Abstract
Patients with attention-deficit/hyperactivity disorder (ADHD) can be slow at switching between stimuli, or between sets of stimuli to control behaviour appropriate to changing situations. We examined clinical and experimental parameters that may influence the speed of such processes measured in the trail-making (TMT) and switch-tasks in cases with ADHD combined-type, their non-affected siblings and unrelated healthy controls. The latency for completion of the trail-making task controlling for psychomotor processing (TMT B–A) was longer for ADHD cases, and correlated with Conners’ ratings of symptom-severity across all subjects. The effect decreased with age. Switch-task responses to questions of “Which number?” and “How many?” between sets of 1/111 or 3/333 elicited differential increases in latency with condition that affected all groups. But there was evidence for increased symptom-related intra-individual variability among the ADHD cases, and across all subjects. Young siblings showed familiality for some measures of TMT and switch-task performance but these were modest. The potential influence of moderator variables on the efficiency of processing stimulus change rather than the speed of processing are discussed.
Keywords: ADHD, attention, heritability, risk, siblings, set, switch, trail-making
To switch attention or behaviour is fundamentally adaptive in environments that change. Switching attention between stimuli (from moment to moment), switching plans (from retreat to approach) can also be rewarding. But switches cost. They need control, effort (energy) and take time compared to a continuation with the ongoing mode of response. If control is efficient the cost can be small. Young people with attention-deficit hyperactivity disorder (ADHD) have disturbances in reinforcement processes (Sonuga-Barke, 2005), controlling effortful processes (Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003; Hurks et al., 2005), and switching attention-related processes (Pearson, Lane, & Swanson, 1991; Mason, Humphreys, & Kent, 2004; White & Shah, 2006).
Neurophysiological and neuroimaging studies report roles for the inferior frontal regions in switching between stimuli (Jemel, Achenbach, Müller, Röpcke, & Oades, 2002; Beck, Rees, Frith, & Lavie, 2001), and parts of the intraparietal sulcus in switching between plans contingent on set (Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Hampshire & Owen, 2006). Activity of the neurotransmitter dopamine facilitates switches between the inputs that are in control of the output of various cortical and subcortical nuclei in the brain (Oades, 1985). Activity in these frontal and parietal loci is reduced in adolescents with ADHD when switches are required (Smith, Taylor, Brammer, Toone, & Rubia, 2006), especially on the right side (Oades, Dittmann-Balcar, Schepker, & Eggers, 1996), and it is widely accepted that dopamine is suboptimally active in ADHD patients (Oades, 2002; Oades, 2006).
There are reports of impaired set shifting in ADHD on the trail-making task (TMT: review (Nigg, 2005) and on the switch-task (Cepeda, Cepeda, & Kramer, 2000; Kramer, Cepeda, & Cepeda, 2001), and that set-shifting is a heritable feature (Nigg, Blaskey, Stawicki, & Sachek, 2004). We therefore proposed to see if a) we could replicate these results in children as well as in adolescents with ADHD, b) we could show a relationship with clinical ratings in these cases and their often symptomatic siblings, and c) if experimental parameters (e.g. stimulus repetition, congruency) modulate response latency and its variability as well in cases of ADHD as in healthy controls.
Our initial emphasis was on the replication of the TMT results. Secondly we looked to see if indicators of increased switch-costs in ADHD cases were at all evident in their siblings. Such an increase would imply shared familial characteristics suggestive of their heritability (Kuntsi, Rogers, Swinard, Borger, van der Meere et al. 2006). An association with ADHD, as opposed to an independent aggregation within a family should become apparent if the costs of switching were also associated with the severity of ADHD features. Thus we would predict that increases of response time would be less than in the ADHD cases but more than in the healthy controls and would correlate with the severity of ADHD-related symptoms.
We felt it would be useful to extend the TMT results to a computer-based test to see if the findings could be generalised. In this test, the switch-task (Cepeda et al., 2000), two numbers are presented (a ‘1’ or a ‘3’) singly or as triplets, and the subject is asked “Which number?” or “How many numbers?”. The task allows a dissection of the cognitive operations that are more difficult for children (e.g. the first post-switch trial – change of instruction, or the congruency/incongruency of stimulus and set). For example Cepeda and colleagues (Cepeda et al., 2000; Kramer et al., 2001) reported a disproportionate increase of response time for ADHD cases on incompatible trials (e.g. the response to a “3” is a “1” after the question “How many?”) that proved sensitive to psychostimulant medication.
On repeated trials the switch-task also permits an investigation of whether the intra-individual variability of response may additionally show characteristics of familiality as predicted by Kuntsi and colleagues (Kuntsi & Asherson, 2005; Kuntsi et al., 2006). The proposal that variability may reflect a heritable vulnerability to ADHD (Castellanos & Tannock, 2002) is supported by associations between risk alleles within the dopamine transporter gene and response variability in ADHD (Bellgrove, Hawi, Kirley, Fitzgerald, Gill et al., 2005). The dopamine transporter is a major target for methylphenidate, a treatment that reduces response times (Kramer et al., 2001) and intra-individual variability (Tannock, Schachar, & Logan, 1995). Thus we predicted that measures of the variability of response time would be larger in ADHD cases, and in siblings would show values intermediate between the cases and unrelated healthy controls.
In summary we predicted higher costs for set switching in ADHD cases on a paper-and-pencil task (the TMT) and a computer based task (the switch-task), and that response times would show greater variability in this group. We sought associations of these measures with symptoms of ADHD and predicted that non-affected siblings of ADHD cases would show modest increases of these measures compared to healthy controls. Lastly we investigated to what extent these measures depended on task presentation features such as stimulus repetition or in/congruency (of the nature of the stimulus presented to the response requested).
1. Method
1.1 Participants
A total of 172 children and adolescents contributed data. The sample consisted of patients referred to the Clinic for Child and Adolescent Psychiatry, their siblings and independent controls who were recruited by advertisement. Families were given a small honorarium and expenses. Following approval by the clinic management and ethics committee of the Faculty of Medicine, written information was provided to the parents/guardians and children, and their verbal and written consent was obtained in accordance with the Helsinki Declaration.
