Abstract
Calpains, Ca2+-activated cysteine proteases, have been implicated in the progression of multiple disease states. We recently identified calpain 10 as a mitochondrial calpain that is involved in Ca2+-induced mitochondrial dysfunction. The goals of this study were to characterize the expression and activity of renal mitochondrial calpain 10 in rabbit, mouse, and rat. Using shRNA technology and immunoblot analysis three previously postulated splice variants of calpain 10 were identified (50, 56, and 75 kDa). SLLVY-AMC zymography and immunoblot analysis was used to directly link calpeptin-sensitive calpain activity to calpain 10 splice variants. Rabbit, mouse, and rat kidney mitochondria contained 75 kDa (calpain 10a), 56 kDa (calpain 10c or 10d), and 50 kDa (calpain 10e). Interestingly, zymography yielded distinct bands of calpain activity containing multiple calpain 10 splice variants in all species. These results provide evidence that several previously postulated splice variants of calpain 10 are localized to the mitochondria in kidneys of rabbits, rats and mice.
Keywords: calpain 10, mitochondria, kidney, SLLVY-AMC, zymography
INTRODUCTION
Calpains are a ubiquitously expressed 15-member family of Ca2+-activated cysteine proteases that have been implicated in many disease states (e.g. muscular dystrophy, gastric cancer, type II diabetes, and renal failure) [1; 2; 3; 4; 5; 6]. The calpain family is divided into two groups, typical and atypical. The first group (calpains 1, 2,3,8,9,11,12,14), are known as typical calpains because they are comprised of four domains including the Ca2+ binding domain (domain IV). The second group (calpains 5,6,7,10,13,15) are known as atypical calpains because they lack the Ca2+ binding domain (domain IV) [1].
Calpain 10 is an atypical calpain that has recently gained attention due to its potential involvement in type 2 diabetes. In 2000 a genome wide scan for type II diabetes susceptibility genes in a population of Mexican Americans identified the calpain 10 gene (CAPN10) as a putative type 2 diabetes susceptibility gene [4]. Since then, multiple other studies involving diverse populations have supported this finding, while others have not [7]. Other investigators have linked this genetic association of CAPN10 and type 2 diabetes to functional roles for calpain 10 in the progression of the diabetic phenotype, including regulation of glucose uptake via GLUT4 vesicles [8; 9] and regulation of mitochondrial metabolism and insulin secretion [10].
While calpains are generally thought to be cytosolic, our laboratory recently identified calpain 10 as a mitochondrial calpain and demonstrated that it plays a role in Ca2+-induced mitochondrial dysfunction [11]. Specifically, rabbit mitochondrial calpain 10 has a mitochondrial targeting sequence and is responsible for Ca+2-induced cleavage of Complex I proteins NDUFV2 and ND6. Horikawa et al [4] described the genetics of human CAPN10, specifically the ability of the gene to undergo alternate splicing, yielding eight potential gene products of varying size. To date there has not been conclusive evidence that the protein products of CAPN10 splice variants are expressed, although multiple investigators have identified immunoreactive bands that correspond to the predicted molecular weight of the splice variants [12; 13; 14]. Thus, the aims of this study were to determine whether multiple calpain 10 splice variants exist in mitochondria and to determine the expression and activity of calpain 10 across species.
MATERIALS AND METHODS
Reagents
Calpain 10 antibody and HRP-conjugated goat anti-rabbit secondary antibody were purchased from Abcam (Cambridge, MA) and Pierce (Rockford, IL), respectively. SLLVY-AMC and Percoll were obtained from Bachem (King of Prussia, PA) and Amersham Biosciences (Piscataway, NJ), respectively. Calpeptin and purified porcine calpain 1 were purchased from Calbiochem (La Jolla, CA). Dulbecco’s Modified Eagle Medium, calf serum, and lipofectamine were obtained from Invitrogen (Carlsbad, CA) and shRNA plasmids targeted to calpain 10 were purchased from Origene (Rockville, MD). All other chemicals were obtained from Sigma (St. Louis, MO).
Calpain 10 shRNA
Normal rat kidney (NRK-52E) cells were cultured as previously decribed [15]. Calpain 10 shRNA was transfected into NRK-52E cells using lipofectamine. After 48 hr cells were lysed and immunoblot analysis was preformed.
