Abstract
Highly reliable biomarkers for the diagnosis of neurological diseases are not widely available. Here we evaluated a luciferase immunoprecipitation technology (LIPS) for the diagnosis of a CNS autoimmune disorder, Stiff-person syndrome (SPS). Analysis by LIPS of 40 sera samples from SPS and control subjects for anti-GAD65 antibodies revealed dramatic titer differences allowing diagnosis of SPS with 100% sensitivity and 100% specificity. Anti-GAD65 antibody titers of SPS were segregated from controls with values greater than 23 standard deviations above the control subject mean. By analyzing patient antibody responses directly to GAD65 sub-fragments, the central region containing the decarboxylase catalytic domain was highly immunoreactive with all of the SPS sera, while the N- and C-terminal regions showed lower antibody titers and only reacted with subsets of SPS sera. Additional profiling revealed that some SPS patients showed autoantibodies against GAD67 and tyrosine hydroxylase, but no significant immunoreactivity was detected with cysteine sulfinic acid decarboxylase or GABA transaminase. This study validates LIPS as a robust method to interrogate autoantibodies for the diagnosis of SPS and potentially other neurological diseases.
Autoantibody profiles are gaining widespread interest as a way to diagnose, predict and monitor a variety of diseases. Efforts are currently underway to identify specific autoantibody profiles associated with neurological disorders such as multiple sclerosis, Parkinson’s and Alzheimer’s disease[1]. Given that the reliable diagnosis of different neurological diseases may require a panel of antigens, a major barrier to the success of using autoantibody profiles for disease biomarker discovery is the inability of current immunoassays to accurately profile multiple antigens. In particular, many solid phase, planar immunoassays such as ELISA and protein chips, fall short of the needed analytical sensitivity because they poorly present and detect conformational epitopes and have high backgrounds due to impure antigen preparations [2; 3]. Liquid phase assays, which often use radioactivity, are useful for detecting conformational epitopes but show a limited dynamic range of antibody titers. These limitations suggest that new methods which are able to detect patient antibody responses with high signals and low backgrounds to panels of autoantigens may be diagnostically useful.
Stiff-Person syndrome (SPS) is a rare, autoimmune CNS disease characterized by a debilitating stiff trunk, epilepsy, spasms and altered startle response [4]. Seminal experiments in the early 1990’s identified the fact that SPS patients had autoantibodies against glutamic acid decarboxylase (GAD65), an enzyme involved in the synthesis of the major inhibitory neurotransmitter, GABA [5]. Subsequent studies revealed that GAD65 is also an autoantigen in insulin-dependent diabetes mellitus (IDDM) [6]. However, IDDM patients typically show 100-fold lower anti-GAD65 titers than SPS patients and have antibodies directed against conformational epitopes rather than linear epitopes [7; 8]. High anti-GAD65 antibody titers are also present in other neurological diseases including cerebellar ataxia [9], Batten disease [10] and autoimmune polyendocrine syndrome type I [11]. While the functional significance of anti-GAD65 antibodies in SPS and in other diseases remains controversial, the high titer anti-GAD65 antibodies in SPS sera block enzymatic activity in vitro [12]. Autoantibodies are directed at a number of other GAD65-related decarboxylases. For example, GAD67, encoded by a separate gene and highly expressed in the nervous system, is an autoantigen in IDDM [13] and SPS [14]. Additional decarboxylases, including aromatic L-amino acid decarboxylase, histidine decarboxylase, and cysteine sulfinic acid decarboyxlase (CSAD), are autoantigens in autoimmune polyendocrine syndrome type I (APS1) [15]. As with GAD65, the physiological reasons for autoantibody production towards these different decarboxylases in various autoimmune diseases is not known.
We recently described LIPS technology that utilizes mammalian cell-produced, recombinant fusion proteins as antigens for efficiently evaluating antibody responses [16; 17]. Here we demonstrate that LIPS can be used to accurately evaluate antibody responses in SPS, an autoimmune CNS disorder. LIPS analysis of the comprehensive humoral response profile to GAD65, GAD65 protein fragments and several other antigens showed that the autoimmune response in SPS centers on the biosynthetic decarboxylase catalytic domain of GAD65 and extends to GAD67, but does not extend to the next most homologous decarboxylase or to the degradative side of the GABA pathway.
