Abstract
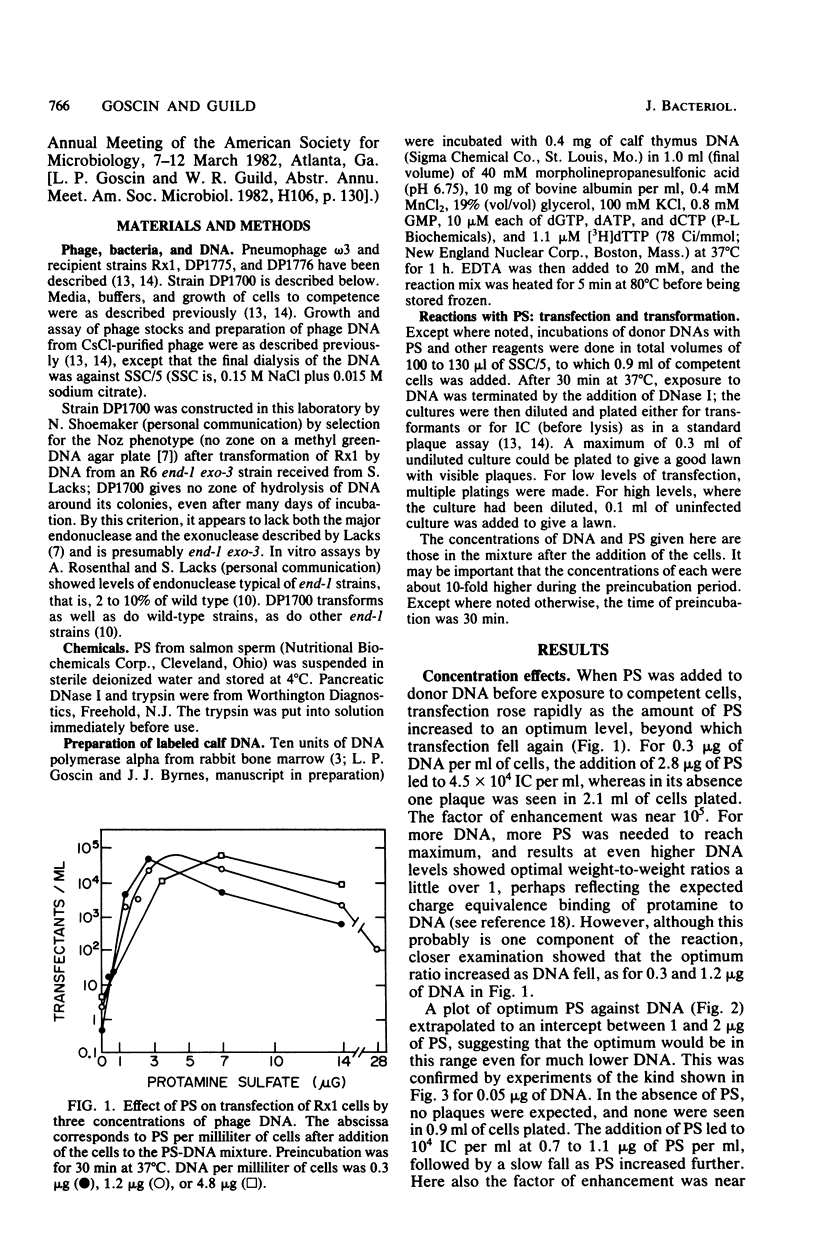
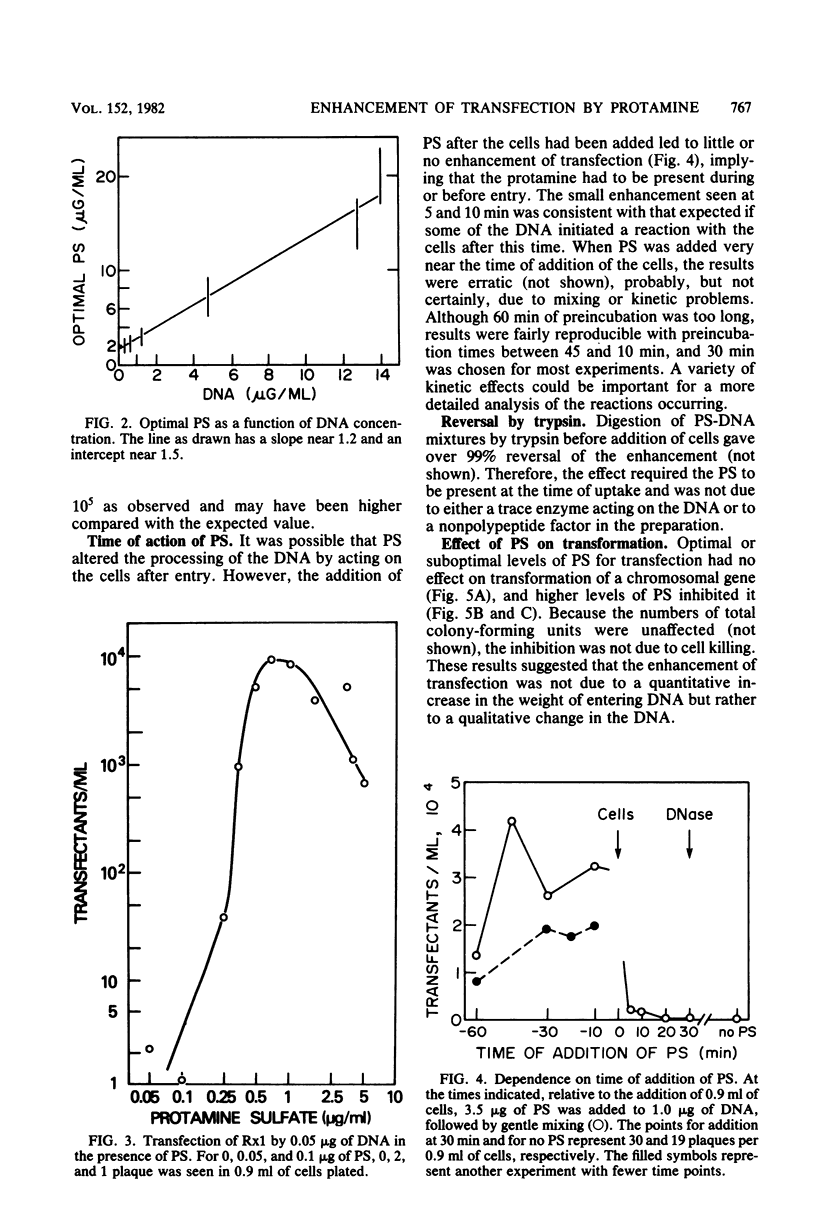
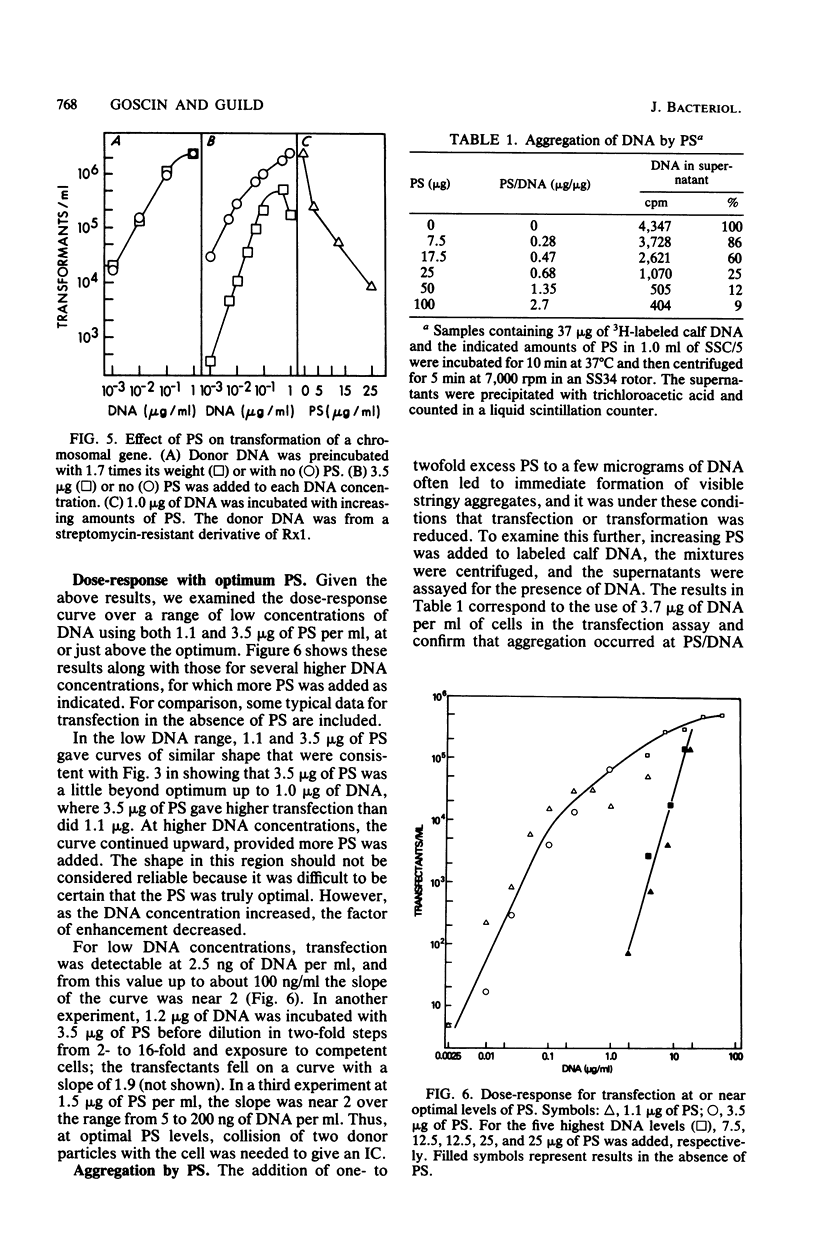
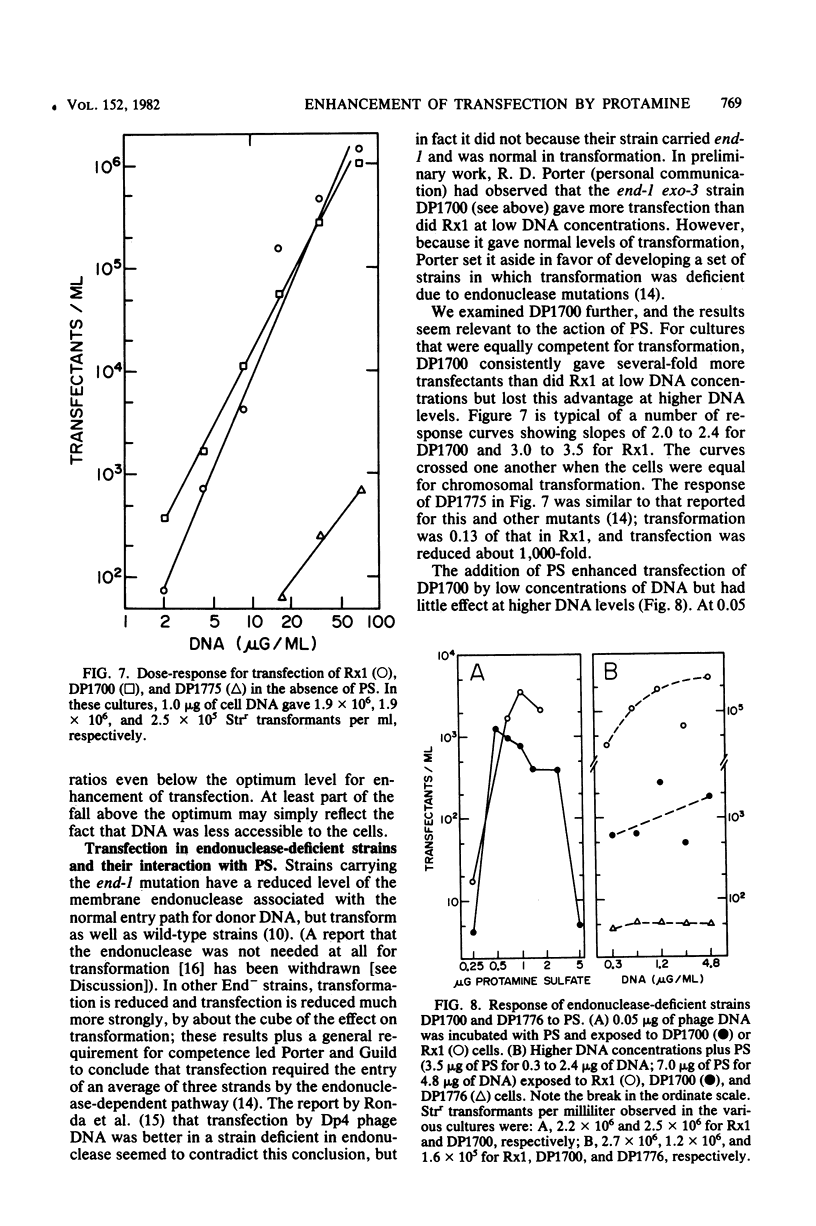
Protamine sulfate enhanced transfection of Streptococcus pneumoniae by DNA of omega 3 phage by factors as large as 10(5)-fold, provided it was present at the time the cells were added to the DNA. For DNA concentrations well below 1 microgram/ml, the optimum amount of protamine sulfate was near 1 microgram/ml of cells. Higher DNA concentrations required more protamine for maximum effect, and in all cases transfection fell when protamine was in excess. Transformation was not enhanced by low protamine levels and was inhibited by higher levels. A recipient strain with low but finite endonuclease activity and normal transformability showed higher transfection than did the wild type at low DNA concentrations but less than did the wild type at high DNA concentrations. Protamine sulfate enhanced its transfection at low, but not high, DNA