Abstract
Purpose
MRL/MpJ mice of substrains MRL/MpJ-fas+/fas+ (MRL/+) and MRL/MpJ-faslpr/faslpr (MRL/lpr) spontaneously develop autoimmune dacryoadenitis and sialadenitis and are a model for the human disorder Sjögren syndrome. The dacryoadenitis in both substrains appears to be Th2 in nature, with little IFN-γ and substantial IL-4 at the site of lacrimal gland inflammation.
Methods
MRL/MpJ mice with a defective IL-4 gene—both MRL/+-IL-4tm/IL-4tm (MRL/+/IL-4tm) and MRL/lpr-IL-4tm/IL-4tm (MRL/lpr-IL-4tm)—that resulted in a loss of IL-4 production were bred and evaluated for dacryoadenitis.
Results
MRL/+/IL-4tm and MRL/lpr/IL-4tm mice developed dacryoadenitis of similar onset, appearance, and severity as found in MRL/MpJ mice with an intact IL-4 gene. Immunohistochemistry examination revealed a substantially greater number of inflammatory cells staining for IFN-γ than for IL-13 in the dacryoadenitis of IL-4 – deficient MRL/MpJ mice (MRL/+/IL-4tm, 66% vs. 0.8%, P = 0.001; MRL/lpr/IL-4tm, 67% vs. 1.2%, P = 0.002). Real-time PCR demonstrated greater amounts of IFN-γ than IL-13 mRNA relative transcripts in lacrimal glands of MRL/lpr/IL-4tm mice (mean difference, 28.6; P = 0.035). Greater CD86 (B7–2) than CD80 (B7–1) expression was present in MRL/+/IL-4tm mice (11% vs. 3%, P = 0.003) and MRL/lpr/IL-4tm mice (10% vs. 3%, P = 0.002).
Conclusions
These results suggest that a Th2 autoimmune process can be converted to a Th1 process in the absence of IL-4.
Sjögren syndrome is an autoimmune disease characterized by the infiltration of mononuclear inflammatory cells into lacrimal and salivary glands, resulting in glandular damage and dysfunction and thereby the characteristic clinical features of dry eyes and dry mouth. Sjögren syndrome may occur as an isolated disorder, in which case it is termed primary Sjögren syndrome, or it may occur hi association with a recognized rheumatic disease, such as rheumatoid arthritis or systemic lupus erythematosus, in which case it is termed secondary Sjögren syndrome. The characteristic autoantibodies seen in the sera of patients with Sjögren syndrome are anti-Ro (or SSA) and anti-La (or SSB).l
The MRL/MpJ mouse is an autoimmune strain that develops lacrimal and salivary gland inflammation (dacryoadenitis and sialadenitis) and is a model for human Sjögren syndrome.2,3 There are two substrains of MRL/MpJ mice, MRL/MpJ-fas+/fas+ (MRL/+) and MRL/MpJ-faslpr/faslpr (MRL/lpr). The faslpr mutation produces a defective Fas protein, defective apoptosis of lymphocytes in peripheral lymphoid organs, and systemic autoimmune disease in MRL/lpr mice (e.g., arthritis, glomerulonephritis, vasculitis).4,5 In addition to dacryoadenitis and sialadenitis, both strains developed antinuclear antibodies. In addition, MRL/lpr mice have antibodies to Ro and La, 6,7 making these mice a model for human Sjögren syndrome.
Previous work from our laboratory has demonstrated that the autoimmune dacryoadenitis in MRL/MpJ mice of both sub-strains appears to be Th2 mediated. 8–11 Most cells in the infiltrates are CD4+ T cells, though aged (12–18 months) MRL/+ mice develop an increasing proportion of B cells.2,3,5 Little or no IFN- γ and IL-12 are present in the lacrimal gland lesions, whereas substantial IL-4 and IL-10 are present, both by quantitative reverse transcription-polymerase chain reaction assays for RNA and by immunohistochemistry of the lesions for protein. There is significantly greater expression of the co-stimulatory molecule CD86 (B7–2) in the lesions than of CD80 (B7–1), a result also consistent with a Th2-mediated process.8,9
Because the dacryoadenitis in MRL/MpJ mice appears to be Th2 in nature, we evaluated the effect of a dysfunctional mutant IL-4 gene, producing a functional IL-4 “knockout” phenotype, on the lacrimal gland inflammation in MRL/MpJ mice.