Material and methods
Subjects and samples
The sera analyzed were derived from 20 well-characterized SPS patients and 20 normal or other neurological disease controls evaluated under institutional review board-approved protocols at the Neuromuscular Disease Section, NIH. The SPS patient cohort (N=20) contained 8 males and 12 females. All SPS patients were evaluated and assigned stiffness and startle indices as described [18; 19; 20]. Twenty additional sera samples served as controls, in which 10 were from normal non-disease control subjects, 5 patients with post-polio syndrome and 5 patients with inclusion body myositis. These forty serum samples were maintained at −80° C, aliquoted, stored at 4° C, and measured as coded samples without pre-knowledge of disease status. The code was revealed only after the anti-GAD-65 antibody titers were established and preliminary categorization of SPS status made. Anti-GAD65 antibodies were also determined by a commercial lab (Mayo Medical Labs) as described [21].
Generation of Ruc-antigen fusion constructs
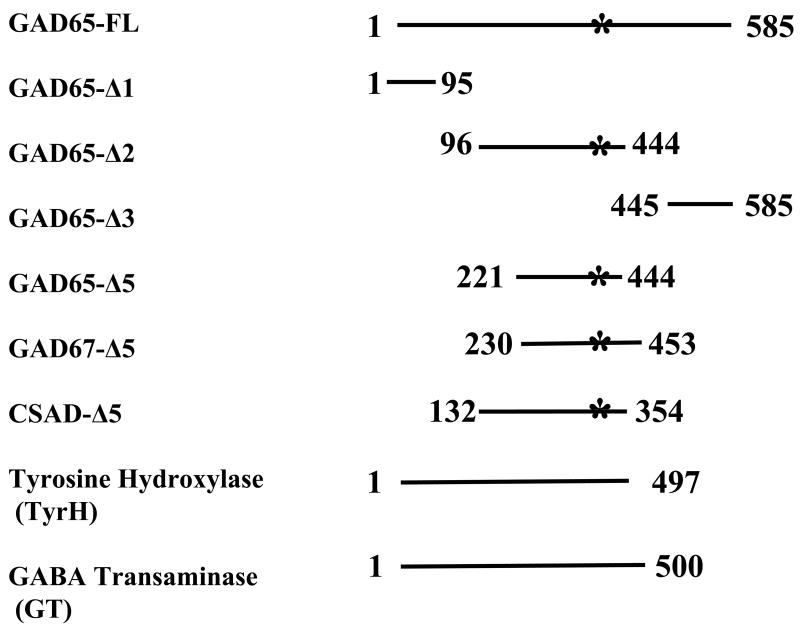
pREN2, a mammalian Renilla luciferase (Ruc) expression vector was used to generate C-terminal antigen fusions [17]. Human cDNA clones were amplified by PCR specific linker-primer adapters (see Online Supporting Information A). For GAD65, tyrosine hydroxylase and GABA transaminase, gene-specific primer-linker adapters were used in PCR amplifications to obtain the full-length coding sequence and a stop codon was included at the end of each coding sequence (Fig. 1) Three GAD65 sub-fragment deletion mutants were constructed using breakpoint as described [22]. Additional deletions mutants corresponding to the central region of GAD65, GAD67 and cysteine sulfinic acid decarboxylase (CSAD) were also generated (Fig. 1).
Fig. 1.
Proteins and protein fragments used as Ruc-fusions for autoantibody detection in SPS. Various coding sequences were amplified using PCR adapter primers and cloned in the pREN2 vector. Numbers represent amino acid residues used for each construct. The asterisk denotes the location of the pyridoxal phosphate-binding sites within GAD65, GAD67 and CSAD. Two other neurotransmitter related enzymes, tyrosine hydroxylase (TyrH) and GABA transaminase (GT) were also constructed.