Data were obtained from 57 youngsters with ADHD combined-type, 44 of their phenotypically unselected siblings, and 71 independent controls, all of European Caucasian descent and aged 5–18y. The gender, age, IQ and Conners parent and teacher ratings (CPRS-R:L, CTRS-R:L: Conners, 2002) for the whole group and for subgroups based on age and ratings are given in Table 1. Diagnoses were made with the Parental Assessment of Child Symptoms (Chen & Taylor, 2006) according to DSM-IV criteria by a trained interviewer. Comorbid oppositional-defiant or conduct disorder was found in 30 cases and there were 17 cases with a mood or anxiety disorder, sometimes in the same individuals. All had an estimated IQ >75 based on 4 WISC-subtests (information, picture arrangement, similarities and block-design: Sattler, 1992). Exclusion criteria for all subjects included autism, epilepsy, general learning difficulties, brain disorders and any genetic or medical disorder associated with externalising behaviour with a similarity to ADHD. All subjects were tested off-medication. For cases normally receiving daily psychostimulants, medication was withdrawn 48 hours prior to testing. Of 166 children who completed the TMT, 120 were tested on the switch-task (see tables 1–5 for the numbers in each group)
Table 1. Group and Subgroup Characteristics.
(Means, with standard deviation, range and number of participants in parentheses)
| Male | Female | Age (years) | Short-IQ | Conners Teacher Ratings | Conners Parent Ratings | |
|---|---|---|---|---|---|---|
| ADHD | 48* | 9 | 10.9 (2.7: 6–16, n57) | 102.3 (14.0: 78–134, n56) | 73.6* (8.4: 57–90, n51) | 76.8* (8.2: 61–90, n52) |
| Siblings | 21 | 23 | 11.3 (3.6: 5–18, n44) | 105.7 (12.5: 88–128, n44) | 56.4** (15.1: 41–90, n43) | 52.7** (12.5: 40–90, n44) |
| Controls | 48 | 23 | 11.0 (2.4: 6–17, n71) | 108.7 (12.5: 78–135, n69) | 47.7 (6.3: 42–75, n55) | 48.6 (7.3: 40–80, n71) |
|
| ||||||
| Siblings’ Conners scale | ||||||
| Rated high T≥65 | 8 | 7 | 11.1 (3.8: 7–18, n15) | 100.9a (12.4: 91–123, n15) | 74.7 (1.9: 53–90, n14) | 61.9 (16.2 42–90, n15) |
| Rated low T<65 | 13 | 16 | 11.3 (3.5: 5–18, n29) | 108.1 (12.1: 88–128 n29) | 47.6 (6.2: 41–63, n29) | 47.9 (6.3: 40–65, n29) |
|
| ||||||
| Younger/Older Participants | ||||||
| ADHD | <12y | 8.6 (1.5: 6–11, n29) | 107.6 (14.6: 87–134, n28) | 71.3## (6.8: 57–90, n24) | 77.1## (7.1: 65–90, n25) | |
| >11y | 13.3 (1.0: 12–16, n28) | 96.9### (11.3: 78–113, n28) | 75.7## (9.2: 63–90, n27) | 76.6## (9.1: 61–90, n27) | ||
| Siblings | <12y | 8.6 (1.8 : 5–11, n25) | 103.0 (13.0: 88–127, n25) | 55.3## (13.7: 41–89, n24) | 55.4 (14.3: 41–90, n25) | |
| >11y | 14.8 (1.9: 12–18, n19) | 109.2 (11.2: 92–128, n19) | 57.9# (17.0: 42–90, n19) | 49.0 (8.8 : 40–77, n19) | ||
| Controls | <12y | 9.2 (1.4 : 6–11, n39) | 110.2 (12.7: 82–134, n37) | 45.9 (4.1: 42–57, n29) | 45.9 (4.1: 40–46, n39) | |
| >11y | 13.1 (1.3 13–17, n32) | 107.0 (12.2: 78–135, n32) | 49.8 (7.7: 42–75, n26) | 51.8 (9.0: 42–80, n32) | ||
First panel -p<.001: more male ADHD cases and higher Conners’ ratings vs. the other groups:
p<.05−.0001, Conners’ ratings distinguish siblings from ADHD cases and controls.
Second panel – p=.05, IQ was lower in siblings with more symptoms: no age differences between sibling groups
Third panel -001<p<.03, older ADHD cases had a lower IQ than other age-groups.
p=.01 & .001, younger and older ADHD cases with higher symptom ratings than all other groups. Younger siblings were more symptomatic than the controls.
p=.09, Older siblings tended to be more symptomatic than controls
Table 5.
A/Comparison of reaction times (ms) and standard deviations (SD) for 3 diagnostic groups where the required response is incongruent or congruent with the stimulus set presented.