Materials and Methods
Mice
Heterozygous MRL/+-IL-4+/IL-4tm mice were obtained from Dr. Joseph Craft at Yale University (New Haven, CT)12 and were shipped to the Jackson Laboratories Custom Breeding Program (Bar Harbor, ME) to produce homozygous MRL/+-IL-4tm/IL-4tm mice. MRL/+-IL-4+/IL-4tm mice were mated to MRL/lpr mice to produce homozygous MRL/lpr-IL-4tm/IL-4tm (MRL/lpr/IL-4tm) mice. Mice were shipped to the Ocular Immunology Laboratories at the Johns Hopkins University School of Medicine (Baltimore, MD) for analysis. Because the targeted mutation in these mice produces a truncated and dysfunctional mRNA, Western blot analysis for the protein product was used to confirm the absence of IL-4 in these mice, including its absence in the lacrimal glands (data not shown). Groups of approximately 10 MRL/+/IL-4tm mice were killed at ages 1,3,5, and 9 months. MRL/lpr mice generally do not survive beyond 6 months of age because of systemic autoimmune disease; therefore, MRL/lpr/IL-4tm mice were killed at ages 1, 3, and 5 months. One lacrimal gland each in mice at each age from each substrain was removed, fixed in formalin, sectioned at 6 μm, and stained with hematoxylin and eosin for histology. The second gland was snap-frozen in optimal cutting temperature compound (OCT; Miles, Elkhart, IN), sectioned at 8 μm, and used for immunohistochemistry. Kidneys also were removed and processed for histology. Lacrimal glands of five more MRL/lpr/IL-4tm mice at each age were removed, snap-frozen, and later used for real-time PCR for mRNA for IFN-γ and IL-13. These results were compared with those of two to four control mice (BALB/c) lacrimal glands at each age. These experiments were approved by the Animal Care and Use Committee at the Johns Hopkins University School of Medicine and were consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Histology
The presence and severity of dacryoadenitis were read by an observer (RAP) who was masked as to substrain and IL-4 status. Lacrimal gland inflammation was graded from 0 to 4+ using our previously described semiquantitative modified focus score scale: 0, no inflammation; 1+, scattered inflammation but no foci of inflammation; 2+, at least one focus of 50 or more inflammatory cells; 3+, multiple foci/gland; and 4+, multiple foci plus lacrimal gland destruction. Lacrimal glands from MRL/MpJ mice of both substrains with intact IL-4 genes were used as controls.3 The severity of renal inflammation in MRL/lpr and MRL/lpr/IL-4tm mice was graded from 0 to 4+ using our previously described semiquantitative system: 0, no inflammation; 1+, mesangial thickening; 2+, focal and local glomerulonephritis; 3+, diffuse glomerulonephritis; and 4+, diffuse proliferative glomerulonephritis.13
Immunohistochemistry
Immunohistochemistry was performed with a panel of antibodies for cell surface markers and for cytokines using indirect staining and the avidin-biotin-peroxidase-complex (ABC) technique, as previously described.2,3,5 Monoclonal antibodies to cell surface markers included rat anti–mouse CD4 (GK1.5; BD PharMingen, San Diego, CA)14 and rat anti–mouse CD8 (anti-Lyt2; BD PharMingen).15,16 The polyclonal antibody to B cells was goat anti–mouse CD19 (Santa Cruz Biotechnology, Santa Cruz, CA).17 Polyclonal antibodies to cytokines included goat anti–mouse IFN-γ (Biosource, Camarillo, CA)18,19 and goat anti–mouse IL-13 (Santa Cruz Biotechnology).20,21 Monoclonal antibodies to co-stimulatory molecules were rat anti–mouse CD80 (anti-B7–1; BD PharMingen)8,22 and rat anti–mouse CD86 (anti-B7–2, PBL; BD Phar-Mingen).8,22
Quantitative Real-Time PCR