LIPS analysis
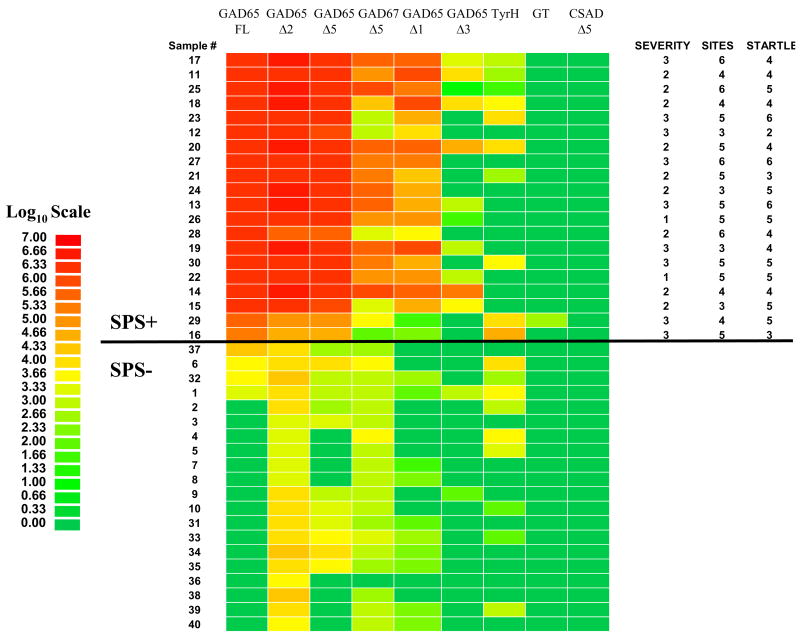
Extracts containing Ruc-antigen fusions were prepared from transfected Cos1 cells as previously described [16]. Unlike our earlier studies, the immunoprecipitation assay was performed at room temperature and adapted to a 96-well plate format (see Online Supporting Information B). All light unit (LU) data presented were obtained from the average of two independent experiments and corrected for background by subtracting LU values of beads incubated with Cos1 cell extract, but no sera. Data presented in Fig. 3 are log10 transformed values and displayed using a heatmap.
Fig. 3.
Detection of antibodies against GAD65 and its sub-fragments in SPS and control sera. Antibody titers to Ruc-antigen fusions for full-length GAD65 (FL) and five GAD65 sub-fragments (Δ1, Δ2, Δ3, and Δ5), GAD67-Δ5, tyrosine hydroxylase (TyrH), GABA transaminase (GT) and CSAD-Δ5 were determined in duplicate from 40 sera samples (1–40) using LIPS. The average titer values for each serum were log10 transformed and the samples were rank ordered from highest to lowest based on the antibody titer to full-length GAD65. Titer levels were color-coded as indicated by the log10 scale on the left, in which signal intensities range from red to green indicating high and low titers, respectively. SPS positive samples are above the black line. At the right is the clinical assessment profile including overall severity scale (1–3; mild, moderate, severe), number of sites affected (1–6) and startle response (1–7). No correlation was found between any of the autoantibody titers and these measures of disease severity.
Results
Using LIPS to analyze anti-GAD65 antibody titers for the diagnosis of SPS
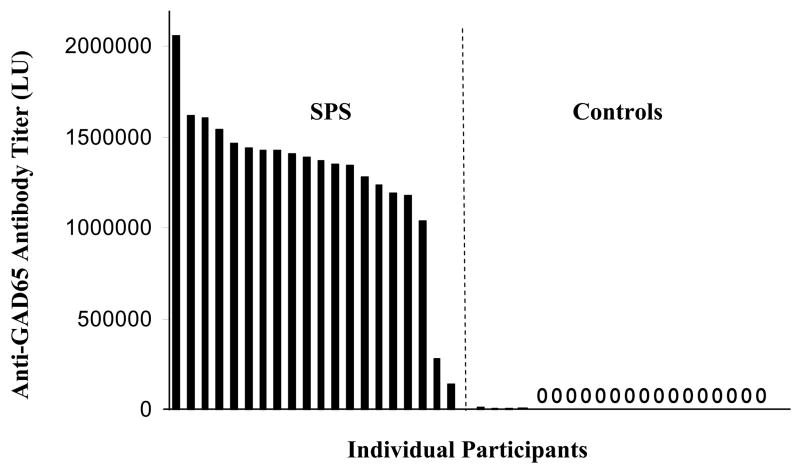
We broadened the application of LIPS to measure antibody titers in an autoimmune neurological disease, SPS. A full-length GAD65 cDNA was constructed as a fusion protein with Ruc. Transfection of one 100 mm2 dish of Cos1 cells with this Ruc-GAD65 fusion construct yielded high expression of the fusion protein producing over 3 × 109 LU per plate. Using this Ruc-GAD65 extract, we tested 40 blinded sera at two separate times in the LIPS format and averaged the values. From these anti-GAD65 tests, the 40 samples showed a wide dynamic range of antibody titers ranging from 0 to 2 million LU (Fig. 2). The 40 blinded samples showed a bimodal distribution and were statistically segregated into two groups containing 20 with a low titer and 20 with a high titer of anti-GAD65 antibodies (P-value = 3.3 × E−16). An investigator blinded to diagnosis, tentatively predicted SPS status: the lowest 20 samples were categorized as “SPS negative” and the highest 20 samples were categorized as “SPS positive.” This conclusion was verified upon subsequent unblinding, yielding correct classification of disease status with 100% sensitivity and 100% specificity. Of the 20 control samples, 16 had GAD65 antibody titers of zero, and the other 4 showed values between 500 and 12,771 LU (Fig. 2). For the SPS patients, the mean value was 1,290,953 ± 424,030 LU. The lowest SPS positive sample #16 was separated from the average control value by the mean plus 23 standard deviations (Fig. 2). Since patients with other neurological diseases such as inclusion body myositis showed no significant anti-GAD65 antibody titers, these results are consistent with other studies showing that elevated anti-GAD65 antibody titers are associated with SPS, diabetes and certain other specific neurological diseases, but are not common to neurological disease in general.
