Abstract
Chronic cocaine abuse induces long-term neurochemical, structural and behavioural changes thought to result from altered gene expression within the nucleus accumbens and other brain regions playing a critical role in addiction. Recent methodological advances now allow the profiling of gene expression in human postmortem brain. In this article, we review studies in which we have used Affymetrix oligonucleotide microarrays to identify transcripts that are differentially expressed in the nucleus accumbens of cocaine abusers in comparison to well-matched control subjects. Of the approximately 39 000 gene transcripts interrogated, the expression of only a fraction of 1% is significantly modified in cocaine abusers. Found within this list are equivalent incidences of increased and decreased transcript abundance, including known gene transcripts clustered into several functional categories. A striking exception is a group of myelin-related genes, consisting of multiple transcripts representing myelin basic protein (MBP), proteolipid protein (PLP) and myelin-associated oligodendrocyte basic protein (MOBP), which as a group are substantially decreased in cocaine abusers compared to controls. These data, suggesting a possible dysregulation of myelin in cocaine abusers, are discussed in the context of myelin-related changes in other human brain disorders. Finally, the effects of cocaine abuse on the profile of gene expression in some other brain regions critical for addiction (the prefrontal cortex and ventral midbrain) are briefly reviewed.
Introduction
Drug addiction, which poses a serious threat to public health in terms of lost productivity and lives (Office of Applied Statistics 2000; National Institute on Drug Abuse 2003), is a multifaceted disorder involving tolerance, dependence, craving and relapse (Nestler, 2002). A better understanding of the molecular mechanisms underlying drug addiction can be expected to facilitate the development of more successful drug treatment strategies. Although the molecular basis of drug abuse is not fully understood, more is known about the neural systems subserving this disorder. In particular, animal studies have identified the nucleus accumbens as a brain region that plays a critical role in addiction (Dackis and O’Brien, 2001; Everitt and Wolf, 2002). Furthermore, in animal models, chronic exposure to cocaine induces structural and functional changes in the nucleus accumbens that are presumably mediated by altered gene expression (Norrholm et al., 2003; Toda et al., 2002). At the same time, it is difficult to model in laboratory animals the uniquely human aspects of cocaine abuse, namely the spontaneous self-administration of cocaine, most often in a binging pattern of abuse, over a period of years or decades.
With the sequencing of the human genome and the advent of microarray technologies, it seems incumbent upon neuroscientists to characterize gene expression patterns within the human brain that underlie complex, presumably polygenic disorders such as drug abuse. Although it is possible in some instances (e.g. neurosurgery patients) to obtain brain tissue biopsies, most human brain tissue becomes available only at autopsy. Fortunately, numerous studies over the last 20 years have demonstrated that mRNA is remarkably stable post mortem, and that changes in the expression of individual transcripts can be assessed readily using autopsy material (Bannon et al., 1992a, 1992b, 2002; Wilson et al., 1996; Segal et al., 1997; Albertson et al., 2004). Analysis of post-mortem brain provides a unique opportunity to examine changes in gene expression in the human drug abuser. We have used microarray technology to investigate changes in gene expression in the nucleus accumbens of chronic cocaine abusers relative to matched control subjects. Of the relatively small number of differentially expressed genes detected, the most robust finding was a decreased expression of several myelin-related genes (Albertson et al., 2004). We review the findings of these studies as well as the other published reports of gene expression profiling in the brains of human cocaine abusers.
Methodologies employed
Tissue acquisition and subject characterization
Brain specimens were collected as part of the routine autopsy process, as described previously (Albertson et al., 2004). Cause and manner of death were determined after medicolegal examination by the Medical Examiner. Study I consisted of five subjects whose deaths were ruled chronic cocaine abuse (based on toxicology, history of drug use and cardiovascular findings (Karch, 2002)) and five drug-free control subjects matched pairwise with cocaine abusers for age, gender and race. Study II consisted of five subjects who exhibited a positive toxicology for cocaine and/or its metabolites (but died of gunshot wound-related trauma) and five controls matched for demographics and cause of death. Post hoc analysis revealed no significant differences between studies of cocaine abusers and control subjects on any demographic, with the exception that Study II subjects were younger than subjects in Study I (Albertson et al., 2004).
Sample preparation and microarray hybridization
Coronal sections (2 – 3 cm thickness) were taken throughout the rostrocaudal extent of the basal ganglia at the time of autopsy. As illustrated in Fig. 1, the nucleus accumbens, defined as the ventral extension of the caudate immediately below the anterior limb of the internal capsule, was dissected free while excluding adjacent external capsule as described (Albertson et al., 2004). Isolation of RNA, elimination of contaminating genomic DNA, and assessment of RNA abundance and quality have been described in detail elsewhere (Albertson et al., 2004).
Figure 1.