Total nucleic acids were isolated from tissues through homogenization with reagent (Trizol; Invitrogen, Carlsbad, CA).10,11 Pure RNA was prepared by treatment of total nucleic acid preparations with DNase1 (RQ1; Promega Life Sciences, Madison, WI), followed by phenol/chloroform extraction and ethanol precipitation. RNA preparations were assessed for residual DNA by standard PCR using primers targeting the β-actin gene. cDNA was prepared for quantitative real-time PCR (qPCR) analyses using the M-MLV reverse transcriptase enzyme (Invitrogen, Carlsbad, CA) and random hexamers as primers, as described previously.10,11 SYBR-green-based qPCR analysis was used to assess relative transcript levels from host genes.10,11 The sequences targeted and the primers used for these studies were generated from GenBank with Gene Runner software. Primers used were as follows: for IFN-γ 5′ primer, 5′-GGATGCATTCATGAGTATTGC-3′ and 3′ primer, 5′-CCTTTTCCGCTTCCTGAGGC-3′ to yield a 126-bp product; and for IL-13 5′ primer, 5′-GCTTATTGAGGAGCTGAGCAACA-3′, and 3′ primer, 5′-GCCAGGTCCACACTCCATA-3′ to yield an 80-bp product.23 Primers were confirmed to amplify the predicted products by testing under qPCR conditions. Each assay typically was performed twice, with each sample run in duplicate each time; signals from each sample were normalized to values obtained for the β-actin gene, which was run as a housekeeping gene simultaneously with the experimental samples. Analyses were conducted in a sequence detector (PE Biosystems model 7700; Applied Biosystems, Foster City, CA), and data were analyzed using the Sequence Detection Software version 1.9. Results from each run for each mouse were averaged and expressed as the relative level of mRNA transcript. Age-matched samples of MRL/lpr/IL-4tm and control BALB/c lacrimal glands were performed in each run to control for interassay variability.10,11
Statistical Analysis
Comparisons between the proportions of cells staining for IFN-γ and IL-13 and between the proportions of cells staining for CD80 and CD86 were made using the sign test, a nonparametric paired analysis that permits comparison to determine whether more cells stain for IFN-γ or IL-13. Comparisons between MRL/+/IL-4tm and MRL/lpr/IL-4tm mice in the percentage of cells staining for a cell surface marker were performed with Student’s t-test. Comparison of the relative amounts of IFN-γ and IL-13 mRNA from real-time PCR was performed using the sign test. Comparison of the amount of mRNA for IFN-γ between MRI/lpr/IL-4tm and BALB/c lacrimal glands was performed using the t-test. Comparison of the severity of renal disease between MRL/lpr and MRL/lpr/IL-4tm mice was performed with the Cochran-Armitage test.24
Results
Dacryoadenitis was present in MRL/+/IL-4tm and MRL/lpr/IL-4tm mice, was characterized by multiple foci of mononuclear inflammatory cells, and histologically did not differ from that in MRL/+ or MRL/lpr mice with an intact IL-4 gene. Onset, histologic appearance, and severity (grade) of inflammation (Table 1) were nearly identical. Renal disease was significantly less severe in MRL/lpr/IL-4tm mice than in MRL/lpr mice with an intact IL-4 gene. Median grades for glomerulonephritis (Table 2) in 3- and 5-month-old MRL/lpr mice with an intact IL-4 gene were 3 for both ages, whereas in MRL/lpr/IL-4tm mice they were 1 and 1.5 for 3- and 5-month-old mice (P < 0.0001 and P = 0.005, respectively).
Table 1.
Lacrimal Gland Inflammation in MRL/MpJ Mice
| Median Grade Inflammation* |
||||
|---|---|---|---|---|
| 1 | 3 | 5–6 | 9 | |
| MRL/MpJ-fas+/fas+-IL-4+/IL-4+ | 0 | 2 | 3 | 3 |
| MRL/MpJ-faslpr/faslpr-IL-4+/IL-4+ | 0 | 2 | 3 | NA† |
| MRL/MpJ-fas+/fas+-IL-4tm/IL-4tm | 0 | 2 | 3 | 3 |
| MRL/MpJ-faslpr/faslpr-IL-4tm/IL-4tm | 0 | 3 | 3 | NA† |
Values shown are mouse ages in months.
Graded 0 to 4+ using modified focus score scale. Median score for 8 to 10 animals per group.
Not applicable (NA) because MRL/lpr mice do not survive past 6 months.
Table 2.
Glomerulonephritis in MRL/MpJ-faslpr/faslpr Mice
| Glomerulonephritis Grade* |
|||
|---|---|---|---|
| Mouse | Age (mo) | Median† | ≥Grade 2, % |
| MRL/MpJ-faslpr/faslpr-IL-4+/IL-4+ | 3 | 3 | 100 |
| 5 | 3 | 100 | |
| MRL/MpJ-faslpr/faslpr-IL-4tm/IL-4tm | 3 | 1 | 0 |
| 5 | 1.5 | 40 | |
Graded 0 to 4+ using a semiquantitative scale as described.
Median of 10 animals per group.