Fig. 2.
Detection of patient antibodies to GAD65. Forty blinded sera samples from controls and SPS patients were utilized to evaluate anti-GAD65 antibody titers. Sera were analyzed using LIPS in two independent assays, the values averaged, and samples rank ordered from high to low titers corresponding to left to right, respectively. By analyzing the anti-GAD65 antibody titers of the 40 coded sera, the samples were statistically segregated (P-value of 3.3 × E−16) into two groups: 20 sera with a high anti-GAD65 antibody titer (left panel) and 20 sera with a low anti-GAD65 antibody titer (right panel). Sixteen of the samples showed values of zero (0). For SPS disease status, samples on the left panel with high anti-GAD65 antibody titer were predicted as positive. Following unblinding, the exact disease status of the 40 sera samples was revealed, showing that the LIPS data predicted SPS status from controls with 100% sensitivity and 100% specificity.
The GAD65 antibody titer values obtained with LIPS were also compared with results from a commercial laboratory that employed, I125-labeled GAD65 in an RIA [21]. While the results from these two different assays are difficult to compare due to commercial assay subtracting titer values of a set of normal patient sera before assigning cut-offs [21], the same positive samples were detected (Online Supplemental Table I). Comparison of the titer values obtained from the RIA with the LIPS showed Pearson and Spearman coefficients of R=0.153 and R=0.54, respectively. The weak correlations observed between the two assays may be due, in part, to the much larger dynamic range of antibody titers detected with LIPS compared to the commercial RIA assay.
Mapping anti-GAD65 autoantibodies against sub-fragments of GAD65
Patient immunoreactivity was also evaluated by LIPS against three non-overlapping fragments of GAD65, spanning amino acids 2-95, 96-444 and 445-585 which were designated GAD65-Δ1, GAD65-Δ2, and GAD65-Δ3, respectively (Fig. 1). To easily evaluate patient immunoreactivity to the different subfragments, the values were logarithmically (log10) transformed and are presented as a color-coded heatmap (Fig. 3) with the subjects rank ordered by antibody titers to full-length GAD65. The GAD65-Δ2, representing the central region of GAD65, was immunoreactive with all SPS samples (Fig. 3). Both the GAD65-Δ1 and GAD65-Δ3 fragments reacted with only a subset of the SPS sera. The GAD65-Δ3 piece yielded the weakest signals and only reacted with 5 of the 20 SPS sera. In contrast, the GAD65-Δ2 fragment reacted with all the SPS samples (Fig. 3) and yielded titer values comparable to that obtained with full-length GAD65. However, the titers values found for the GAD65-Δ2 were not strongly correlated (R=0.57) with the values obtained with full-length GAD65 protein. Nevertheless, these results suggest that the central region of GAD65 contains many antigenic epitopes recognized by SPS sera.