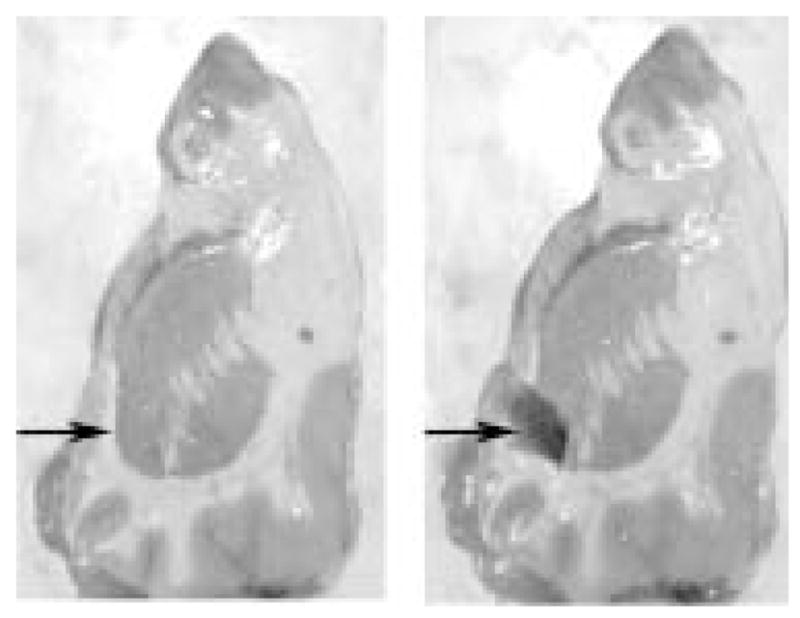
Coronal section of human forebrain illustrating the dissection of the nucleus accumbens. Arrow on the midline indicates the area dissected.
Affymetrix oligonucleotide arrays (Santa Clara, CA, USA) were used in all studies. Before use in full-scale experiments, the quality of all RNA samples was verified by test array hybridization (test 2 or test 3). The 3′/5′ ratios of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, a housekeeping gene) were generated from these test chips as a further measure of sample quality and efficiency of the reverse transcription-polymerase chain reaction (RT-PCR) and in vitro transcription. According to Affymetrix quality control parameters, this ratio should not be more than 3.0. For subsequent full-scale analysis, Study I samples were hybridized separately to five oligonucleotide arrays representing distinct probe sets: human u95Av2, u95B, u95C, u133A and u133B, for a total of 50 microarrays. Study II samples were analysed on both the u133A and u133B arrays. The sample labelling, hybridization and scanning followed the Affymetrix GeneChip® Expression Analysis Technical Manual (www.affymetrix.com).
Microarray data analysis
Microarray data were analysed with the Affymetrix Microarray Suite 5.0 software package. Images were scaled for signal intensity to account for any differences in hybridization efficiencies. Data were analysed in pairs, comparing each cocaine sample with its matched control. Significant differences between subject pairs were calculated using the Wilcoxon signed rank test (p ≤ 0.05); marginal calls were considered non-significant. Although initially analysed separately, data from Study I and Study II were pooled for increased statistical power. Only transcripts that were increased or decreased in the majority (⩾ 6 of 10) of subject pairs, representing 0.2% of the total transcripts, were considered differentially expressed. Transcripts meeting this criterion were examined post hoc for the statistical significance of differences between groups using Mann – Whitney U-tests (p ≤ 0.05). Functional groups were created using probe information provided by Affymetrix.
In order to look for similar patterns of gene expression across subjects, hierarchical clustering was performed with Spotfire Decision Site software (Somerville, MA, USA). Microarray signal data were converted to Z scores for comparison across studies. The clustering was carried out using unweighted averages, giving equal importance to each gene, and the Euclidian distance default with subsequent logistic regression analysis with SPSS version 10.0.5 (Chicago, IL, USA).
Methods for validation of microarray findings
RNA from all 20 subjects was used for verification of the microarray data. Equivalent amounts of RNA from each subject were pooled to create standard curves assayed in parallel with replicate samples consisting of 5 ng RNA from individual subjects. RT was performed (Sensiscript RT Kit, Qiagen, Valencia, CA, USA) using random hexamers, followed by PCR using the Qiagen SYBR Green PCR Kit and specific primers for myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated oligodendrocyte basic protein (MOBP), cocaine- and amphetamine-regulated transcript (CART) or β-actin. LightCycler3 Data Analysis software (Roche, Palo Alto, CA, USA) used standard curve data to create a regression model from which transcript concentrations were calculated. For sample normalization, MBP, PLP, MOBP or CART values were divided by the subject’s β-actin values. Details of this assay are published (Albertson et al., 2004). In addition, representative subject pairs were assessed for the level of MBP-immunoreactivity as described (Albertson et al., 2004).