Immunohistochemistry for cell surface markers revealed that the predominant cell type in lacrimal gland inflammation in MRL/+/IL-4tm and MRL/lpr/IL-4 tm mice was the CD4+ T cell, with approximately two thirds of the infiltrate at various ages composed of these cells (Table 3). CD8+ T cells constituted 12% of the infiltrates in MRL/+/IL-4tm and 16% in MRL/lpr/IL-4tm mice (P = 0.048). B cells were present in greater abundance in MRL/+/IL-4tm (30%) mice than in MRL/lpr/IL-4tm mice (18%; P = 0.0002). Immunohistochemistry for IFN-γ and IL-13 (Fig. 1) demonstrated that IFN-γ was detected on a significantly greater proportion of inflammatory cells in the lacrimal gland than was IL-13 in MRL/+/IL-4tm and MRL/lpr/IL-4tm mice (Table 3). Mean percentages of inflammatory cells staining for IFN-γ in MRL/+/IL-4tm and MRL/lpr/IL-4tm mice were 66% and 67%, respectively, whereas mean percentages of cells staining for IL-13 were 0.8% and 1.2%, respectively. Mean differences between IFN-γ and IL-13 staining were 72% for MRL/+/IL-4tm mice (P = 0.001) and 67% for MRL/lpr/IL-4tm mice (P = 0.002). CD86 (B7–2) expression (Fig. 2) was observed on more mononuclear inflammatory cells in the lacrimal glands than was CD80 (B7–1) in MRL/+/IL-4tm mice (11% vs. 3%) and MRL/lpr/IL-4tm mice (10% vs. 3%). Mean differences between paired CD86 and CD80 staining were 7% for MRL/lpr/IL-4tm mice (P = 0.003) and 5% for MRL/lpr/IL-4tm mice (P = 0.002).
Table 3.
Immunohistochemistry of Lacrimal Gland Inflammation in IL-4-Deficient MRL/MpJ Mice
| Mononuclear Inflammatory Cell Staining, %
|
|||||||
|---|---|---|---|---|---|---|---|
| CD4 | CD8 | B cells | IFN-γ | IL-13 | CD80 | CD86 | |
| MRL/MpJ-fas+/fas+-IL-4tm/IL-4tm | |||||||
| 3 | 63 ± 2 | 11 ± 4 | 32 ± 5 | 70 ± 11 | 0.6 ± 0.4 | 3 ± 1 | 9 ± 2 |
| 5 | 66 ± 3 | 13 ± 6 | 31 ± 6 | 76 ± 3 | 0.5 ± 0.2 | 3 ± 1 | 14 ± 3 |
| 9 | 65 ± 10 | 10 ± 4 | 10 ± 4 | 48 ± 5 | 1.3 ± 0.3 | 3 ± 2 | 8 ± 4 |
| All ages | 65 ± 6 | 12 ± 5 | 30 ± 5 | 66 ± 14 | 0.8 ± 0.5 | 3 ± 2 | 11 ± 4 |
| MRL/MpJ-faslpr/faslpr-IL-4tm/IL-4tm | |||||||
| 3 | 67 ± 11 | 18 ± 5 | 14 ± 5 | 63 ± 10 | 1.7 ± 1.2 | 3 ± 1 | 8 ± 2 |
| 5 | 36 ± 4 | 13 ± 3 | 22 ± 10 | 71 ± 4 | 0.7 ± 0.3 | 4 ± 1 | 12 ± 3 |
| All ages | 65 ± 8 | 16 ± 5 | 18 ± 8 | 67 ± 8 | 1.2 ± 0.9 | 3 ± 1 | 10 ± 3 |
Values are mouse ages in months, shown as mean ± SD of 5 animals per age group.
Figure 1.

Lacrimal gland inflammation in 5-month-old IL-4-deficient MRL/MpJ mice. (A) Stained for IFN-γ. showing positive staining. (B) Stained for IL-13, showing minimal staining. Original magnification, ×160.
Figure 2.
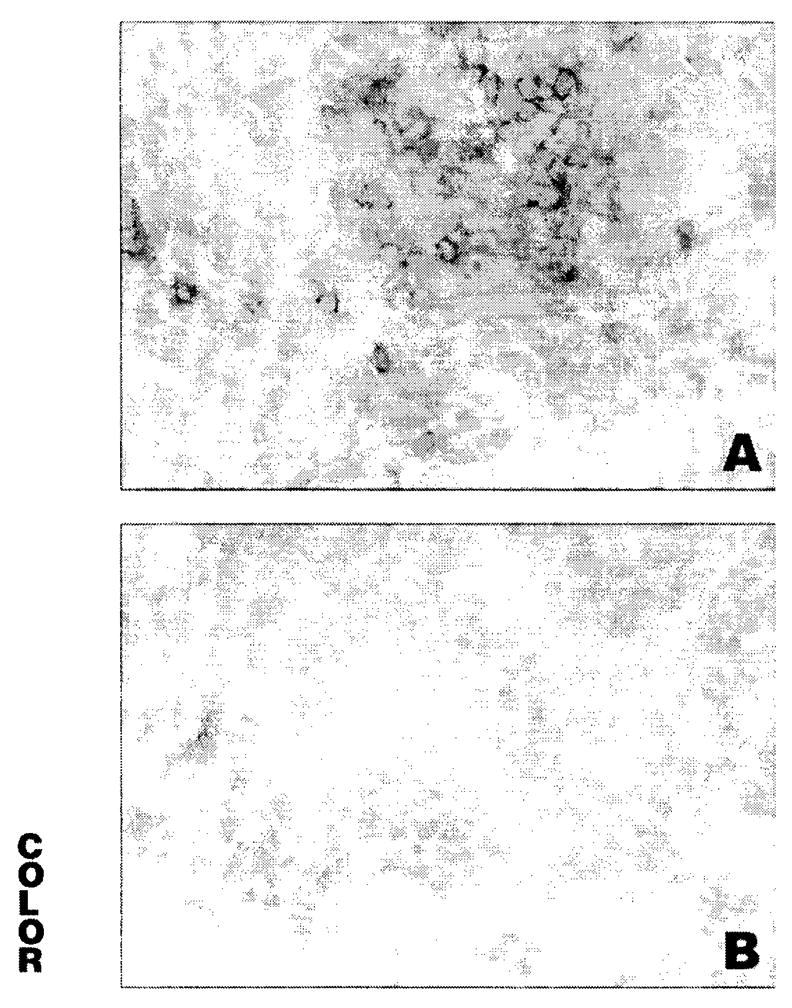
Lacrimal gland inflammation in 5-month-old IL-4-deficient MRL/MpJ mice. (A) Stained for CD86, showing large number positive cells. (B) Stained for CD80, showing minimal staining. Original magnification, ×160.