Due to the high positive reactivity with the central GAD65-Δ2 portion, this region was sub-divided into two additional fragments designated GAD65-Δ4 and GAD65-Δ5, spanning amino acids 96- 220, and 221-444, respectively. Tests with GAD65-Δ4 yielded values less than 500 LU in both the SPS and control samples (data not shown). In contrast, high levels of immunoreactivity were observed with the GAD65-Δ5 fusion protein with sera from SPS patients, but not with control sera (Fig. 3). The GAD65-Δ5 antibody titers in the SPS sera correlated strongly with values obtained with GAD65-Δ2 (R=0.91; P=0.001). Additional analysis revealed that the antibody titers to full-length GAD65 or GAD65/67 sub-fragments did not correlate with disease severity (Fig. 3) or disease duration (data not shown) That is, much higher antibody titers were found in some patients with mild disease (e.g. #22 and 26) compared to those patients with much lower titers with severe disease (e.g. patients #29 and #16).
SPS immunoreactivity to the catalytic region of two other GAD-65 related decarboxylases
Since high SPS immunoreactivities were observed for the GAD65-Δ5 region spanning part of the catalytic domain, we speculated that SPS patients might exhibit immunoreactivity to other related decarboxylases. Sequence alignment of the GAD65-Δ5 region with the two most closely related decarboxylases, GAD67 and CSAD, revealed 75% and 57% identity, respectively (Fig. 4). The corresponding Δ5 fragments from these two decarboxylases were cloned, expressed and tested. From these assays, we found that the GAD67 fragment (designated GAD67-Δ5) reacted with sera from 14 of the 20 SPS patients, but did not react with any of the control sera (Fig. 3). The anti-GAD67-Δ5 titers correlated well (R=0.71; P<0.04) with values observed for GAD65-Δ5. However, the corresponding region of CSAD, designated CSAD-Δ5, did not show any immunoreactivity with the different sera tested (Fig. 3). These results suggest that epitopes within this region of GAD65 and GAD67, but not CSAD are autoantigen targets in SPS. The amino acid sequences of the Δ5 fragments are aligned in Fig. 4.
Fig. 4.
Amino acid sequence homology within the corresponding regions of the GAD65-Δ5, GAD67-Δ5 and CSAD-Δ5 deletion mutants. The corresponding amino acid residues within each protein are shown. Identical amino acids residues are denoted by a dash line and the + denotes conservative amino acid substitutions. The active site lysine involved in pyridoxal phosphate binding is shown in bold.
Few autoantibodies in SPS to tyrosine hydroxylase and GABA transaminase
Based on the possibility that other neurotransmitter enzymes might be autoantigens in SPS, we tested for antibodies against GABA transaminase and tyrosine hydroxylase. None of the SPS or control sera showed immunoreactivity with GABA transaminase (Fig. 3). For tyrosine hydroxylase, several sera from both SPS and the controls yielded reproducible signals but the titers were typically much lower compared to the anti-GAD65 antibody titers (Fig. 3). The two individuals showing the highest anti-tyrosine hydroxylase antibody titers were found in SPS patients corresponding to SPS sample #16 and #29 and these same two SPS patients had the lowest anti-GAD65 antibodies titers.
Discussion
In this study, a molecular profile of autoantibodies to GAD65 and closely associated decarboxylases was determined using a highly sensitive, non-radioactive, liquid phase immunoprecipitation assay. The LIPS assay detected anti-GAD65 titers spanning more than 6 log10 differences in the SPS and controls sera samples and this allowed accurate diagnosis of SPS from control subjects with 100% sensitivity and 100% specificity. While statistical analysis revealed that the lowest SPS sera sample was separated by the mean plus 23 standard deviations of the anti-GAD65 antibody titers of the controls, existing immunoassay formats show differences of only 3 or 4 standard deviations [14; 21]. Despite the large dynamic range of titers and the use of a large panel of GAD65 antigen fragments, no significant correlation of anti-GAD65 antibodies was observed with any of the clinical parameters, such as disease severity, and duration. These results are consistent with studies showing that anti-GAD65 antibody titers do not correlate with severity or duration of SPS [20; 23].