Mitochondrial isolation
Mitochondria were isolated from the kidney cortex of female New Zealand White rabbits (2 kg), kidney cortex of male Sprague-Dawley rats (250 g) and whole kidney of male C57BL/6 mice (20–30 g) as previously described [11; 16]. Following isolation of kidney mitochondria from the rabbit, further fractionation was performed to yield a mitochondrial matrix fraction as previously described [11; 17].
Cellular Fractionation
Renal proximal tubules were isolated from New Zealand White rabbits (2 kg) using the iron oxide perfusion method and grown in 35-mm culture dishes as previously described [18; 19]. Cells were grown to confluence and harvested for isolation of mitochondrial and cytosolic fractions as previously described [20].
Zymography
Zymography was preformed as previously described with modifications [11; 21]. Briefly, zymogram gels were cast just prior to use and were composed of 8% acrylamide for resolving and 6% acrylamide for stacking. Resolving gels were copolymerized with the calpain substrate SLLVY-AMC (5 μM). Mitochondrial protein samples, briefly sonicated prior to electrophoresis to release proteins (50–100 μg), and purified porcine calpain 1 (10 μg) were electrophoretically separated under nondenaturing conditions (running buffer – 25 mM Tris base, 125 mM glycine, 1 mM EDTA, 10 mM 2-mercaptoethanol, pH 8.0) at 125 V for 3 hours at 4°C. Following electrophoresis, gels were incubated in a Ca2+ buffer (50 mM Tris-HCl, 5mM CaCl2, 10 mM 2-mercaptoethanol, pH 7.0) for 10 min at room temperature and imaged on an Alpha Innotech imaging station. The presence of fluorescent bands indicated proteolytic activity. Bands of activity were excised from the zymogram and proteins were eluted from the gel slices in a denaturing elution buffer (25 mM Tris-base, 192 mM glycine, 1 mM thioglycolic acid, and 0.1% SDS, pH 8.8). Eluted proteins were concentrated in protein concentrating microcentrifuge tubes (Millipore) and subjected to immunoblot analysis.
Immunoblot analysis
Protein samples were separated by SDS-PAGE (4–12% acrylamide) and transferred to nitrocellulose membranes. Membranes were blocked in 2% non-fat milk in TBST-20 (Tris-buffered saline Tween-20), incubated with an antibody to Domain III of calpain 10 (1:1000) for 2 h, and then incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000) for 1 h. Immunoreactive proteins were visualized by enhanced chemiluminescence detection on an Alpha Innotech imaging system.
Calpain Activity
Calpain activity was measured spectrofluorometrically using the calpain substrate SLLVY-AMC as previously described [11; 22]. Mitochondria or mitochondrial matrix fractions were incubated with SLLVY-AMC (50 μM) in the absence and presence of the calpain inhibitor, calpeptin (10 μM), in a buffer (130 mM KCl, 9mM Tris-PO4, 4 mM Tris-HCl, and 1 mM EGTA, pH 7.4). Activity was measured under linear conditions as a function of SLLVY-AMC hydrolysis using excitation and emission wavelengths of 355 nm and 444 nm respectively.
Statistical analysis
Mitochondria or matrix isolated from one animal represents one experiment (n=1). Data are presented as means ± SE and were subjected to student’s t-test. A value of p<0.05 was considered a statistically different.
RESULTS AND DISCUSSION
NRK-52E cells were transfected with a shRNA construct targeted to calpain 10 or scramble control, and immunoblot analysis was performed on cell lysates 48 h later. Using a calpain 10 specific antibody, three postulated splice variants were identified; 75 kDa, 56 kDa, and 50 kDa (fig 1a). The molecular weight of these postulated splice variants are consistent with molecular weight predictions of the gene products of the splice variants calpain 10a (75 kDa, full-length), calpain 10c or calpain 10d (56 kDa), and calpain 10e (50 kDa). In cells transfected with calpain 10 targeted shRNA, there was a decrease in protein expression of the 75 kDa (81%), 56 kDa (71%), and 50 kDa (60%) (fig 1a). There was no change in the levels of these proteins in cells transfected with the scramble control. The differences in the relative “knockdown” of the calpain 10 splice variants could be due to different protein stability and turnover or the efficiency of the shRNA to bind to and interfere with the mRNA of the splice variants. Although different antibodies and constructs were used, similar knockdown of the 75 kDa and 56 kDa splice variants was seen in primary cultures of human skeletal muscle cells that were transfected with siRNA targeted to calpain 10 [14]. These results provide strong evidence that the three immunoreactive bands recognized by the calpain 10 specific antibody are indeed three splice variants of calpain 10.
