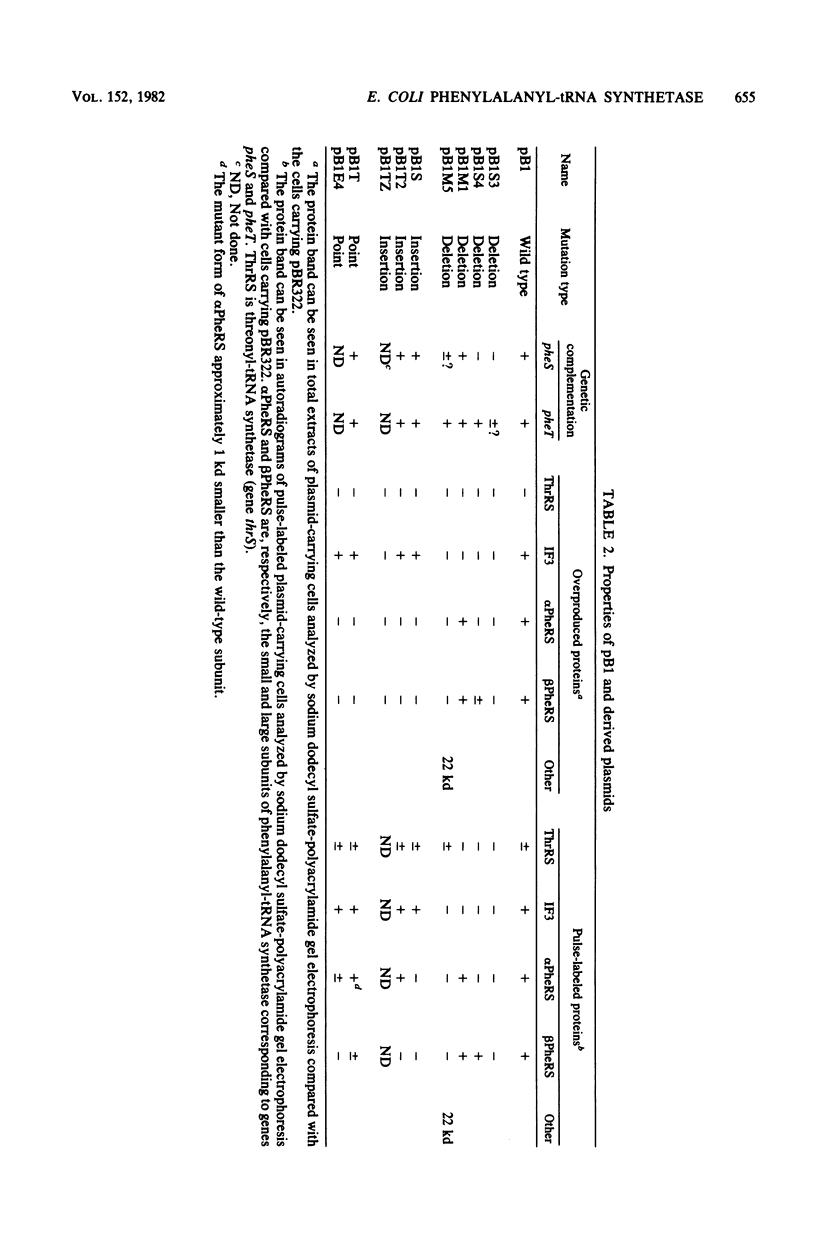
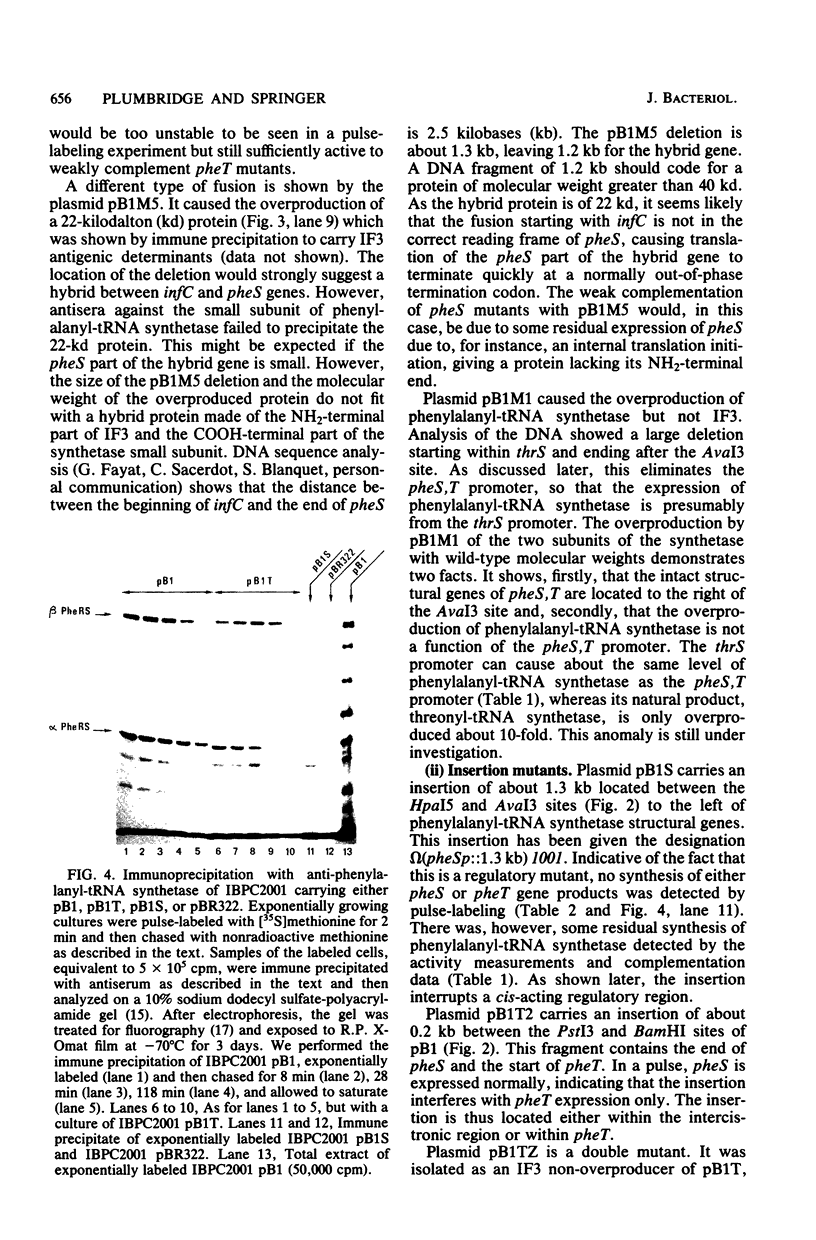
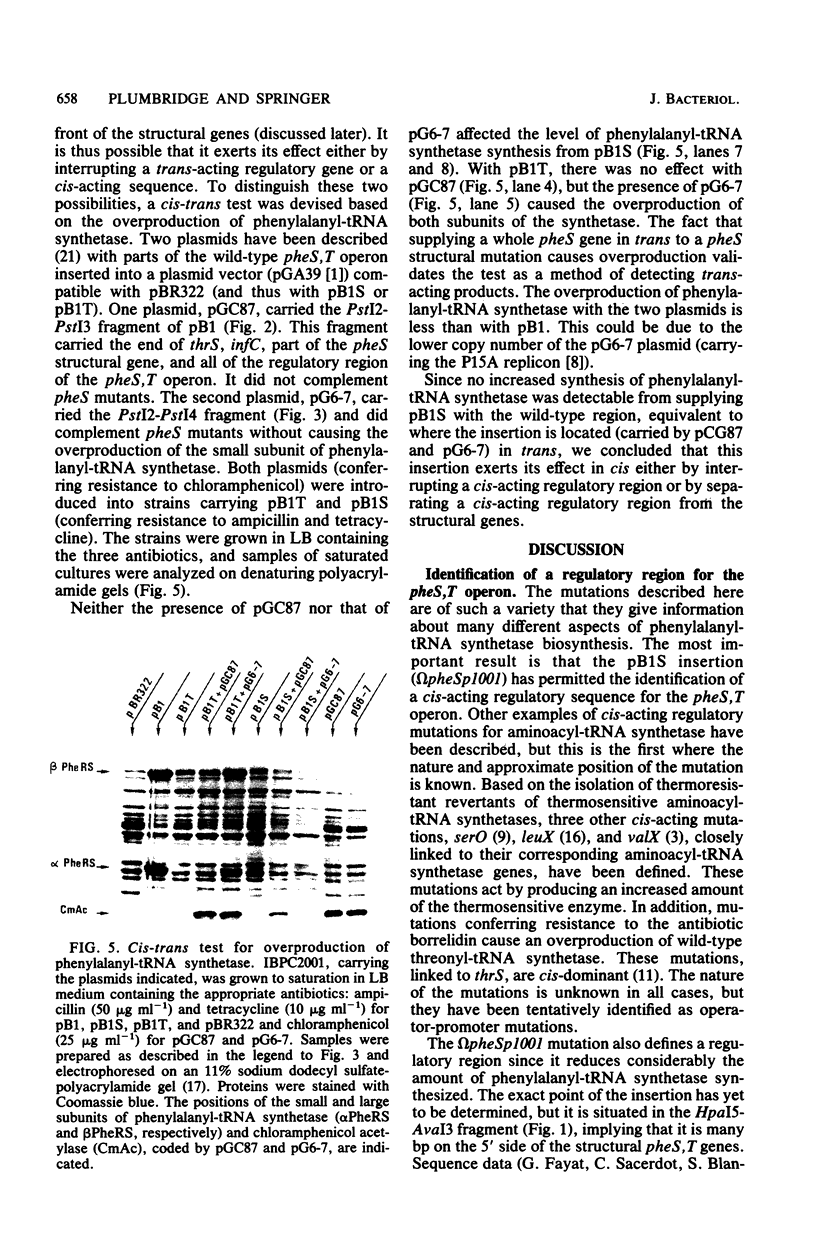
Plasmid pB1 carries the genes for threonyl-tRNA synthetase, phenylalanyl-tRNA synthetase, and translation initiation factor IF3. Strains carrying this plasmid overproduce phenylalanyl-tRNA synthetase about 100-fold. Spontaneous mutant plasmids were obtained which no longer caused the overproduction of the enzyme. Three classes of mutations were found. (i) Deletion mutations were found, some of which had the interesting property of fusing different genes together, e.g., putting phenylalanyl-tRNA synthetase under the control of the threonyl-tRNA synthetase promoter. (ii) Insertion mutations were found; one insertion in particular was studied. This insertion is located in front of the structural gene for phenylalanyl-tRNA synthetase and is shown to interrupt a cis-acting regulatory region. (iii) Mutations that showed no major change in DNA structure were found. One of these mutations is apparently purely structural, as it produces a small subunit of phenylalanyl-tRNA synthetase with a reduced molecular weight. This protein is less stable than the wild-type enzyme. These mutations represent useful tools to investigate how the phenylalanyl-tRNA synthetase operon is regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer M., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-transfer ribonucleic acid synthetases and of transfer ribonucleic acid in Escherichia coli. V. Mutants with increased levels of valyl-transfer ribonucleic acid synthetase. J Bacteriol. 