Our simple system of expressing cloned GAD65 fragments as Ruc fusion proteins and testing them with patient sera in a standard format was used to efficiently map GAD65 immunoreactivity. Overall, the direct cloning and testing of GAD65 fragments in the LIPS format was highly successful, whereby 5 of the 6 constructs derived from GAD65 were reactive with at least some SPS sera. One construct, GAD65-Δ4, did not react with any sera, supporting the notion that this GAD65 protein piece may not fold properly or is partially proteolyzed in Cos1 cells. Nevertheless, deletion analysis of GAD65 revealed that the central region of GAD65 had the highest immunoreactivity. Less immunoreactivity was observed with the C-terminus, and the N-terminus of GAD65 showed a still poorer signal and reacted with even fewer SPS sera. By further sub-dividing the central region of GAD65, we identified a 220 amino acid fragment (GAD65-Δ5) that mapped to the decarboxylase catalytic site and showed immunoreactivity with SPS sera equivalent to full-length protein. These results are consistent with a small study using 2 SPS sera showing that a competitive peptide derived from the catalytic site of GAD65 blocked immunoreactivity with SPS sera [24].
Two other decarboxylases, GAD67 and cysteine sulfinic acid decarboxylase were also tested for immunoreactivity in SPS. One of the proteins tested, GAD67, has previously been identified as an antigen in about 60% of SPS patient and in 1% of controls [14]. While only a 223 amino acid piece of GAD67 was tested here, significant immunoreactivity was detected with 14 of the 20 SPS patients (70%) and none of the controls. While the corresponding region of CSAD has 57% identity with GAD65, none of the SPS patients showed immunoreactivity. Comparison of the amino acid sequences within this corresponding region of the three proteins showed that CSAD was considerably more divergent in this region, which likely explains, in part, the lack of observed SPS immunoreactivity. Thus, these results suggest that small differences in protein structure have dramatic effects on altering the antibody recognition of these relatively similar decarboxylases.
Studies with GABA transaminase did not show immunoreactivity with any of the sera tested and rules out the simple idea that in SPS the antibodies are generated against molecules involved in GABA metabolism. Interestingly, several sera showed significant immunoreactivity with tyrosine hydroxylase, a known autoantigen in autoimmune pituitary disease [25]. The two highest titers against tyrosine hydroxylase were found in SPS patients and these same sera showed the lowest anti-GAD65 antibodies titers among the SPS samples. While these findings are difficult to interpret due to the limited number of samples that showed this combination of being low positives for GAD65 and positive for tyrosine hydroxylase, these two samples may represent a different stage of the disease or a unique subset of SPS.
While there are many reports demonstrating unique autoantibody profiles associated with different neurological diseases, the diagnostic specificity and sensitivity of many of these immunoassay tests is not optimal [1]. The results presented here demonstrate that LIPS produces highly robust values for identifying SPS cases. We anticipate that the application of LIPS with different panels of neuronal antigens may yield high discriminative power and be useful for the diagnosis and monitoring of many other neurological diseases.
Supplementary Material
Acknowledgments
We are also grateful to Jason Keller and Hannah Leahy for helpful technical assistance, comments and reading of the manuscript. This research was supported by the Intramural Research Program of NIDCR and NINDS, National Institutes of Health.
Footnotes
COMPETING FINIANCIAL INTERESTS
Georgetown University has filed a patent application based on LIPS and one of the inventors (P.D.B.) is entitled to future royalties received by the University in accordance with university conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turck CW, Maccarrone G, Sayan-Ayata E, Jacob AM, Ditzen C, Kronsbein H, Birg I, Doertbudak CC, Haegler K, Lebar M, Teplytska L, Kolb N, Uwaje N, Zollinger R. The quest for brain disorder biomarkers. J Med Invest. 2005;52(Suppl):231–5. doi: 10.2152/jmi.52.231. [DOI] [PubMed] [Google Scholar]
- 2.Sharp V, Utz PJ. Technology insight: can autoantibody profiling improve clinical practice? Nat Clin Pract Rheumatol. 2007;3:96–103. doi: 10.1038/ncprheum0404. [DOI] [PubMed] [Google Scholar]
- 3.Sodoyez-Goffaux F, Koch M, Dozio N, Brandenburg D, Sodoyez JC. Advantages and pitfalls of radioimmune and enzyme linked immunosorbent assays of insulin antibodies. Diabetologia. 1988;31:694–702. doi: 10.1007/BF00278754. [DOI] [PubMed] [Google Scholar]