Figure 1. shRNA knockdown of Calpain 10 in NRK-52E cells and mitochondrial calpain activity.
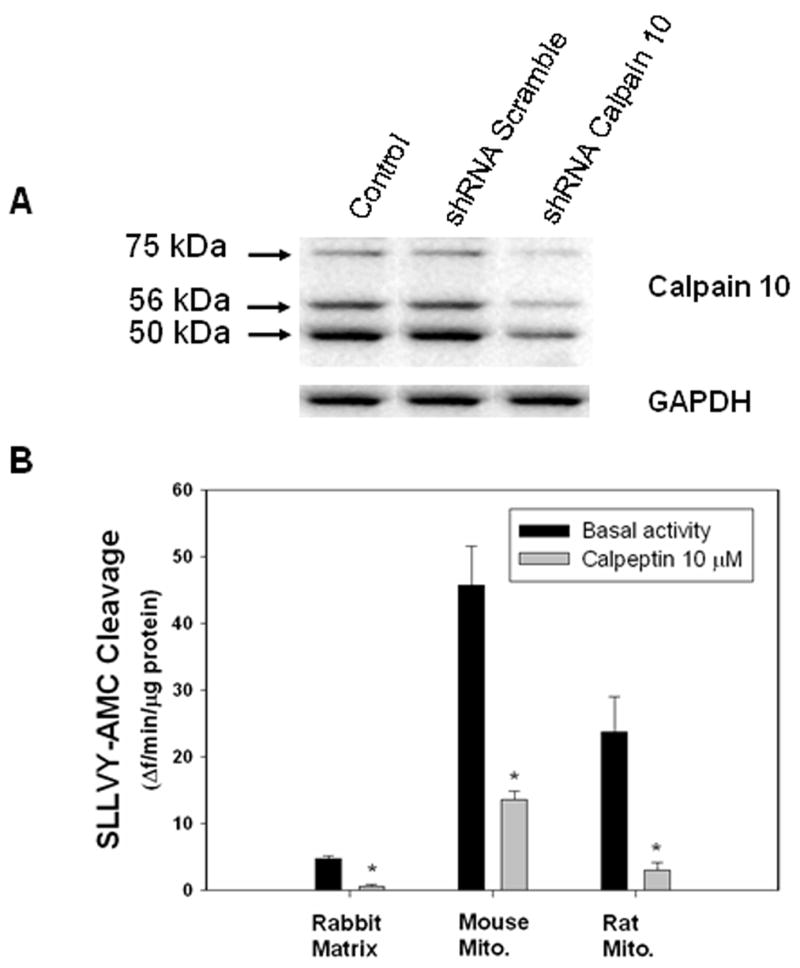
A) NRK52-E cells were transfected with shRNA constructs targeted to calpain 10 or scramble, and lysed after 48 h. Cell proteins were separated by SDS-PAGE and subjected to immunoblot analysis for CAPN10 and GAPDH. Representative of at least 3 experiments. B) Renal mitochondria from rabbit, mouse and rat were assayed for calpain activity using the fluorogenic calpain substrate SLLVY-AMC (50 μM) in the presence and absence of calpeptin. Data are expressed as means ± SE, n=3. Asterisks indicate means that are statistically significant from basal activity, p<0.05.
To examine the mitochondrial calpain activity in different species, the fluorogenic calpain substrate SLLVY-AMC was utilized. Because SLLVY-AMC is not specific for calpains [23; 24; 25; 26], we used the calpain inhibitor calpeptin to confirm calpain activity. Isolated mitochondria or matrix from rabbit, mouse, and rat were assayed for SLLVY-AMC cleavage over time in the presence or absence of 10 μM calpeptin (fig 1b). Calpeptin inhibited 86±5% (rabbit), 70±1% (mouse) and 85±7% (rat) of total SLLVY-AMC cleavage, indicating that substrate cleavage is primarily calpain mediated.