Quantitative real-time PCR for mRNA demonstrated an increase in lacrimal gland IFN-γ relative to that in BALB/c control mice and greater amounts of IFN-γ than of IL-13 in the lacrimal glands of MRL/lpr/IL-4tm mice (Table 4). The mean difference in relative transcripts between IFN-γ and IL-13 in MRL/lpr/IL-4tm mice was 28.6 (P = 0.035). Insufficient material was available for analysis of real-time PCR of lacrimal glands from MRL/+/IL-4tm mice. Among control BALB/c mice there were no changes with increasing age in the amounts of IFN-γ and IL-13 (data not shown). For the sake of analysis, the results from BALB/c mice of different ages were combined. These qPCR results confirmed those of immunohistochemistry in MRL/lpr/IL-4tm mice, and IFN-γ mRNA levels were greater in MRL/lpr/IL-4tm mice than in control BALB/c mice (mean 43.6 vs. 1.7; P < 0.0001). Although IL-13 mRNA levels appeared to be modestly greater in MRL/lpr/IL-4tm lacrimal glands than in those of BALB/c controls, the elevation of IFN-γ was substantially greater than that of IL-13, consistent with the results of immunohistochemistry.
Table 4.
Real-Time PCU for IFN-γ and IL-13 mRNA in Lacrimal Glands from IL-4-Deficient MRL/MpJ-faslpr/faslpr-IL-4tm/IL-4tm Mice
| Relative Transcript Levels* |
||
|---|---|---|
| Age (mo) | IFN-γ (Median) | IL-13 (Median) |
| MRL/MpJ-faslpr/faslpr-IL-4tm/IL-4tm | ||
| 1 | 16.5 | 0.6 |
| 3 | 74.0 | 2.1 |
| 5 | 47.5 | 2.1 |
| All ages | 47.5 | 2.1 |
| BALB/c controls | 3.0 | 0.2 |
Normalized to β-actin, a housekeeping gene. IFN- γ and IL-13 were assessed in same real-time PCU runs for each animal.
Discussion
Previous work has suggested that the lacrimal and salivary gland inflammation in MRL/MpJ mice of both substrains with an intact IL-4 gene is mediated by CD4+ T cells and appears to be Th2 in nature. The predominant mononuclear inflammatory cell in the infiltrates in the lacrimal and salivary glands is the CD4+ T cell (Table 5), 2,3,5,25 and the lacrimal and salivary gland inflammation can be transferred to SCID mice by CD4+ T cells isolated from the salivary glands of MRL/lpr mice.26 Using both immunohistochemistry (Table 5) and quantitative PCR, we previously detected little or no IFN-γ but substantial IL-4 in the lacrimal gland lesions of MRL/+ and MRL/lpr mice with an intact IL-4 gene, suggesting a Th2-mediated process.8,9 In addition, there was little to no IL-12 but detectable IL-10 in the lacrimal gland lesions of MRL/MpJ mice of both substrains with an intact IL-4 gene,7 also consistent with a Th2-mediated process. Although tissue damage in autoimmune diseases typically is said to be a Th1 process, there are other examples of Th2-mediated tissue damage, such as the vasculitic lesions of Palmerston North mice27 and experimental autoimmune uveitis in IFN-γ-deficient mice.28
Table 5.