concentrations. The behavior of this strain and the enhancement of transfection by protamine sulfate of wild-type cells were each consistent with less cutting of the donor DNA at the cell surface, which is part of the normal entry process in naturally competent gram-positive bacteria. Less cutting would lead to entry of fewer but longer strands that would be more efficient in reconstruction of the 33-megadalton phage replicon. We suggest that in this system protamine enhances transfection by inhibition of the surface nuclease action that is part of the normal entry process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzinger R., Kleber I., Huskey R. Transfection of Escherichia coli spheroplasts. I. General facilitation of double-stranded deoxyribonucleic acid infectivity by protamine sulfate. J Virol. 1971 May;7(5):646–650. doi: 10.1128/jvi.7.5.646-650.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R. Transfection of Enterobacteriaceae and its applications. Microbiol Rev. 1978 Mar;42(1):194–236. doi: 10.1128/mr.42.1.194-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J. J., Black V. L. Comparison of DNA polymerase alpha and delta from bone marrow. Biochemistry. 1978 Oct 3;17(20):4226–4231. doi: 10.1021/bi00613a018. [DOI] [PubMed] [Google Scholar]
- Dubes G. R. Methods for transfecting cells with nucleic acids of animal viruses: a review. Experientia Suppl. 1971;16:3–82. doi: 10.1007/978-3-0348-5773-4. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz G., Mauser R. Infectious DNA from coliphage T1. I. Some properties of the spheroplast assay system. Mol Gen Genet. 1969;104(2):178–194. doi: 10.1007/BF00272800. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Identification of a deoxyribonuclease implicated in genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1975 Jul;123(1):222–232. doi: 10.1128/jb.123.1.222-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Structure of deoxyribonucleic acid on the cell surface during uptake by pneumococcus. J Bacteriol. 1973 Sep;115(3):1055–1062. doi: 10.1128/jb.115.3.1055-1062.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Transfection in pneumococcus: single-strand intermediates in the formation of infective centers. J Virol. 1978 Jan;25(1):60–72. doi: 10.1128/jvi.25.1.60-72.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C., Lopez R., Tomasz A., Portoles A. Transfection of Streptococcus pneumoniae with bacteriophage DNA. J Virol. 1978 May;26(2):221–225. doi: 10.1128/jvi.26.2.221-225.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Asmus A., Tomasz A. Induction of normal levels of genetic transformation in a class of endonuclease-defective mutants of Pneumococci. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1067–1076. doi: 10.1016/0006-291x(78)91504-8. [DOI] [PubMed] [Google Scholar]
- Warrant R. W., Kim S. H. alpha-Helix-double helix interaction shown in the structure of a protamine-transfer RNA complex and a nucleoprotamine model. Nature. 1978 Jan 12;271(5641):130–135. doi: 10.1038/271130a0. [DOI] [PubMed] [Google Scholar]