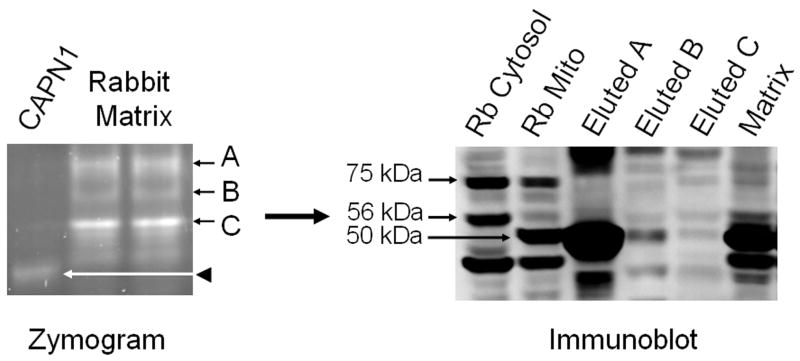
To further examine rabbit renal mitochondrial calpain activity, mitochondrial matrix proteins were separated under native conditions by SLLVY-AMC zymography. Following electrophoresis, gels were bathed in a Ca2+ buffer and imaged. Purified cytosolic calpain 1 was used as a control to visualize the migration pattern of this typical calpain. As previously reported matrix fractions yielded three major bands of activity that did not co-migrate with purified cytosolic calpain 1 (fig 2, A,B,C), indicating that the mitochondrial matrix calpain is not calpain 1 in rabbit renal cortical mitochondrial matrix [11]. Bands of activity were excised from the gel and proteins were eluted, denatured and subjected to immunoblot analysis with a calpain 10 specific antibody. Mitochondrial and cytosolic fractions from primary cultures of renal proximal tubular cells (RPTC) were used as positive controls for the identification of calpain 10 splice variants, revealing the presence of the 75 kDa, 56 kDa, and 50 kDa (fig 2). Excised band A from the zymogram yielded the 56 kDa and the 50 kDa splice variants of calpain 10. Excised bands B and C from the zymogram contained the 75 kDa, 56 kDa and the 50 kDa splice variants. These results reveal that rabbit renal cortical mitochondrial matrix calpain activity is the result of one or more of the three calpain 10 splice variants.
Figure 2. Rabbit renal cortical mitochondrial matrix contains multiple calpain 10 splice variants.
Rabbit renal cortical mitochondrial matrix was separated on native-PAGE zymography gels. In matrix, three bands of activity (A, B, and C) were excised from the zymogram, and proteins eluted, separated by SDS-PAGE and subjected to immunoblot analysis for calpain 10. Rabbit RPTC cytosolic and mitochondrial fractions were used as positive controls for calpain 10 splice variants, 75 kDa (full length), 56 kDa, and 50 kDa. Lanes A, B, and C (immunoblot) correspond to the bands of activity A, B, and C from the zymogram. Representative of at least 3 experiments.
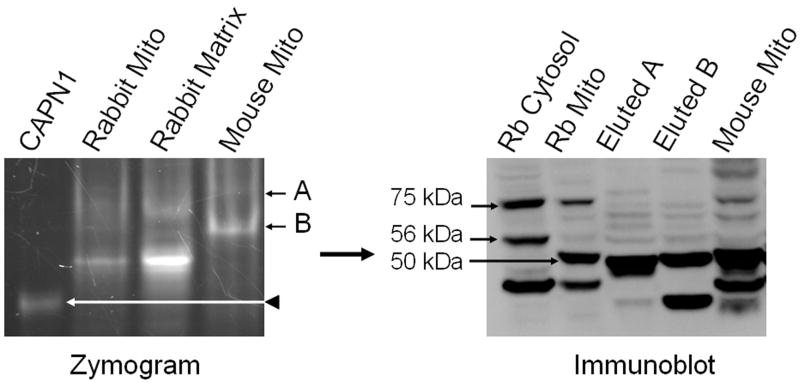
Next we investigated calpain activity in mouse kidney mitochondria using zymography as described above. Two major bands of activity that did not co-migrate with cytosolic calpain 1 were identified (fig 3.). Interestingly, the bands from mouse mitochondria did not co-migrate with the bands from rabbit matrix, with the exception that band B in mouse mitochondria migrates close to band B in the rabbit matrix. Bands of activity were excised and subjected to immunoblot analysis as described above. Excised bands A and B from the mouse zymogram yielded the 75 kDa (runs slightly lower than the control), 56 kDa, and the 50 kDa splice variants of calpain 10 (fig 3). These results reveal that mouse renal mitochondrial calpain activity is the result of one or more of the three calpain 10 splice variants.
Figure 3. Mouse renal mitochondria contain multiple calpain 10 splice variants.
Mouse renal mitochondria were separated on native-PAGE zymography gels. Two bands of activity (A and B) were excised from the zymogram, and proteins eluted, separated by SDS-PAGE and subjected to immunoblot analysis for CAPN10. Rabbit RPTC cytosolic and mitochondrial fractions were used as positive controls for calpain 10 splice variants, 75 kDa (full length), 56 kDa, and 50 kDa. Lanes A and B (immunoblot) correspond to the bands of activity (A and B) from the zymogram. Representative of at least 3 experiments.