Immunohistochemistry of Lacrimal Gland Inflammatory Lesions in MRL/MpJ Mice with Intact IL-4
| Mononuclear Inflammatory Cell Staining, %
|
|||||||
|---|---|---|---|---|---|---|---|
| Age (mo) | CD4 | CDS 8 | B | IFN-γ | IL-4 | CD80 | CD86 |
| MRL/MpJ-fas+/fas+-IL-4+/IL-4+ | |||||||
| 3 | 56 ± 9 | 32 ± 8 | 8 ± 6 | 0 | 46 | 2 | 22 |
| 5–6 | 51 ± 7 | 31 ± 9 | 11 ± 7 | 3 | 55 | 4 | 28 |
| 9–12 | 46 ± 10 | 28 ± 6 | 14 ± 14 | NA | NA | NA | NA |
| MRL/MpJ-faslpr/faslpr-IL-4+/IL-4+ | |||||||
| 2–3 | 68 ± 8 | 22 ± 7 | 9 ± 7 | 1 | 67 | 10 | 20 |
| 5 | 60 ± 9 | 14 ± 5 | 10 ± 2 | 3 | 40 | 6 | 20 |
Our data in IL-4 - deficient MRL/MpJ mice are consistent with a Th1-mediated process in which there is a substantial number of inflammatory cells staining for IFN-γ. These mice show little evidence of a Th2 mechanism, as indicated by the absence of IL-13. Our results (Tables 3 and 4) contrast with those in MRL/MpJ mice with an intact IL-4 gene (Table 5) in which almost no IFN-γ was detected.
Traditionally, Th1 processes have been thought to be involved in cell-mediated responses such as delayed-type hypersensitivity, and Th2 processes have been thought to be involved in humoral responses such as allergy. Furthermore, these two types of responses mutually inhibit each other through their respective cytokine profiles. CD80 (B7–1) and CD86 (B7–2) are costimulatory molecules that interact with CD28 and induce Th1 and Th2 responses, respectively.29,30 Interventions that interfere with CD80 can convert a Th1 response into a Th2 response, and those that interfere with CD86 can convert a Th2 response into a Th1 response.29 Furthermore, suppressing Th2-mediated pulmonary allergic responses with CpG oligodeoxynucleotides during antigen challenge in a murine model is associated with increases in IFN-γ, decreases in IL-4 and IL-13, increases in CD80 mRNA expression, and decreases in CD86 expression,30 all consistent with the reciprocal activities of CD80/CD86 on Th1/Th2 responses.
In Th1-mediated autoimmune models, deficiency of IFN-γ may result in conversion of the disease process to one that is Th2 mediated.28 However, deficiency or inhibition of IL-4 alone in Th2-mediated models often results in a Th2-mediated process in which IL-13 (rather than IL-4) appears to drive the process.31,32 Therefore, our results showing that a Th2-mediated process in MRL/MpJ mice converts to a Th1-mediated process in the absence of IL-4 are less typical. Nevertheless, the absence of IL-13 and the presence of IFN-γ in the lacrimal glands of MRL/+/IL-4tm and MRL/lpr/IL-4tm mice are consistent with a Th1-mediated process in the dacryoadenitis of IL-4 deficient MRL/MpJ mice.
MRL/lpr mice also develop systemic autoimmune disease. The pathogenic mechanisms involved in the systemic autoimmune disease in MRL/lpr mice are complicated with evidence of Th1- and Th2-mediated processes. Previous work by others reported that IFN-γ- and IL-4-deficient mice develop significantly reduced lymphadenopathy and end organ disease, including glomerulonephritis, compared with MRL/lpr mice with intact IFN-γ and IL-4 genes.12 Our data showing reduced severity of renal disease in MRL/lpr/IL-4tm mice are consistent with those reported by Peng et al.12
Although IFN-γ and IL-4 appear to play important roles in the development of systemic disease in MRL/lpr mice, CD86 appears to play a critical role in disease induction. MRL/lpr mice deficient in CD86 have milder or absent renal disease than do MRL/lpr mice within intact CD86 expression. Conversely, MRL/lpr mice deficient in CD80 develop more severe end-organ disease.33 Our results suggest that CD86 may also play a key role in lacrimal gland disease. Expression of CD86 is greater than that of CD80 in MRL/MpJ mice of both substrains, regardless of whether IL-4 production is intact8 or deficient. The surprising aspect of our results is the conversion of a Th2 response to a Th1 response in IL-4 – deficient MRL/MpJ/IL-4tm mice, despite the greater expression of CD86 than of CD80 in these mice. It is possible that despite the greater expression of CD86 than of CD80 in the absence of IL-4, it is possible that CD80 costimulation drives the immune response toward a Th1-mediated process; our results cannot exclude this possibility. However, the demonstrated critical role of CD86 in systemic disease production in MRL/lpr mice and the absence of IL-13 in the lacrimal glands suggest that CD86 costimulation could produce the Th1 response in the absence of IL-4. Although CD86 costimulation typically does not induce a Th1 response, a role for CD86 induction of a Th1-mediated process has been described in the lacrimal and salivary glands of IqI/Jic mice, another model for primary Sjögren syndrome.34
Studies of minor salivary gland biopsy specimens of humans with Sjögren syndrome have yielded variable results and have suggested Th1 and Th2 processes35–37 Although MRL/MpJ mice appear to have Th2-mediated dacryoadenitis, IL-4-deficient MRL/MpJ mice appear to have a Th1-mediated process, and both models are consistent with the variable results in humans.35–37 In addition, the two substrains of MRL/MpJ mice—MRL+ and MRL/lpr—could be considered models for primary and secondary Sjögren syndrome given the presence of dacryoadenitis, sialadenitis, and autoantibodies (including anti-Ro and anti-La) and the relative absence and presence of systemic autoimmune disease in the two substrains, respectively.