Results of nucleus accumbens gene profiling studies
Quality control experiments
The quality of the post-mortem samples used in these experiments was assessed initially by brain pH (a reliable indicator of RNA quality and stability; Kingsbury et al., 1995) followed by RNA analysis using spectrophotometric and electropherographic techniques (Albertson et al., 2004). Sample quality was further demonstrated by hybridization to Affymetrix oligonucleotide test arrays. The 3′/5′ GAPDH ratios of all samples ( <2.0) documented the quality of input RNA as well as efficiency of the RT-PCR and in vitro transcription reactions preceding hybridization to microarrays. Thus all samples passed multiple quality measures before inclusion in the subsequent analyses. No significant differences were found among studies or groups in terms of brain pH, 3′/5′ GAPDH ratios, tissue weight, RNA yield or 260/280 ratios (Albertson et al., 2004).
In order to determine test – retest reliability for the overall microarray procedure an experiment was performed by splitting human nucleus accumbens RNA (not otherwise used in subsequent experiments) into two aliquots that were then treated as unique samples. These samples were labelled several months apart with separate RT, PCR and in vitro transcription reactions and hybridized onto two individual u95Av2 arrays. The reproducibility of the entire procedure is shown in Fig. 2 and demonstrates a significant correlation in the abundance of present transcripts (n = 5558) between the two samples as determined by Pearson’s product moment (R2 = 0.98, p = 0.002). None the less, because of the enormous number of genes interrogated, even a small residual variance means that scores of genes may be detected as differentially expressed when they are not, thus necessitating multiple microarray experiments and validation of targets using QRT-PCR.
Figure 2.
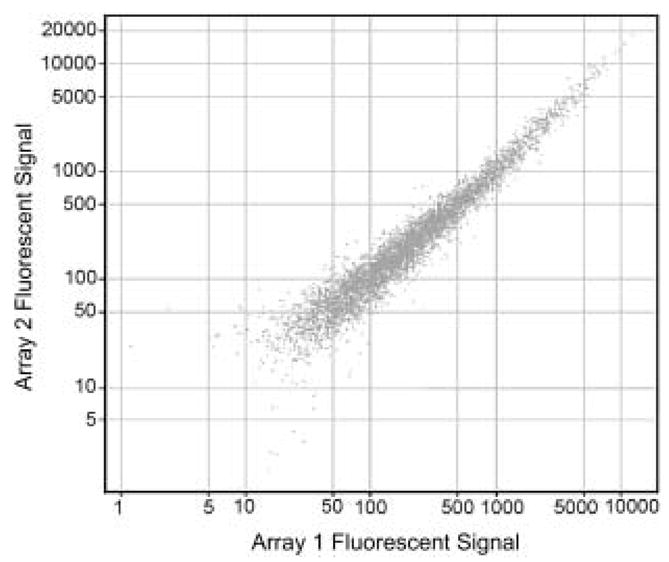
Validation of microarray procedure using human tissue. Sample derived from human nucleus accumbens was hybridized to separate Affymetrix U95A chips as described in the text.
Comparison of gene expression in cocaine abusers and matched controls
Transcripts detected in the nucleus accumbens of all subject pairs and differentially expressed in the majority of cocaine abusers relative to their matched controls represent only approximately 0.2% of the ~ 45 000 oligonucleotide probes on the u133 series Affymetrix microarrays (a total of 110 genes; see Albertson et al., 2004 for details). One-half of these were unannotated; the remainder could be clustered into broad functional categories (Fig. 3). The largest groups of annotated genes were related to signal transduction, translation/transcription/RNA processing, neurotransmission/synaptic function/membrane recycling, glia and structural/cell adhesion. Post-hoc examination (by Mann – Whitney U test) confirmed the statistical significance of the differential expression for the majority of transcripts (Albertson et al., 2004). Most functional categories encompassed both increases and decreases in cocaine-related expression patterns, such that there was little overall change seen by functional group. This could be due to a number of factors, not least of which are the broad categories employed and current limitations in gene annotation and functional clustering tools.
Figure 3.
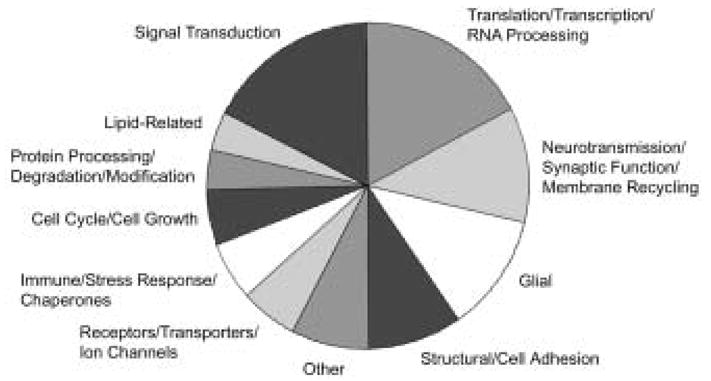
Functional categorization of annotated genes whose expression was significantly changed in cocaine abusers. As most categories encompassed both increased and decreased transcripts, there was little overall change by category.