1979 Jul;139(1):165–175. doi: 10.1128/jb.139.1.165-175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. Isolation and characterization of a regulatory mutant of an aminoacyl-transfer ribonucleic acid synthetase in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1096–1103. doi: 10.1128/jb.113.3.1096-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhler J., Rechenmacher A., Thomale J., Nass G., Böck A. Genetic analysis of mutations causing borrelidin resistance by overproduction of threonyl-transfer ribonucleic acid synthetase. J Bacteriol. 1980 Sep;143(3):1135–1141. doi: 10.1128/jb.143.3.1135-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Böck A., Thomale J., Nass G. Threonyl-transfer ribonucleic acid synthetase from Escherichia coli: subunit structure and genetic analysis of the structural gene by means of a mutated enzyme and of a specialized transducing lambda bacteriophage. J Bacteriol. 1977 Sep;131(3):943–950. doi: 10.1128/jb.131.3.943-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Springer M., Böck A. A specialized transducing lambda phage carrying the Escherichia coli genes for phenylalanyl-tRNA synthetase. Mol Gen Genet. 1977 Apr 29;152(3):205–210. doi: 10.1007/BF00268819. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Perricaudet M., Gros D., Garapin A., Gottesman M., Fritsch A., Tiollais P. Description and properties of bacteriophage lambda vectors useful for the cloning of EcoRI DNA fragments. Biochimie. 1978;60(2):183–187. doi: 10.1016/s0300-9084(78)80752-4. [DOI] [PubMed] [Google Scholar]