B/Comparison of the standard deviations (sd) and coefficients of variation (cv) of the reaction times (intra-individual variance)
| “Which number?” Incongruent : 111 | Congruent : 1 | “How many?” Incongruent : 3 | Congruent : 333 | |||||
|---|---|---|---|---|---|---|---|---|
| ADHD | 1546 | 1260** | 1217 | 1364* | ||||
| SD/N | (592: 39) | (490: 39) | (534: 41) | (549: 41) | ||||
| Siblings | 1616 | 1252 # | 1510 | 1550 | ||||
| SD/N | (652: 31) | (571: 31) | (846: 32) | (709: 32) | ||||
| Controls | 1565 | 1283** | 1446 | 1344 | ||||
| SD/N | (618: 39) | (526: 39) | (703: 39) | (504: 39) | ||||
| ADHD sd | 1160 | (961) | 982 | (618) | 799 | (602) | 1065## | (736) |
| cv | .69 | (.31) | .72 | (.21) | .60 | (.22) | .71 §§ | (.24) |
| Siblings sd | 1130 | (871) | 940 | (736) | 1003 | (1164) | 1306 | (1116) |
| cv | .61 | (.21) | .67+ | (.25) | .55 | (.30) | .70 §§ | (.27) |
| Controlssd | 1196 | (1314) | 977 | (810) | 923 | (985) | 941 | (637) |
| cv | .64 | (.30) | .67 | (.30) | .55 | (.34) | .66 § | (.28) |
t-test (congruent vs. incongruent),
t >4.62, p <.0001:
t =2.66, p =.01:
t =−2.20, p =.03
t =3.14, p =.003;
t =2.24, p =.03;
t = 3.3–4.0, p <.002; (congruent vs. incongruent)
1.2 Trail-Making Test (TMT) Procedure
Form A of the TMT, a paper and pencil test, is a measure of psychomotor speed, and form B adds the requirement of alternating the attentional set (Reitan, 1955). TMT-A was preceded by a short practice - connecting the printed digits 1 to 8, in sequence. The black numbers were 1 cm high dispersed in a 8.5 × 13 cm outline on white paper. The test requires joining the digits 1–25. In the TMT-B practice digits (1–4) and letters (A–D) were presented on 21 x 30 cm paper for connection in the ascending sequence 1-A-2-B-3 and so on. The test had 12 digits and 12 letters. The instructions were to work as fast as possible but to make as few mistakes as possible. Mistakes in the practice session were corrected. Test measures were the latency to completion and the number of errors made. Data were analysed with a MANOVA with covariates of age, IQ and gender as appropriate. We sought Pearson and partial correlations of set shifting on the TMT with Conners ratings and indices of switching under different conditions in the switch-test, taking age, gender and IQ into consideration.
1.3 Switch-task Procedure
We used a modified version of the programme for running the switch-task (Cepeda et al., 2000). Subjects were presented with either the number 1 or 3, which appeared singly (1 or 3) or as a row of 3 numbers (111, 333). Before the numbers were presented the subject was instructed to respond according to the question of either “Which number?” or “How many numbers?”. The responses were executed on the appropriate computer keys numbered 1 and 3 with two fingers from the dominant hand. (The other keys on the keyboard were masked.)
The task consisted of 3 conditions, each with a practice and a test sequence of trials. The first two conditions resulted in baseline responses to “Which number?” and to “How many numbers?” (12 practice and 25 test trials each). The third was the “Switch” condition in which each trial was preceded by the question “Which number?” or “How many numbers?” on the screen. The same question could appear just once or be repeated on 2–3 trials in a pseudo-random order. There were 30 practice trials and 8 blocks of 75 test trials with short pauses.
Subjects were encouraged to respond both fast and correctly. Instructions were presented verbally and on the screen before the first two conditions: (e.g. press the number that you see, when 1, press the ‘1’, when 3 press the ‘3’; or, count how many numbers you see, press the ‘1’ for a single number, press the ‘3’ for 3 numbers). Errors were pointed out during the practice sequences. Before the third condition subjects were told that the question of “which number?” or “how many?” would appear on the screen before each trial requiring them to change from pressing for the identity of the number seen to the number of these numbers presented and vice versa. Two types of feedback were given: errors were followed by a brief beep-tone, and after the end of each condition the accuracy and mean response time were presented. (If performance was judged to be poor, the subjects were asked for improvement.)
The subjects sat 60 cm from the screen on which 1.4 cm numbers were presented. Both the intervals between trials and between the screen instruction and the appearance of the number(s) varied orthogonally and were 100 or 1200 ms. The combined presentation remained on screen until a response was made. Response times <100 or >4000 ms were discarded before analysis.
The analysis was planned to contrast the performance of the cases, their siblings and the controls. Secondly, the effect of gender was considered. Thirdly, the siblings were divided into those with high vs. low ratings of symptoms (Conners scales T≥65 vs. T<65). To reduce the variance in the data MANOVAS were repeated with covariates for age and IQ. Tukey post-hoc tests are cited. Fourthly, we compared data for younger cases, siblings and controls with those older than 12 years. (a) We compared baseline errors and response times for comparison of the costs of switching (correct responses). (b) The costs of switching were then examined separately for the “Which number?” and “How many?” conditions (first trial after a switch of instruction: the externally imposed set). (c) These costs were contrasted with the benefits (positive priming) of repetition of the same instruction (last trial of a run of up to 4 trials of the same condition or instructional set (“Which number?” and “How many?”). (d) Then we examined the strength of the stimulus set in the instructional set of “Which number?” This involves comparing the response times when the subject sees a ‘1’ and presses a ‘1’ with the subject seeing a ‘111’ and pressing a ‘1’. A similar comparison was made after asking ‘How many?’ for seeing a ‘3’/pressing a ‘3’ and seeing a ‘333’/pressing a ‘3’.) e) Lastly the standard deviations (sd) were also examined as indices of intra-individual variability, and differences checked with the coefficient of variation (response-time-sd/response-time) to take into account the different levels of performance (Klein, Wendling, Huettner, Ruder, & Peper, 2006). Significant results (α <.05) and trends where appropriate (α <.1) are reported.
2. Results
2.1 Group Characteristics
There were no significant differences between the ADHD, sibling and control groups on mean age or IQ (Table 1). However, there were significant differences in IQ between the younger and older subgroups: F(5,133) = 3.8, p <.003, due to the lower IQ of the older ADHD cases. The proportion of males to females and the two Conners’ sets of ratings were higher in the ADHD than the other two groups: F(2,146–169) = 8–153, p<.0005. For siblings, compared with controls, there were fewer males but higher Conners’ ratings: F(1,96–113) = 4.6–15.0, p<.04. Sibling groups rated high and low on the Conners’ scales differed only on these ratings: F(1,32) = 17–91, p<.0003. Significant differences in the ANOVA for Conners’ ratings for younger and older subjects: F (5,143) = 36.4, p<.0001: were explained in part by the clearly higher ratings for the cases, but also by a tendency for higher ratings in both the younger (p=.01) and the older siblings (p=.09) than for the matched controls.