- 4.Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. 1999;131:522–30. doi: 10.7326/0003-4819-131-7-199910050-00008. [DOI] [PubMed] [Google Scholar]
- 5.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322:1555–60. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- 6.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Namchuk M, Bugawan T, Fu Q, Jaffe M, Shi Y, Aanstoot HJ, Turck CW, Erlich H, Lennon V, Baekkeskov S. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180:595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjork E, Velloso LA, Kampe O, Karlsson FA. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes. 1994;43:161–5. doi: 10.2337/diab.43.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki H, Sato R, Shichiri M, Hirata Y. A patient with type 1 diabetes mellitus and cerebellar ataxia associated with high titer of circulating anti-glutamic acid decarboxylase antibodies. Endocr J. 2001;48:261–8. doi: 10.1507/endocrj.48.261. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay S, Kriscenski-Perry E, Wenger DA, Pearce DA. An autoantibody to GAD65 in sera of patients with juvenile neuronal ceroid lipofuscinoses. Neurology. 2002;59:1816–7. doi: 10.1212/01.wnl.0000041913.97883.8b. [DOI] [PubMed] [Google Scholar]
- 11.Tuomi T, Bjorses P, Falorni A, Partanen J, Perheentupa J, Lernmark A, Miettinen A. Antibodies to glutamic acid decarboxylase and insulin-dependent diabetes in patients with autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 1996;81:1488–94. doi: 10.1210/jcem.81.4.8636356. [DOI] [PubMed] [Google Scholar]
- 12.Dinkel K, Meinck HM, Jury KM, Karges W, Richter W. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol. 1998;44:194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 13.Hagopian WA, Michelsen B, Karlsen AE, Larsen F, Moody A, Grubin CE, Rowe R, Petersen J, McEvoy R, Lernmark A. Autoantibodies in IDDM primarily recognize the 65,000-M(r) rather than the 67,000-M(r) isoform of glutamic acid decarboxylase. Diabetes. 1993;42:631–6. doi: 10.2337/diab.42.4.631. [DOI] [PubMed] [Google Scholar]
- 14.Meinck HM, Faber L, Morgenthaler N, Seissler J, Maile S, Butler M, Solimena M, DeCamilli P, Scherbaum WA. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry. 2001;71:100–3. doi: 10.1136/jnnp.71.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, Gustafsson J, Husebye ES, Perheentupa J, Gylling M, Manns MP, Rorsman F, Kampe O, Nilsson T. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89:557–62. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 16.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–95. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 17.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnology. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalakas MC, Fujii M, Li M, McElroy B. The clinical spectrum of anti-GAD antibody-positive patients with stiff-person syndrome. Neurology. 2000;55:1531–5. doi: 10.1212/wnl.55.10.1531. [DOI] [PubMed] [Google Scholar]
- 19.Dalakas MC, Li M, Fujii M, Jacobowitz DM. Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology. 2001;57:780–4. doi: 10.1212/wnl.57.5.780. [DOI] [PubMed] [Google Scholar]
- 20.Rakocevic G, Raju R, Dalakas MC. Anti-glutamic acid decarboxylase antibodies in the serum and cerebrospinal fluid of patients with stiff-person syndrome: correlation with clinical severity. Arch Neurol. 2004;61:902–4. doi: 10.1001/archneur.61.6.902. [DOI] [PubMed] [Google Scholar]
- 21.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73:1161–6. doi: 10.4065/73.12.1161. [DOI] [PubMed] [Google Scholar]
- 22.Primo ME, Anton EA, Villanueva AL, Poskus E, Ermacora MR. Engineered variants of human glutamic acid decarboxylase (GAD) and autoantibody epitope recognition. Clin Immunol. 2003;108:38–45. doi: 10.1016/s1521-6616(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 23.Murinson BB, Butler M, Marfurt K, Gleason S, De Camilli P, Solimena M. Markedly elevated GAD antibodies in SPS: effects of age and illness duration. Neurology. 2004;63:2146–8. doi: 10.1212/01.wnl.0000145661.01675.a8. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Hagopian WA, Brashear HR, Daniels T, Lernmark A. Identification of autoantibody epitopes of glutamic acid decarboxylase in stiff-man syndrome patients. J Immunol. 1994;152:930–4. [PubMed] [Google Scholar]
- 25.Hedstrand H, Ekwall O, Haavik J, Landgren E, Betterle C, Perheentupa J, Gustafsson J, Husebye E, Rorsman F, Kampe O. Identification of tyrosine hydroxylase as an autoantigen in autoimmune polyendocrine syndrome type I. Biochem Biophys Res Commun. 2000;267:456–61. doi: 10.1006/bbrc.1999.1945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.