It was interesting to note that cocaine- and amphetamine-regulated transcript (CART) mRNA levels were increased in the nucleus accumbens of human cocaine abusers, a finding confirmed by hybridization to a second distinct (u95) array as well as by RT-PCR (Albertson et al., 2004). CART represents a gene whose expression is induced by cocaine in rodent forebrain (Douglass et al., 1995), supporting the notion that at least some of the gene expression changes seen in the cocaine-exposed human brain are consonant with previous animal model data.
Although cocaine abuse was associated with equivalent incidences of increased and decreased gene expression overall (seen as positive and negative signal log ratios, respectively; Fig. 4), a striking exception was the group of myelin-related genes, consisting of multiple transcripts representing myelin basic protein (MBP), proteolipid protein (PLP) and myelin associated oligodendrocyte basic protein (MOBP). As a group, these transcripts showed a substantial decrease in cocaine abusers compared to controls (Fig. 4). Changes in myelin-related transcript abundance found using the u133 microarrays were confirmed in separate analyses using u95 series microarrays and RNA from Study I subjects (Albertson et al., 2004).
Figure 4.
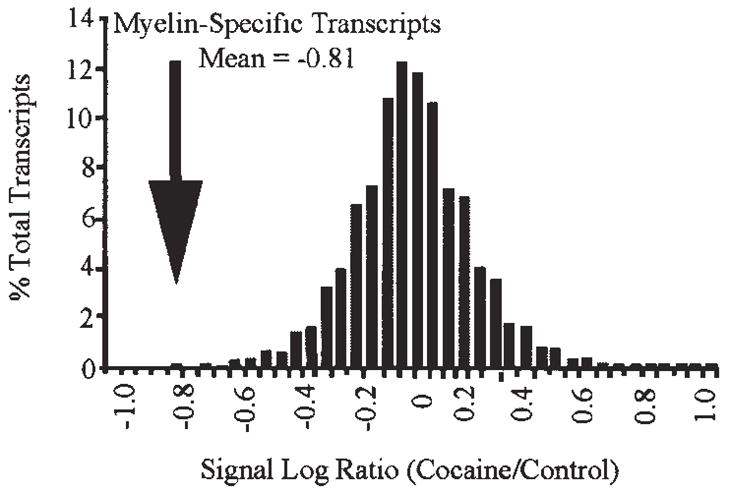
Decreased expression of myelin-related transcripts in cocaine abusers. The signal log ratio (base 2) distribution of all transcripts detected in the majority of subject pairs was plotted after binning averages into groups of 0.05 (n = 18055, mean = 0.01). The myelin-specific group (n = 5, mean = −0.81) was found to be significantly decreased from the mean of all transcripts present (t(4) = −7.95, p = 0.001).
We performed hierarchical clustering (Fig. 5) to illustrate the relatedness and relative expression levels of transcripts differentially expressed between cocaine abusers and control subjects. Microarray signal data were converted into Z scores for this analysis, with each column representing transcript expression from individual subjects. When looking at overall patterns of expression (Fig. 5, left side), some clusters of genes with both increased expression (red) and decreased expression (green) are evident in the cocaine subjects. The tight branching of MBP and MOBP (Fig. 5, right side) is indicative of a decline in cocaine subjects and suggests a similarity in their transcriptional regulation. Two genes not associated previously with MBP and MOBP, namely dishevelled associated activator of morphogenesis 2 (DAAM2) and peanut-like 2 (PNUTL2) were also found in this cluster. We performed a logistic regression, creating a mathematical model for the prediction of group affiliation (cocaine vs. control). This analysis revealed that the relative abundance of MBP and MOBP was sufficient to classify with 90% accuracy our subjects as either cocaine abusers or controls (χ2(2) = 14.14, p = 0.001). When data from all four genes in this cluster (MBP, MOBP, DAAM2 and PNUTL2) were used, the classification of cocaine abusers and controls was 100% accurate (χ2(4) = 27.73, p = 0.0001) regardless of cause of death. No such predictive value was seen with a randomly chosen set of transcripts (Albertson et al., 2004). The ability of this limited cluster of genes to identify samples derived from cocaine abusers supports the microarray data and suggests that changes in myelin-related gene expression are a fundamental characteristic of cocaine abusers.
Figure 5.