In conclusion, our results demonstrate that in the absence of functional IL-4, a Th2-mediated end organ disease in MRL/MpJ mice of both substrains is converted to a Th1-mediated disease. Furthermore, they suggest that in the absence of IL-4, CD86 may have a role in this conversion to a Th1-mediated process. Finally, understanding the pathogenesis of the dacryoadenitis and sialadenitis in MRL/MpJ mice may lead to better understanding of the pathogenesis of dacryoadenitis and sialadenitis in human Sjögren syndrome.
Acknowledgments
The authors thank Joseph Craft at Yale University School of Medicine for the MRL/MpJ-fas+/fas+-IL-4+/IL-4tm mice.
Supported by National Eye Institute Grant EY-05912 (DAJ), National Institute of Allergy and Infectious Disease Grant AI-44493 (JAW-H), National Institute of Arthritis and Musculoskeletal Diseases/National Institutes of Health Grants AR-48331 (JAW-H), AR-42541 (APH), and AR-47186 (HCG), and by the Wilmer Eye Institute Moser-Stark Cornea Research Fund. DAJ is a recipient of a Research to Prevent Blindness Senior Scientific Investigator Award. EKA is a recipient of the Research to Prevent Blindness William and Mary Greve Scholarship.
Footnotes
Disclosure: D.A. Jabs, None; R.A. Prendergast, None; A.L. Campbell, None; B. Lee, None; E.K. Akpek, None; H.C. Gérard, None; A.P. Hudson, None; J.A. Whittum-Hudson, None
References
- 1.Jabs DA. Ocular manifestations of the rheumatic diseases. In: Tasman W, Jaeger EA, editors. Duane’s Clinical Ophthalmology. Baltimore, MD: Williams & Wilkins; 1992. pp. 1–40. [Google Scholar]
- 2.Jabs DA, Prendergast RA. Murine models of Sjögren syndrome: immunohistologic analysis of different strains. Invest Ophthalmol Vis Sci. 1988;29:1437–1443. [PubMed] [Google Scholar]
- 3.Jabs DA, Enger C, Prendergast RA. Murine models of Sjögren syndrome: evolution of the lacrimal gland inflammatory lesions. Invest Ophthalmol Vis Sci. 1991;32:371–380. [PubMed] [Google Scholar]
- 4.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA, Prendergast RA. Reactive lymphocytes in lacrimal gland and renal vasculitic lesions of autoimmune MRL/lpr mice express L3T4. J Exp Med. 1987;166:1198–1203. doi: 10.1084/jem.166.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahren M, Skarsrein K, Blange I, Petersson I, Johsson R. MRL/lpr mice produce anti-Ro 52,000 MW antibodies: detection analysis of specificity and site of production. Immunology. 1994;83:9–15. [PMC free article] [PubMed] [Google Scholar]
- 7.St Clair EW, Kenan D, Burch JA, Keene JD, Pisetsky DS. Anti-La antibody production by MRL-lpr/lpr mice: analysis of fine specificity. J Immunol. 1991;146:1885–1892. [PubMed] [Google Scholar]
- 8.Jabs DA, Lee B, Whittum-Hudson JA, Prendergast RA. Th1 versus Th2 immune responses in autoimnume lacrimal gland disease in MRL/Mp mice. Invest Ophthalmol Vis Sci. 2000;41:826–831. [PubMed] [Google Scholar]
- 9.Jabs DA, Prendergast RA, Rorer EM, Hudson AP, Whittum-Hudson JA. Cytokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2001;42:2567–2571. [PubMed] [Google Scholar]
- 10.Jabs DA, Gerard HC, Yuewang W, et al. Inflammatory mediators in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2004;45:2293–2298. doi: 10.1167/iovs.03-0958. [DOI] [PubMed] [Google Scholar]
- 11.Akpek EK, Jabs DA, Gérard HC, et al. Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest Ophthalmol Vis Sci. 2004;45:185–190. doi: 10.1167/iovs.03-0812. [DOI] [PubMed] [Google Scholar]
- 12.Peng SL, Moslehi J, Craft J. Roles of interferon-γ and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabs DA, Burek CL, Hu Q, et al. Anti-CD4 monoclonal antibody therapy suppresses autoimmune disease in MRL/Mp-lpr/lpr mice. Cell Immunol. 1992;141:496–507. doi: 10.1016/0008-8749(92)90166-m. [DOI] [PubMed] [Google Scholar]