2.2 Trail-Making (TMT)
A multivariate analysis showed no significant group differences for the few errors made (table 1). But a similar analysis of TMT latencies showed a main effect of group: Wilks’ Λ = 0.92, F(6,322) = 2.32, p=.03, η2 = .04, power .80. The cases were psychomotorically slower than the other two groups (TMT-A): F(2, 165) = 4.5, p=.01, η2 = .05. They were even slower with the addition of set-switching (TMT-B): F(2,165) = 5.8, p<.004, η2 = .07. The TMT B–A difference-measure illustrates the contribution of slower cognitive switching to the slower latency in the ADHD group: F(2,165) = 5.9, p=.003, η2=.07, power .87: table 2. The latencies of siblings did not differ significantly from those of controls on any TMT measure, and were unaffected by high or low ratings on the Conners’ scales (TMT-A 41/41 sec, TMT-B 118/107 sec, TMT B–A 77/66 sec, respectively).
Table 2.
Mean latencies (sec) and mean number of errors recorded on the Trail-Making test (TMT: standard deviation in parentheses) by diagnostic group
| TMT-A | TMT-B | TMT B–A | ||||
|---|---|---|---|---|---|---|
| Group | N | Latency | Errors | Latency | Errors | Increased Latency |
| ADHD | 56 | 52.4 1, 3 | 0.16 | 150.1 2,4 | 0.89 | 99.9 1,4 |
| (30.3) | (0.06) | (101.6) | (0.18) | (78.6) | ||
| Siblings | 40 | 41.1 | 0.18 | 110.8 | 0.85 | 69.7 |
| (18.5) | (0.18) | (70.7) | (0.21) | (56.2) | ||
| Controls | 70 | 41.6 | 0.09 | 107.4 | 0.56 | 65.8 |
| (16.1) | (0.05) | (46.1) | (0.16) | (36.4) | ||
p <.04,
p<.03, ADHD vs. siblings:
p<.03,
p<.005, ADHD vs. controls
Age and IQ as covariates marginally increased and decreased the significance of the main analysis (TMT B–A: F= 6.9 and 4.4, respectively). Gender as a covariate marginally reduced the significance further (e.g. TMT B–A: F=4.1, p=.02). This latter result reflected the slower responses of male versus the relatively normal latencies shown by female cases (F(1,160) = 3.7, p = .057, η2 = .023: Table 3a), siblings (F = 9.2, p = .003, η2 = .054) and controls (F = 10.2, p < .002, η2 = .061, power .89). Nonetheless there was no significant group interaction with gender (F<0.9), that likely reflects the small number of female ADHD cases.
Table 3.
Mean latencies (sec) recorded on the Trail-Making test (TMT: standard deviation in parentheses) for 3 subject groups divided by gender (A) and age (B)
| TMT-A | TMT-B | TMT B–A | |||||
|---|---|---|---|---|---|---|---|
| N | |||||||
|
| |||||||
| A/Gender | Latency | (sd) | Latency | (sd) | Latency difference (sd) | ||
| ADHD | M 47 | 54.1 | (32.0) | 160.0 | (106.6) | 108.51 | (82.0) |
| F 9 | 43.4 | (17.4) | 98.4 | (46.3) | 55.0 | (33.9) | |
| Sibs | M 19 | 45.9 | (20.4) | 129.5 | (87.4) | 83.62 | (70.3) |
| F 21 | 36.7 | (15.8) | 93.8 | (47.1) | 57.0 | (37.0) | |
| Cons | M 48 | 42.7 | (17.6) | 114.9 | (50.3) | 72.23 | (38.9) |
| F 22 | 39.1 | (12.3) | 91.0 | (30.0) | 51.8 | (25.9) | |
|
| |||||||
| B/Age | Latency | (sd) | Latency | (sd) | Latency difference (sd) | ||
| ADHD | <12y (29) | 62.6a | (36.7) | 188.1a,b | (122.8) | 126.2a,c | (93.2) |
| >11y (27) | 41.5 | (15.7) | 109.4 | (47.7) | 71.7 | (46.1) | |
| Sibs | <12y (25) | 65.6 | (58.8) | 145.2 | (76.0) | 94.1 | (63.3) |
| >11y (17) | 27.5 | (7.3) | 64.1 | (14.4) | 36.6 | (13.3) | |
| Cons | <12y (38) | 48.9 | (16.7) | 129.9 | (49.6) | 80.9 | (40.3) |
| >11y (32) | 32.9 | (10.2) | 80.7 | (20.9) | 47.7 | (19.8) | |
A/males (M) vs. females (F) within each group.
p = .057;
p = .003;
p = .002,
B/ younger (<12y) vs. older cases (>11y) .0001 < p < .05;
ADHD vs. Controls, p = .007;
ADHD vs. Controls, p = .01.
With regard to age, adolescents in each group were faster than the younger children: F(5, 165) = 9.2–11.5, p<.0001, η2 = .22–.26, (Table 3b). Covariation for IQ and gender made little difference (F= 7.7–12.5). Among the younger subjects there were no group differences on TMT-A. On TMT-B younger ADHD cases did not differ from their siblings, but showed significantly longer latencies than the controls. Both effects were confirmed by the TMT B–A measures. Among the older subjects there were again no group differences on the TMT-A. Although the older cases showed descriptively longer TMT-B latencies than the siblings and the controls, these comparisons were not significant.
Group performance differences were more marked for tests of cognitive (TMT-B) than psychomotor function (TMT-A), but the size of the effect was larger in the young and decreased with increasing age. While the effect sizes for the increased costs of switching (especially the cognitive component) were moderate for the cases versus the siblings and controls in turn, those showing a decrease of cost with age were by contrast small. The effects of gender and IQ on group differences were negligible.