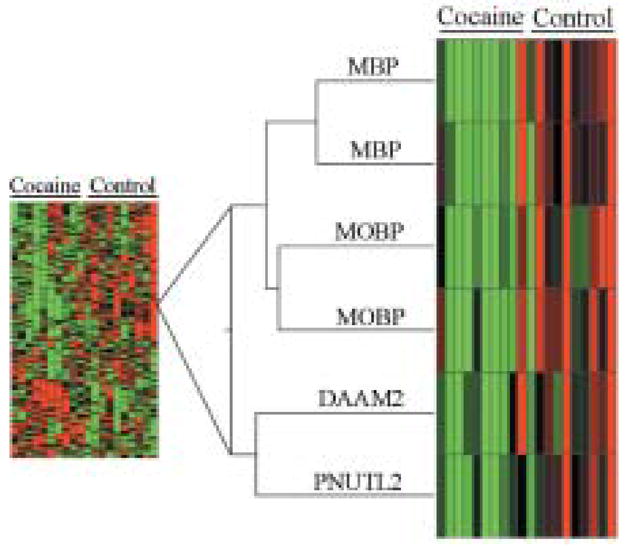
Hierarchical cluster showing the relatedness and relative expression levels of transcripts differentially expressed between cocaine and control groups. Each column represents transcript expression from individual subjects. Z-score data were converted to color for ease of interpretation: black represents the mean across subjects, with red for values above the mean, while green indicates expression below the mean. Branch lengths represent relatedness of signal expression across subjects. Right side of the figure shows the parallel changes in expression of four myelin-related transcripts (two MBP, two MOBP), and two closely clustered transcripts, as a function of cocaine abuse. MBP: myelin basic protein; MOBP: myelin-associated oligodendrocyte basic protein; DAAM2: dishevelled associated activator of morphogenesis 2; PNUTL2: peanut-like 2.
Confirmation of changes in myelin-related transcripts was obtained using quantitative RT-PCR. Cocaine abusers who exhibited decreases in MBP, MOBP and PLP relative to their controls on the microarrays were also found to have decreased abundances of these mRNAs by QRT-PCR (Albertson et al., 2004). Pearson’s correlation revealed a significant correlation between the subject pairs’ QRT-PCR and microarray data (Fig. 6; MBP for Study II subjects shown; R2 = 0.98). In addition, tissue sections from representative subject pairs processed for immunohistochemistry revealed a significant decrease in the number of MBP-immunoreactive oligodendrocytes within the nucleus accumbens (Albertson et al., 2004).
Figure 6.
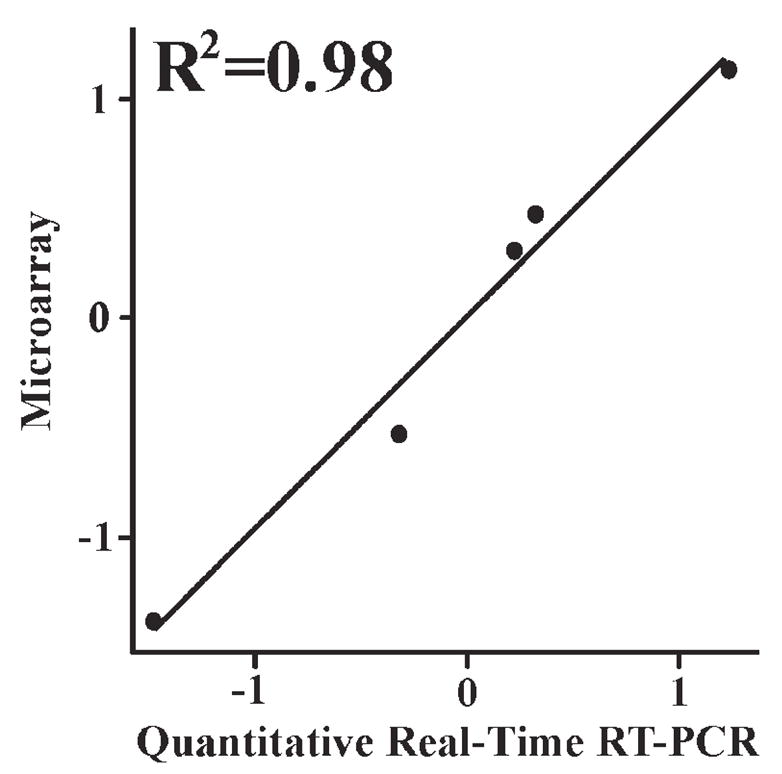
Scatter plot demonstrating the correlation between MBP microarray and quantitative RT-PCR data. Difference scores for each Study II subject pair were converted to Z-scores and correlated by Pearson’s product moment.
Discussion
Gene expression profiles in the nucleus accumbens of human cocaine abusers: dysregulation of myelin-related genes
Human cocaine abusers typically self-administer cocaine in a binging pattern over a period of years or decades. To the extent that animal models fail to duplicate these and other aspects of human cocaine abuse, they may fall short in revealing relevant changes in gene expression. This has led us to investigate changes in gene expression in human postmortem brain using microarray. This unbiased approach allowed us to explore fully differences in the nucleus accumbens of human cocaine abusers unconstrained by a priori hypotheses. Previous studies have demonstrated the stability of human brain RNA post mortem (Perrett et al., 1988; Kobayashi et al., 1990; Bannon et al., 1992a,b; Kingsbury et al., 1995) and its suitability for use with microarray (Mirnics et al., 2000; Hakak et al., 2001; Hemby et al., 2002). In the experiments described here, subject pairs were matched carefully and sample quality was confirmed by multiple measures (brain pH, RNA characterization and test array hybridization). With these samples, we found that a very small percentage of all the transcripts expressed in the nucleus accumbens was affected by chronic cocaine abuse. A major finding of these studies is the decreased expression of myelin-related genes in the nucleus accumbens of human cocaine abusers, accompanied by an apparent loss of MBP-positive oligodendrocytes. These myelin-related findings were the most robust and consistent findings from our study, cross-validated with different microarray types, multiple experimental techniques and two independent cohorts of cocaine-abusing individuals with different causes of death.