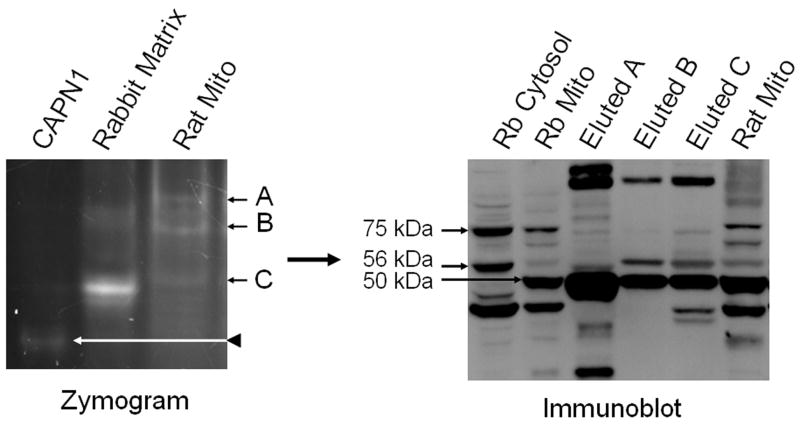
Rat kidney mitochondrial proteins were subjected to zymography as described above and calpain activity examined. Three major bands of activity, that did not co-migrate with purified cytosolic calpain 1, were identified (fig 4). These bands of activity did not co-migrate with any of the bands found in the rabbit matrix. Bands of activity from the rat zymogram were excised and subjected to immunoblot analysis. Excised band A from the rat zymogram yielded the 75 kDa (runs slightly lower than control) and the 50 kDa splice variants of calpain 10 (fig 4). Excised band B from the zymogram yielded the 56 kDa and the 50 kDa calpain 10 splice variants. Excised band C from the zymogram yielded the 75 kDa (runs slightly lower than control), the 56 kDa, and the 50 kDa splice variants. These results reveal that rat renal mitochondria calpain activity is the result of one or more of the three calpain 10 splice variants.
Figure 4. Rat renal mitochondria contain multiple calpain 10 splice variants.
Rat renal mitochondria were separated on native-PAGE zymography gels. Three bands of activity (A, B, and C) were excised from the zymogram, and proteins eluted, separated by SDS-PAGE and subjected to immunoblot analysis for CAPN10 Rabbit RPTC cytosolic and mitochondrial fractions were used as positive controls for calpain 10 splice variants, 75 kDa (full length), 56 kDa, and 50 kDa. Lanes A, B, and C (immunoblot) correspond to the bands of activity (A, B, and C) from the zymogram. Representative of at least 3 experiments.
Taken together, these results reveal that multiple splice variants of calpain 10 are expressed and proteolytically active in renal mitochondria across three species. Interestingly, mitochondrial calpain 10 in each species seems to display a unique pattern of migration in native gels, while the migration patterns of denatured mitochondrial calpain 10 are similar. A number of reasons may contribute to this observation. For example, differences in amino acid composition, post-translational modifications, autolytic activation, or protein binding partners may alter calpain 10 migration in native gels. Further studies are needed to explore these possibilities.
Ideally, one would expect to see one band of activity from the zymogram be composed of one splice variant of calpain 10; however this was not the case. Multiple splice variants are present in each band of activity from the zymogram. Protein separation in native gel electrophoresis is dependent on the charge and shape of the protein, as well as its size [27], and therefore a 50 kDa and a 75 kDa calpain 10 splice variant may reasonably co-migrate. As noted above, protein binding partners also could explain this finding. In this case, each calpain 10 splice variant could independently bind to a common binding partner that imparts similar shape and charge on each splice variant, resulting in co-migration. Post-translational modifications also could influence charge characteristics such that separate splice variants would have similar native electrophoretic mobility.
We have identified three splice variants of calpain 10 using a calpain 10 specific shRNA knockdown approach. Kidney mitochondria from three species contained mitochondrial calpain activity that was inhibited by the calpain inhibitor, calpeptin. All sources of mitochondrial calpain activity are immunoreactive with calpain 10 antibodies, revealing that kidney mitochondria contain at least 3 splice variants of calpain 10 (75 kDa, 56 kDa, and 50 kDa). Additional studies are needed to elucidate the function and regulation of the calpain 10 splice variants.