Did TMT performance vary with symptoms, as predicted? Partial correlations taking the obvious effect of age and the putative effect of IQ on a cognitive measure into account, showed a modestly significant positive association: for TMT A/B/B–A, CTRS, r = .15/.19/.20, p = .010–.036; CPRS, r = .15/.13/.12, p = .04–.07, df = 140. The teacher rated symptoms across all subjects (r = .20, r2 =.10, p = .02) accounted for 10% of the variance.
2.3 Switch-Task
The mean number of errors made on the baseline conditions of “Which number?” and “How many?” ranged from 0.4 in controls to 1.4 for cases. These were too few for analysis. However, on the “Switch” condition cases and their siblings made more than twice the number of errors of controls (X=6.7, sd=10.6; X=7.3, sd=19.6; X=2.9, sd=5.2, respectively: F(2,112) = 2.2, p=.08, η2 =.04, power .5. The “Switch” condition appeared more effortful. It tended to lead to less accurate responses in the cases and their siblings. While there was no group-condition interaction for response time there was trend for cases to show long latencies on the “Switch” condition supporting this interpretation (mean + 60 ms, p = .067, table 4).
Table 4.
Mean response time (RT: ms) and standard deviations (sd) in 3 conditions of the switch-task in ADHD cases, their siblings and controls: coefficient of variation in the “Switch” condition
| Group: | ADHD | Siblings | Controls | |||
|---|---|---|---|---|---|---|
| Mean | (sd) | Mean | (sd) | Mean | (sd) | |
| N: | 39 | 33 | 38 | |||
| Condition | ||||||
| “Which number?” | 278.4 | (149.5) | 286.4 | (200.6) | 268.8 | (251.5) |
| “How many?” | 343.7 | (187.2) | 440.4 | (403.0) | 360.8 | (270.9) |
| “Switch” | 703.91 | (147.6) | 662.2 | (156.7) | 642.9 | (140.1) |
|
| ||||||
| Coefficient of variation | ||||||
| “Switch” | .512,3 | (.06) | .48 | (.06) | .46 | (.06) |
Coefficient of variation = SD of mean RT/RT:
ADHD vs. controls, t = 1.9, p = .067:
ADHD vs. Siblings, t = 2.25, p = .028:
ADHD vs. controls, t = 3.28, p = .002
The older members (>11y) of each group had shorter response latencies than the younger ones (<12y): Wilks Λ=0.66, F(15,279) = 3.04, p<.0001, η2 =.13, power .99. This was significant for the “How-many” and “Switch” conditions for siblings and controls (Tukey, p < .007/.013 and p < .0001/.007, respectively). The comparison was only significant for the ADHD group in the “Switch” condition (e.g. p = .02: Figure 1). The significant development with age in the two comparison groups but not in the cases was unaltered by covarying for gender or IQ. The effect size for the influence of age was much larger than with the TMT.
Figure 1.
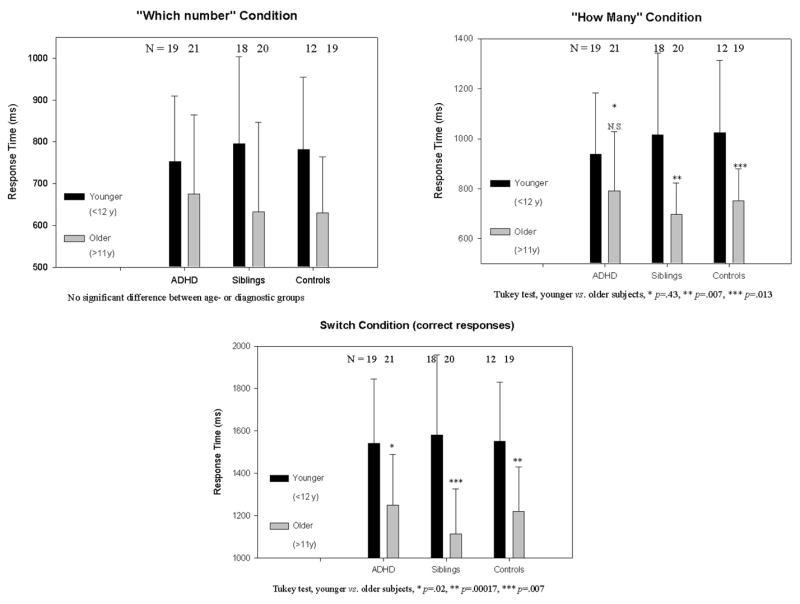
The response time (RT and standard deviation [sd]) for ADHD cases, their siblings and independent controls aged less than 12.0 years or over 11.9 years on three conditions of the switch-task shows a greater developmental decrease with increasing age in the comparison groups.
Considering the results from the TMT task, the lack of major group differences in response latency in the switch-test was unexpected. This absence is consistent with both the lack of a difference between sibling-groups with high vs. low Conners’ ratings, and the absence of an association between Conners’ teacher or parent ratings and response times in the “Switch” condition: r = .02 –.16 (p=.2–.9) n = 68, controlled for IQ and age. Despite this, the trend toward longer response times specific to the “Switch” condition is supported by a significant partial correlation between the comparable latency measures in the TMT B–A and the “Switch” condition of the switch-task: r = 0.36, p<.002, with an overlap accounting for about 15% of the variance.
Was the performance of ADHD cases in terms of response latency as consistent as in the comparison groups? A MANCOVA (age and IQ) of the standard deviations of response latency provided a tentative indication that the cases responded more variably: Wilks Λ=.87, F(6,152) = 1.9, p =.09, η2 =.07, power .68. When the load of the task increased from the simple “Which number?” and “How many?” to the “Switch” condition then the variability of response timing (indicated by the standard deviation) was increased more for cases than for the siblings and controls: F(2,83) = 3.5, p=0.037. The MANOVA data in table 4 illustrate this, and the increased variability in the “Switch” condition was confirmed by a significantly larger coefficient of variation than in either the sibling or control groups. The comparison by age groups was significant: F(5,110)=3.74, p<.005, η2 =.15, power .92. The effect was shown for the comparison of the older cases and controls (Tukey p=.007: younger/older subjects for ADHD .49/.53, siblings .46/.50, controls .47/.46). Controlling for age and IQ it was found that the coefficient of variation in the “Switch” condition correlated clearly with Conners’ teacher ratings, df 93, r = .41, r2 = .17, p = .005, and with the parental ratings: df 104, r = .34, r2 = .12, p=.02. In contrast, correlations with variation on the baseline conditions were not significant.