Our observations find support in several studies suggesting that drug administration in animals or humans may change myelin expression (Volkow et al., 1988a; Filley et al., 1990; Wiggins and Ruiz, 1990; Korbo, 1999; Kittler et al., 2000; Mayfield et al., 2002; Saito et al., 2002). In neuroimaging studies, white matter hyperintensities and chemical changes indicative of white matter pathology are often seen in cocaine abusers (Chang et al., 1997, 1999; Bartzokis et al., 1999a,b). More recently, two studies have demonstrated a loss of white matter volume (Bartzokis et al., 2002) and microstructure integrity in chronic human cocaine abusers (Lim et al., 2002). Paralleling our findings, Lehrmann et al. (2003) have reported decreased PLP mRNA in the prefrontal cortex of human cocaine abusers (discussed below).
One possible explanation for these documented effects on white matter might be the vasoconstrictive effects of cocaine (Kaufman et al., 1998). The normal adult human brain contains oligodendrocyte progenitors with the capacity for extensive continued myelination through the fourth decade of life (Chang et al., 2000; Bartzokis et al., 2001). Cerebral vasoconstriction has been linked to hypoperfusion, which in turn has been shown to decrease MBP over time (Kurumatani et al., 1998). It is possible that the vascular effects of cocaine could interfere with the continued myelination in adult brain, accounting for our findings of decreased myelin transcripts and decreased MBP-immunoreactive oligodendrocytes (Albertson et al., 2004). Alternatively, the effects of cocaine on myelin-related gene expression may be directly related to the increases in extracellular dopamine (DA) that cocaine elicits in the nucleus accumbens (Pettit and Justice, 1989; Hemby et al., 1997). Oligodendrocytes express D2 and D3 DA receptors (Bogarzone et al., 1998; Howard et al., 1998), and it has been reported that DA receptor stimulation decreases the conversion from immature to mature oligodendrocytes (Bogarzone et al., 1998). It is plausible that in the DA-rich nucleus accumbens, cocaine diminishes generation of mature myelin-producing oligodendrocytes through the overstimulation of oligodendrocyte DA receptors. The extent to which these effects are localized solely to the nucleus accumbens is unknown, as we also observed an apparent decrease in MBP-immunoreactive oligodendrocytes in the white matter immediately surrounding the nucleus accumbens of cocaine abusers (data not shown). Further studies are required to distinguish between these possibilities. Although the literature connecting myelin and cocaine is relatively modest, perhaps reflective of the unexpectedness of the association, a link between altered myelination and the cognitive and motoric deficits associated with cocaine abuse (Bauer, 1996; Strickland et al., 1998; Robinson et al., 1999; Fillmore and Rush, 2002) has face validity. It has been reported that the majority of long-term cocaine users have focal perfusion defects, a subtle form of cerebrovascular dysfunction, which have been associated with moderate to severe cognitive impairment (Volkow et al., 1988b; Holman et al., 1991; Strickland et al., 1993). Both the cognitive and focal vascular defects reportedly persist in periods of abstinence.
‘Another possibility that cannot be discounted at present is that the decreased expression of myelin-associated genes is not secondary to cocaine’s neurochemical effects, but represents instead a predisposing phenotype associated with cocaine abuse. In this regard, it may be worth noting that chronic cocaine abusers such as the subjects in our study (half of whom died directly as a result of drug abuse), represent a very small proportion of individuals in the general population who have self-administered cocaine at some time. The dysregulation of myelin in a number of other central nervous system (CNS) disorders is discussed below.
Gene expression profiles in other brain regions from human cocaine abusers
Another recent gene expression profiling study has focused on changes occurring within the ventral midbrain, the site of origin for DA neurones that project to the nucleus accumbens and other forebrain areas (Tang et al., 2003). In this study, the midbrain ventral tegmental area and lateral substantia nigra from 10 cocaine abusers and 11 matched controls were analysed using custom neural macroarray blots consisting of 81 cDNAs corresponding to DA, glutamate and GABA receptors, regulators of G protein signalling and a few other transcripts. The predominant effect observed in this small subset of genes was an up-regulation of several glutamate receptor transcripts in the ventral tegmental area, a finding broadly supported by immunoblot data. It is also interesting to note that the neurotransmitter CART is down-regulated in the ventral tegmental area of cocaine abusers (Tang et al., 2003) although up-regulated in the nucleus accumbens (Albertson et al., 2004).