- LaRossa R., Vögell G., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. II. Isolation of regulatory mutants affecting leucyl-tRNA synthetase levels. J Mol Biol. 1977 Dec 25;117(4):1033–1048. doi: 10.1016/s0022-2836(77)80011-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Escherichia coli phenylalanyl-tRNA synthetase operon: transcription studies of wild-type and mutated operons on multicopy plasmids. J Bacteriol. 1982 Nov;152(2):661–668. doi: 10.1128/jb.152.2.661-668.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Genes for the two subunits of phenylalanyl-tRNA synthesis of Escherichia coli are transcribed from the same promoter. J Mol Biol. 1980 Dec 25;144(4):595–600. doi: 10.1016/0022-2836(80)90341-1. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Sacerdot C., Fayat G., Dessen P., Springer M., Plumbridge J. A., Grunberg-Manago M., Blanquet S. Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon. EMBO J. 1982;1(3):311–315. doi: 10.1002/j.1460-2075.1982.tb01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Genetic organization of the E. coli chromosome around the structural gene for initiation factor IF3 (infC). Mol Gen Genet. 1979 Feb 1;169(3):337–343. doi: 10.1007/BF00382279. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Hennecke H. Specialized transducing phage for the initiation factor 3 gene in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3970–3974. doi: 10.1073/pnas.74.9.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Plumbridge J. A., Trudel M., Graffe M., Grunberg-Manago M. Transcription units around the gene for E. coli translation initiation factor IF3 (infC). Mol Gen Genet. 1982;186(2):247–252. doi: 10.1007/BF00331857. [DOI] [PubMed] [Google Scholar]