An examination of the costs of switching, measured by increased response time for the first trial of a new set (“Which number?” to “How many?” or vice versa), and of the benefits of repeat trials (repetition of “Which number?” or “How many?”) showed no between-group differences (e.g. “How many”: ADHD 1514, Siblings 1492, Controls 1420 ms). But, against expectations within-group comparisons for latencies after a switch (costs) with those at the end of several repeated trials (benefits of repetition) showed least change for controls (“How many”: 1420 vs. 1480 ms) and large decreases reflecting the benefits of repetition for the siblings (“Which number”: 1704 vs. 1402 ms: t = 2.57, p<.015) and the ADHD cases (“How many”: 1514 vs. 1378 ms: t = 1.79 p =.08). However, the variability (sd) measures were remarkably stable for the post-switch and repeat trials, suggesting an insensitivity of response variability to small changes in the costs and benefits of switch- or repeat-trials.
Another form of switching that requires effort is the requirement of a response that is not congruent with the perceptual representation of the stimulus presented (e.g. “Which number?” where “111” is presented, and “How many?” where a “3” is presented). The cost of resolving the incongruence between the perceptual and the conceptual set is given by the comparison with the response latencies in the congruent conditions (e.g. “Which number?” see a “1”, and “How many?” see “333”). There were no significant differences between groups on any of these 4 measures. Each group showed a similarly reduced latency and standard deviation for the simpler, congruent (vs. incongruent) presentation of “Which number?” (table 5), but not for the “How many?” presentation. Here, all subjects showed an increase of response time and measures of variability: these were more marked and statistically significant for the ADHD cases (table 5).
3. Discussion
3.1 Trail-Making
On the TMT ADHD cases, independent of psychomotor slowness, took longer to shift attentional set than either their siblings or the controls, who responded similarly to each other. A comparison of the data for cases with controls showed a moderate effect size (d) of 0.54, which is consistent with recent reports that range from 0.55–0.75 (Nigg, 2005; Boonstra, Oosterlaan, Sergeant, & Buitelaar, 2005). The effect tended to be smaller in girls (see also Lockwood, Marcotte, & Stern, 2001), and to decrease with age. Boucugnani and Jones (1989) also reported improvements on the TMT-B with age in ADHD and normal children. As a percentage they appeared larger in the ADHD group, yet (as here) showed no significant interaction with age.
Among the siblings, the young and the male children showed TMT response latencies and errors intermediate between the cases and controls independent of high or low Conners’ symptom ratings. As symptom severity correlated with latency across the whole population, we propose that the apparent familiality of siblings’ performance likely reflects a feature of the shared environment rather than of the disorder. Young non-ADHD children are vulnerable in a family with an ADHD case, but unlike ADHD cases (White and Shah, 2006) this is not maintained with development. We believe this is the first time that data on symptoms, age and gender have been reported for TMT performance in ADHD cases and their non-ADHD siblings. Overall performance was moderated more by diagnosis and less by symptom severity. But there was a modest detrimental influence on the cost of switching when ADHD symptoms were expressed, with 10% of the variance explained.
3.2 Switch-Task
On the switch-task, in terms of response time and errors, all subjects had increasing difficulty with the conditions “Which number?”, “How many?” and “Switch”. The older members of each group were faster than the younger ones, but this improvement was notably less evident in the adolescent cases, consistent with delayed maturational processes (Cantwell, 1985).
The tendency for ADHD cases to show longer response latencies and more errors in the “Switch” condition than the other children may reflect the increased effort required in this condition over the baseline. This interpretation is supported by the significantly increased coefficient of variation in the ADHD group, a measure that takes the level of response into account. This measure of variability distinguishes ADHD performance on at least 5 other tests of sustained attention and executive function (Klein et al., 2006).
Unlike the TMT, overall response times did not correlate with symptom ratings. Further, siblings’ performance was highly variable and did not sit between the other two groups. But as with the TMT, siblings’ performance was independent of the level of symptom severity. However the characteristic of response time variability was associated with symptom expression, with some 17% of the variance explained. Indeed, the performance on the switch task-forms (TMT B–A and “Switch” condition) was correlated. While the overlap of the variance explained was a modest 15%, it was highly significant. Together these results on the switch-task suggest that the underlying moderator of performance in cases and siblings alike may reflect the consistency of response rather than the speed of processing itself. This parallels interpretations of Kuntsi and colleagues (2006) that emphasised intra-individual variability of response as a core feature or phenotype (with potential heritability) based on studies with a Go/no-go task and the “Fast-task” (Andreou et al., 2007).
Where might the basis for the increased sensitivity to the costs of switching in ADHD lie? Against initial expectations the benefit of repeated trials of the same set versus the cost of switching set (e.g. “Which number?” to “How many?”) proved to be similar for each group. By contrast, the problem for most subjects lay more with the conflict or congruence between the set initiated by the instruction and the perceptual nature of the stimulus seen (e.g. “Which number?” vs. presentation of three ‘ones’ i.e. 111). The parallel condition for the instruction “How many?” was treated likewise by the comparison subjects but was more effortful for ADHD cases in terms of response time and variability (standard deviation and coefficient of variation). This suggests that decisions were more difficult for the ADHD cases when several stimuli were presented.