In addition to the nucleus accumbens, the prefrontal cortex is thought to play an important role in mediating responses to cocaine in animals and man (Lehrmann et al., 2003). The profile of gene expression in the dorsolateral prefrontal cortex has been examined using samples from seven cocaine abusers and matched controls with a pairwise design and an 1100 clone neuroarray (Lehrmann et al., 2003). Sixty-five differentially expressed transcripts were identified, representing changes in broad functional categories including energy metabolism, cytoskeleton, cell signalling, transcription and oligodendrocyte function. The authors noted that each subject pair fell into one of two distinct patterns of gene expression, with the subgroups often showing opposite directions of change for any given gene. The presence of anhydroecgonine (a cocaine metabolite generated by smoking crack cocaine) in most of the cocaine abusers from one of the two subgroups led to the suggestion that the distinct patterns of gene expression might reflect an acute response to crack cocaine exposure in one group vs. a post-drug refractory state in the other cohort. This intriguing hypothesis warrants further investigation.
In this latter study, a significant decrease in PLP expression was seen in the prefrontal cortex of a subset of cocaine abusers (Lehrmann et al., 2003), paralleling the striking changes we have seen in PLP and other myelin-related genes in the nucleus accumbens (Albertson et al., 2004). Surprisingly, decreased expression of PLP and numerous other myelin-related genes have also been observed in postmortem brains from alcoholics (Lewohl et al., 2000; Mayfield et al., 2002), schizophrenics (Hakak et al., 2001; Pongrac et al., 2002; Tkachev et al., 2003) and bipolar disorder patients (Tkachev et al., 2003). The common factors among these disparate diseases that could underlie similar changes in myelin-related gene expression are, at present, obscure. The fact that several other brain diseases do not exhibit changes in myelin-related gene expression (Tkachev et al., 2003) suggests some specificity to this finding. Clearly, this intriguing commonality of decreased myelin-related gene expression warrants further investigation.
The difficulties in comparing data across CNS gene profiling studies are manifold. One obvious difficulty with any study of the CNS is that each brain region encompasses a different complement of neural types with distinct interneuronal connections and responsivity to stimuli. Another potential confound of human addiction studies is the cohorts studied, their history of drug exposure and comorbidity for other brain disorders. Of course, the nature of the gene expression platform employed (macroarray vs. microarray, custom designed vs. commercial) is critical, as expression changes cannot be detected for genes that are not assessed. Less obvious to the scientist not engaged in these types of studies is the importance of experimental design (e.g. pairwise vs. group analysis) and statistical analyses on the outcome of these studies. Hopefully, many of these limitations will be overcome successfully through sample and data sharing arrangements and further standardization of experimental approaches. Even with these limitations, gene expression profiling of human postmortem brain holds great promise for providing new and unanticipated insights into the pathogenesis of addictive disorders, ultimately suggesting novel therapeutic strategies.
Acknowledgments
The authors would like to extend their gratitude to Drs John Kamholz, Robert Skoff, Anthony Campagnoni, Robin Fisher and Sharon K. Michelhaugh for helpful discussions and Donald M. Kuhn, Carl J. Schmidt and Barb Pruetz for their participation in the studies reviewed above. We would also like to thank Dr Susan Land, Daniel Lott, Tara Twomey, Bin Yao and Dan Liu at Wayne State University’s Applied Genomics Technology Center.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Poosch MS, Haverstick DM, Mandall A, Xue IC-H, Shibata K, Dragovic LJ. Preprotachykinin gene expression in the human basal ganglia: characterization of mRNAs and pre-mRNAs produced by alternate RNA splicing. Brain Res Mol Brain Res. 1992a;12:225–231. doi: 10.1016/0169-328x(92)90088-s. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci USA. 1992b;89:7095–7099. doi: 10.1073/pnas.89.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Pruetz B, Manning-Bog AB, Whitty CJ, Michelhaugh SK, Sacchetti P, Granneman JG, Mash DC, Schmidt CJ. Decreased expression of the transcription factor NURR1 in dopamine neurons of cocaine abusers. Proc Natl Acad Sci USA. 2002;99:6382–6385. doi: 10.1073/pnas.092654299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Hance DB, Lu PH, Foster JA, Mintz J, Ling W, Bridge P. Magnetic resonance imaging evidence of ‘silent’ cerebrovascular toxicity in cocaine dependence. Biol Psychiatry. 1999a;45:1203–1211. doi: 10.1016/s0006-3223(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Goldstein IB, Hance DB, Beckson M, Shapiro D, Lu PH, Edwards N, Mintz J, Bridge P. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. Am J Neuroradiol. 1999b;20:1628–1635. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Resting hand tremor in abstinent cocaine-dependent, alcohol-dependent, and polydrug-dependent patients. Alcohol Clin Exp Res. 1996;20:1196–1201. doi: 10.1111/j.1530-0277.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Bogarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18:5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Mehringer CM, Ernst T, Melchor R, Myers H, Forney D, Satz P. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;42:1105–1114. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Dopamine release in the dorsal striatum during cocaine-seeking behaviour under the control of a drug-associated cue. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Heaton RK, Rosenberg NL. White matter dementia in chronic toluene abuse. Neurology. 1990;40:532–534. doi: 10.1212/wnl.40.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behaviour in chronic cocaine users. Drug Alcohol Dep. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch Gen Psychiatry. 2002;59:631–640. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Holman BL, Carvalho PA, Mendelson JH, Teoh SK, Nardin R, Hallgring E, Hebben N, Johnson KA. Brain perfusion is abnormal in cocaine-dependent polydrug users: a study using technetium-99m-HMPAO and ASPECT. J Nucl Med. 1991;32:1206–1210. [PubMed] [Google Scholar]
- Howard S, Landry C, Fisher R, Bezouglaia O, Handley V, Campagnoni A. Postnatal localization and morphogenesis of cells expressing the dopaminergic D2 receptor gene in rat brain: expression in non-neuronal cells. J Comp Neurol. 1998;391:87–98. doi: 10.1002/(sici)1096-9861(19980202)391:1<87::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Karch SB. Karch’s pathology of drug abuse. 3. Washington, DC: CRC Press; 2002. pp. 1–186. [Google Scholar]
- Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ, Mendelson JH, Lukas SE, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. [PubMed] [Google Scholar]
- Kingsbury AE, Foster OJF, Nisbet AP, Cairns N, Bray L, Eve DJ, Lees AJ, Marsden CD. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–318. doi: 10.1016/0169-328x(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Grigorenko EV, Clayton C, Zhuang S-Y, Bundey SC, Trowler MM, Wallace D, Hampson R, Deadwyler S. Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics. 2000;3:175–185. doi: 10.1152/physiolgenomics.2000.3.3.175. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sakimura K, Kuwano R, Sato S, Ikuta F, Takahashi Y, Miyatake T, Tsuji S. Stability of messenger RNA in postmortem human brains and construction of human brain cDNA libraries. J Mol Neurosci. 1990;2:29–34. doi: 10.1007/BF02896923. [DOI] [PubMed] [Google Scholar]
- Korbo L. Glial cell loss in the hippocampus of alcoholics. Alcohol Clin Exp Res. 1999;23:164–168. [PubMed] [Google Scholar]
- Kurumatani T, Kudo T, Ikura Y, Takeda M. White matter changes in the gerbil brain under chronic cerebral hypoperfusion. Stroke. 1998;29:1058–1062. doi: 10.1161/01.str.29.5.1058. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Oyler J, Vawter MP, Hyde TM, Kolachana B, Kleinman JE, Huestis MA, Becker KG, Freed WJ. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochem. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) NIDA info facts: costs to society. Rockville, MD: National Institutes of Health, US Department of Health and Human Services; 2003. [Google Scholar]
- Nestler EJ. From neurobiology to treatment: progress against addiction. Nature Neurosci. 2002;5:1076–1079. doi: 10.1038/nn945. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Applied Statistics. Drug abuse warning network annual medical examiner data 1998. Rockville, MD: SAMHSA, US Department of Health and Human Services; 2000. [Google Scholar]
- Perrett CW, Marchbanks RM, Whatley SA. Characterisation of messenger RNA extracted post-mortem from the brains of schizophrenic, depressed and control subjects. J Neurol Neurosurg Psychiatry. 1988;51:325–331. doi: 10.1136/jnnp.51.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pongrac J, Middleton FA, Lewis DA, Levitt P, Mirnics K. Gene expression profiling with DNA microarrays: advancing our understanding of psychiatric disorders. Neurochem Res. 2002;27:1049–1063. doi: 10.1023/a:1020904821237. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Heaton RK, O’Malley SS. Neuropsychological functioning in cocaine abusers with and without alcohol dependence. J Int Neuropsychol Soc. 1999;5:10–19. doi: 10.1017/s1355617799511028. [DOI] [PubMed] [Google Scholar]
- Saito M, Smiley J, Toth R, Vadasz C. Microarray analysis of gene expression in rat hippocampus after chronic ethanol treatment. Neurochem Res. 2002;27:1221–1229. doi: 10.1023/a:1020937728506. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Strickland TL, Miller BL, Kowell A, Stein R. Neurobiology of cocaine-induced organic brain impairment: contributions from functional neuroimaging. Neuropsychol Rev. 1998;8:1–9. doi: 10.1023/a:1025613322003. [DOI] [PubMed] [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Toda S, McGinty JF, Kalivas PW. Repeated cocaine administration alters the expression of genes in corticolimbic circuitry after a 3-week withdrawal: a DNA macroarray study. J Neurochem. 2002;82:1290–1299. doi: 10.1046/j.1471-4159.2002.01083.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Valentine A, Kulkarni M. Radiological and neurological changes in the drug abuse patient: a study with MRI. J Neuroradiol. 1988a;15:288–293. [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988b;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Wiggins RC, Ruiz B. Development under the influence of cocaine. II. Comparison of the effects of maternal cocaine and associated undernutrition on brain myelin development in the offspring. Metab Brain Dis. 1990;5:101–109. doi: 10.1007/BF01001050. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niznik HB, Kish SJ. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol. 1996;40:428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]