- 14.Dialynas DP, Quan ZS, Wall KA, et al. Characterization of murine T cell surface molecule, designated T3T4, identified by monoclonal antibody GK-1.5: similarity of L3T4 to human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 15.Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 16.Ledbetter JA, Rouse RV, Micklem HS, Herzenberg LA. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens: two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980;152:280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes: a new member of the immunoglobulin superfamily. J Immunol. 1989;143:712–717. [PubMed] [Google Scholar]
- 18.Mosmann TR, Cherwinski HM, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 19.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clones. III: further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vries JE, Zurawski G. Immunoregulatory properties of IL-13: its potential role in atopic disease. Int Arch Allergy Immunol. 1995;106:175–179. doi: 10.1159/000236842. [DOI] [PubMed] [Google Scholar]
- 21.Zurawski G, De Vries JE. Jnterleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells. 1994;12:169–174. doi: 10.1002/stem.5530120204. [DOI] [PubMed] [Google Scholar]
- 22.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7–1 and B7–2 costimulatory ligands; expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 24.Snedecor GW, Cochran WG. Statistical Methods. 6. Ames, IA: Iowa State University Press; 1967. [Google Scholar]
- 25.Jonsson R, Tarkowski A, Backman K, Holmdahl R, Klareskog L. Sialadenitis in the MRL-1 mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60:611–616. [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi Y, Hanji N, Hamano H, Yangi K. Transfer of Sjögren-like autoimmune lesions into SCID mice and prevention of lesions by anti-CD4 and anti-T cell receptor antibody treatment. Eur J Immunol. 1994;24:2826–2831. doi: 10.1002/eji.1830241137. [DOI] [PubMed] [Google Scholar]
- 27.Luzina IG, Knitzer RH, Atamas SP, et al. Vasculitis in the Palmerston North mouse model of lupus: phenotype and cytokine production of infiltrating cells. Arthritis Rheum. 1999;42:561–568. doi: 10.1002/1529-0131(199904)42:3<561::AID-ANR22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Jones LS, Rizzo LV, Agarwal RK, et al. IFN-γ deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158:5997–6005. [PubMed] [Google Scholar]
- 29.Kuchroo VK, Das MP, Brown JA, et al. B7–1 and B7–2 costimulatoty molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 30.Serebrisky D, Teper AA, Huang CK, et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol. 2000;165:5906–5912. doi: 10.4049/jimmunol.165.10.5906. [DOI] [PubMed] [Google Scholar]
- 31.Noben-Trauth N, Shultz LD, Brombacher F, et al. An interleukin (IL)-4 independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor deficient mice. Proc Nat Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4R alpha-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–672. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- 33.Liang B, Kashgarian MJ, Sharpe AH, Mamula MJ. Autoantibody responses and pathology regulated by B7–1 and B7–2 costimulation in MRL/lpr lupus. J Immunol. 2000;165:3436–3443. doi: 10.4049/jimmunol.165.6.3436. [DOI] [PubMed] [Google Scholar]
- 34.Konno A, Takada K, Saegusa J, Takiguchi M. Presence of B7–2+ dendritic cells and expression of Th1 cytokines in the early development of sialodacryoadenitis in the Iql/Jic mouse model of primary Sjögren syndrome. Autoimmunity. 2003;36:247–254. doi: 10.1080/0891693031000141077. [DOI] [PubMed] [Google Scholar]
- 35.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren syndrome. J Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 36.Ohyama Y, Nakamura S, Matsuzzaki G, et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren syndrome. Arthritis Rheum. 1996;39:1376–1384. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 37.Aziz KE, Markovi B, McCluskey PJ, Wakefield D. A study of cytokines and minor salivary glands. In: Nussenblatt RB, Whitcup SM, Casbi RR, Gerry I, editors. Advances in Ocular Immunology. New York: Elsevier; 1994. pp. 303–306. [Google Scholar]