3.3 Comparison with previous switch-task reports
Our study of the switch-task does not clearly replicate the results of Cepeda and colleagues (Cepeda et al., 2000; Kramer et al., 2001), although some aspects are similar. Their ADHD and control samples had an age similar to our younger subgroups, but a 14 point lower IQ. Their samples contained more girls (38%), non-white Caucasians (44%) and non-combined type diagnoses (19%: Cepeda et al., 2000) and responded more slowly than our groups. They concentrated on comparing compatible and incompatible responses in terms of a switch between trials where the question altered but the number for response (1 or 3) remained the same. They reported that ADHD response latencies were normal on non-switch trials but increased by nearly 300 ms on compatible switches, and about 450 ms on incompatible switches over those of controls. These changes are much larger than in the present study.
In contrast to Cepeda et al. we found no group differences on response latency either by comparing conditions (figure 1), nor with analyses of the “Switch” condition (table 4). Both of our analyses showed that response times increased between the “Which number?” and “How many?” conditions to a comparable extent in each group. Unfortunately Cepeda et al. did not present separate analyses for the different congruent/incongruent presentations of each condition. Further, the benefit of repeating trials of the same type, not reported by Cepeda, was evident in each group, albeit to an extent that differed between the two conditions.
However, the conclusions from Cepeda, Kramer and colleagues receive some support from our analysis of response variability. We noted here that the ADHD cases showed increased intra-individual variability on the “Switch” condition, and on the incongruent vs. congruent presentations of the “How many?” condition. The reasons for the differences between studies could lie with the group selection (above), but could also reflect the way the task was run. In order to have enough trials to analyse what happened after a switch or at the end of a repeated sequence of trials, the “Switch” condition proved to be long for young children. Despite welcome pauses they needed encouragement to continue. Problems with vigilance are not usually found for ADHD children (van der Meere & Sergeant, 1988). Indeed, Klein et al. (2006) did not find any effect of declining vigilance or changes of the standard deviation of response with up to 15 repeated trial blocks of a Go/no-go task. But we cannot exclude the possibility that to a degree some subjects went off task, and responded partially at random. An alternative explanation comes from the neuroimaging study of Smith et al. (2006). They reported group differences in regional brain activation between switch and repeated conditions on the Meiran task but found no performance differences between 13–14 year-old ADHD cases and controls. Thus, in our hands the computer test was perhaps not sensitive enough to record the costs of switching, while the demands of the pencil-and-paper TMT were overall higher.
3.4 Conclusions
The switch-test – where performance correlated with the TMT - could be used for studies of intra-individual variability in a modified form to retain interest and task engagement (e.g. presentation in segments separated by other activities). It may be preferred to the Meiran test, which may not yield group performance differences, or other tasks that involve switching (e.g. card-sorting) that sometimes indicate problems with the flexibility of set (Barkley, 1997) and sometimes not (Grodzinsky et al., 1992; Denckla, 1996). These may not be suitable for demonstrating impairment in ADHD cases in summoning the energy and effort for efficient cognitive switching in terms of response latency (Greve, Williams, Haas, Littell, & Reinoso, 1996). In the present experiments the switch-test was valuable in showing up the role of inconsistent responses, of the intra-individual variability of response latency. The measures of the variability of response time proved more sensitive to diagnosis than mean response latency measures of the costs of cognitive switching.
The TMT is a convenient short task that can demonstrate the cost of cognitive switching in terms of response times. Costs for cases increased by about 50%, comparable to (Gorenstein, Mammato, & Sandy, 1989) or slightly longer than other studies of ADHD (Grodzinsky & Diamond, 1992), but shorter than those of the inattentive subtype of ADHD (Chhabildas, Pennington, & Willcutt, 2001) or with comorbid oppositional disorder (van Goozen et al., 2004). However, the latencies in these studies are much less than the 3-fold increases reported for brain-damaged children (Reitan & Wolfson, 2004). From a developmental perspective one notes that the latencies here were more than twice those of students (Tombaugh, 2003).
The results on the switch-task support those from the TMT, but the generalization from one to the other is not robust. There were tendencies for an increased intra-individual variability of response in ADHD cases performing the switch task, and to a lesser extent among their siblings (compared to controls). Further, siblings (like cases) took longer on the TMT B–A when young, made more errors on the switch-task, and showed more variability on some conditions than the controls. The switch-task variability but not the TMT latencies were associated with symptom expression. Thus, although the severity of symptoms may be related to the increased costs (slower responses) experienced by the cases with ADHD there may be another moderator of performance in ADHD associated with maintaining attention and organizing appropriate responses, and this concerns the consistency or variability of the speed of processing
Acknowledgments
We are extremely grateful to Nicholas Cepeda (University of Illinois) for allowing us to use his switching task programme and advice on its implementation. We also thank Kristina van Leewen (University of Duisburg-Essen) for help in running the tests, Rodney Davies (University of Wollongong) for assistance with the analysis and not least we thank all the families who took part in the test sessions. We acknowledge support from the International Multicentre ADHD Genetics project (IMAGE), NIH grant R01MH62873 to Dr. S.V. Faraone, and for an investigator-initiated grant from Janssen-Cilag.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007 doi: 10.1017/S0033291707000815. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention and executive functions: constructing a unifying theory of ADHD. Psychology Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nature Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Fitzgerald M, Gill M, Robertson IH. Association between Dopamine Transporter (DAT1) Genotype, Left-Sided Inattention, and an Enhanced Response to Methylphenidate in Attention-Deficit Hyperactivity Disorder. Neuropsychopharmacology. 2005;30:2290–2297. doi: 10.1038/sj.npp.1300839. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK. Executive functioning in adult ADHD: a meta-analytic review. Psychological Medicine. 2005;35:1097–1108. doi: 10.1017/s003329170500499x. [DOI] [PubMed] [Google Scholar]
- Boucugnani LL, Jones RW. Behaviours analogous to frontal lobe dysfunction in children with attention deficit hyperactivity disorder. Archives of Clinical Neuropsychology. 1989;4:161–173. [Google Scholar]
- Cantwell DP. Hyperactive children have grown up: what have we learned about what happens to them? Archives of General Psychiatry. 1985;42:1026–1028. doi: 10.1001/archpsyc.1985.01790330110013. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews: Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Cepeda ML, Kramer AF. Task switching and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:213–226. doi: 10.1023/a:1005143419092. [DOI] [PubMed] [Google Scholar]
- Chen W, Taylor EA. Parental Account of Children’s Symptoms (PACS), ADHD phenotypes and its application to molecular genetic studies. In: Oades RD, editor. Attention-Deficit/Hyperactivity Disorder and the Hyperkinetic Syndrome: Current Ideas and Ways Forward. Hauppauge, NY 11788: Nova Science Publishing Inc; 2006. pp. 3–20. [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Conners CK. Manual for Conners’ rating scales. Revised. N. Tonoawanda, NY: Multi-Health Systems Inc; 2002. [Google Scholar]
- Denckla MB. Research on executive function in a neurodevelopmental context: application of clinical measures. Developmental Neuropsychology. 1996;12:5–15. [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Mammato CA, Sandy JM. Performance of inattentive-overactive children on selected measures of prefrontal-type function. Journal of Clinical Psychology. 1989;45:619–632. doi: 10.1002/1097-4679(198907)45:4<619::aid-jclp2270450419>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Greve KW, Williams MC, Haas WG, Littell RR, Reinoso C. The role of attention in wisconsin card sorting test performance. Archives of Clinical Neuropsychology. 1996;11:215–222. [PubMed] [Google Scholar]
- Grodzinsky GM, Diamond R. Frontal lobe functioning in boys with attention- deficit hyperactivity disorder. Developmental Neuropsychology. 1992;8:427–445. [Google Scholar]
- Hampshire A, Owen AM. Feature Article Fractionating Attentional Control Using Event-Related fMRI. Cerebral Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hurks PPM, Adam JJ, Hendriksen JGM, Vles JSH, Feron FJM, Kalff AC, et al. Controlled Visuomotor Preparation Deficits in Attention-Deficit/Hyperactivity Disorder. Neuropsychology. 2005;19:66–76. doi: 10.1037/0894-4105.19.1.66. [DOI] [PubMed] [Google Scholar]
- Jemel B, Achenbach C, Müller B, Röpcke B, Oades RD. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topography. 2002;15:13–27. doi: 10.1023/a:1019944805499. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder (ADHD) Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Cepeda NJ, Cepeda ML. Methylphenidate effects on task switching performance in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1277–1284. doi: 10.1097/00004583-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Asherson P. An interdisciplinary approach to ADHD. In: Larimer MP, editor. Attention Deficit Hyperactivity Disorder Research Developments. Hauppauge, NY: Nova Science Publishers, Inc; 2005. pp. 1–30. [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger NA, van der Meere JJ, Rijsdijk F, et al. Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychological Medicine. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood KA, Marcotte AC, Stern C. Differentiation of attention-deficit/hyperactivity disorder subtypes: application of a neuropsychological model of attention. Journal of Clinical and Experimental Neuropsychology. 2001;23:317–330. doi: 10.1076/jcen.23.3.317.1179. [DOI] [PubMed] [Google Scholar]
- Mason D, Humphreys G, Kent L. Visual search, singleton capture, and the control of attentional set in ADHD. Cognitive Neuropsychology. 2004;21:661–687. doi: 10.1080/02643290342000267. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic Theory and Findings in Attention-Deficit/Hyperactivity Disorder: The State of the Field and Salient Challenges for the Coming Decade. Biological Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology. 2004;113:614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Oades RD. Dopamine may be ‘hyper’ with respect to noradrenaline metabolism, but ‘hypo’ with respect to serotonin metabolism in children with ADHD. Behavioural Brain Research. 2002;130:97–101. doi: 10.1016/s0166-4328(01)00440-5. [DOI] [PubMed] [Google Scholar]
- Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neuroscience and Biobehavioral Reviews. 1985;9:261–283. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Oades RD. Function and dysfunction of monoamine interactions in children and adolescents with AD/HD. In: Levin ED, editor. Neurotransmitter interactions and cognitive function. Basel: Birkhäuser Verlag; 2006. pp. 207–244. [DOI] [PubMed] [Google Scholar]
- Oades RD, Dittmann-Balcar A, Schepker R, Eggers C. Auditory event-related potentials and mismatch negativity in healthy children and those with attention-deficit- or Tourette-like symptoms. Biological Psychology. 1996;43:163–185. doi: 10.1016/0301-0511(96)05189-7. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Lane DM, Swanson JM. Auditory attention switching in hyperactive children. Journal of Abnormal Child Psychology. 1991;19:479–492. doi: 10.1007/BF00919090. [DOI] [PubMed] [Google Scholar]
- Reitan RM. The relation of the trail making test to organic brain damage. Journal of Consultative Psychology. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Archives of Clinical Neuropsychology. 2004;19:281–288. doi: 10.1016/S0887-6177(03)00042-8. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of Children: Behavioral and Clinical Applications. 3. San Diego: J.M. Sattler Publ. Inc; 1992. [Google Scholar]
- Sergeant JA, Geurts HM, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor EA, Brammer M, Toone B, Rubia K. Task-Specific Hypoactivation in Prefrontal and Temporoparietal Brain Regions During Motor Inhibition and Task Switching in Medication-Naive Children and Adolescents With Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Logan GD. Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology. 1995;23:235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2003;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- van der Meere JJ, Sergeant JA. Controlled processing and vigilance in hyperactivity: time will tell. Journal of Abnormal Child Psychology. 1988;16:641–655. doi: 10.1007/BF00913475. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Cohen-Kettenis PT, Snoek H, Matthys W, Swaab-Barneveld H, van Engeland H. Executive functioning in children: a comparison of hospitalised ODD and ODD/ADHD children and normal controls. Journal of Child Psychology and Psychiatry. 2004;45:284–292. doi: 10.1111/j.1469-7610.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- White HA, Shah P. Training attention-switching ability in adults with ADHD. Journal of Attention Disorders. 2006;10:44–53. doi: 10.1177/1087054705286063. [DOI] [PubMed] [Google Scholar]