Acknowledgments
This study was supported by National Institute of Health Sciences Grant ES012239 and National Institutes of Health Training Grant T32 HL007260.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 2.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–63. doi: 10.1111/j.1349-7006.2000.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Horikawa Y, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–75. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee PK, Brown PA, Cuzzocrea S, Zacharowski K, Stewart KN, Mota-Filipe H, McDonald MC, Thiemermann C. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59:2073–83. doi: 10.1046/j.1523-1755.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Rainey JJ, Harriman JF, Schnellmann RG. Calpains mediate acute renal cell death: role of autolysis and translocation. Am J Physiol Renal Physiol. 2001;281:F728–38. doi: 10.1152/ajprenal.2001.281.4.F728. [DOI] [PubMed] [Google Scholar]
- 7.Turner MD, Cassell PG, Hitman GA. Calpain-10: from genome search to function. Diabetes Metab Res Rev. 2005;21:505–14. doi: 10.1002/dmrr.578. [DOI] [PubMed] [Google Scholar]
- 8.Otani K, Han DH, Ford EL, Garcia-Roves PM, Ye H, Horikawa Y, Bell GI, Holloszy JO, Polonsky KS. Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J Biol Chem. 2004;279:20915–20. doi: 10.1074/jbc.M400213200. [DOI] [PubMed] [Google Scholar]
- 9.Paul DS, Harmon AW, Winston CP, Patel YM. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem J. 2003;376:625–32. doi: 10.1042/BJ20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou YP, Sreenan S, Pan CY, Currie KP, Bindokas VP, Horikawa Y, Lee JP, Ostrega D, Ahmed N, Baldwin AC, Cox NJ, Fox AP, Miller RJ, Bell GI, Polonsky KS. A 48-hour exposure of pancreatic islets to calpain inhibitors impairs mitochondrial fuel metabolism and the exocytosis of insulin. Metabolism. 2003;52:528–34. doi: 10.1053/meta.2003.50091. [DOI] [PubMed] [Google Scholar]
- 11.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–71. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Villasenor A, Hiriart M, Cebrian ME, Zacarias-Castillo R, Ostrosky-Wegman P. The activity of calpains in lymphocytes is glucose-dependent and is decreased in diabetic patients. Blood Cells Mol Dis. 2007 doi: 10.1016/j.bcmd.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Turner MD, Fulcher FK, Jones CV, Smith BT, Aganna E, Partridge CJ, Hitman GA, Clark A, Patel YM. Calpain facilitates actin reorganization during glucose-stimulated insulin secretion. Biochem Biophys Res Commun. 2007;352:650–5. doi: 10.1016/j.bbrc.2006.11.077. [DOI] [PubMed] [Google Scholar]
- 14.Brown AE, Yeaman SJ, Walker M. Targeted suppression of calpain-10 expression impairs insulin-stimulated glucose uptake in cultured primary human skeletal muscle cells. Mol Genet Metab. 2007;91:318–24. doi: 10.1016/j.ymgme.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Covington MD, Bayless KJ, Burghardt RC, Davis GE, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells: evidence for a role of metalloproteinases. Am J Physiol Renal Physiol. 2005;289:F280–8. doi: 10.1152/ajprenal.00351.2004. [DOI] [PubMed] [Google Scholar]
- 16.Schnellmann RG, Cross TJ, Lock EA. Pentachlorobutadienyl-L-cysteine uncouples oxidative phosphorylation by dissipating the proton gradient. Toxicol Appl Pharmacol. 1989;100:498–505. doi: 10.1016/0041-008x(89)90297-4. [DOI] [PubMed] [Google Scholar]
- 17.Williams SD, Gottlieb RA. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak G, Schnellmann RG. L-ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol. 1996;271:C2072–80. doi: 10.1152/ajpcell.1996.271.6.C2072. [DOI] [PubMed] [Google Scholar]
- 19.Nowak G, Schnellmann RG. Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol. 1995;268:C1053–61. doi: 10.1152/ajpcell.1995.268.4.C1053. [DOI] [PubMed] [Google Scholar]
- 20.Nowak G, Clifton GL, Godwin ML, Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am J Physiol Renal Physiol. 2006;291:F840–55. doi: 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur JS, Mykles DL. Calpain zymography with casein or fluorescein isothiocyanate casein. Methods Mol Biol. 2000;144:109–16. doi: 10.1385/1-59259-050-0:109. [DOI] [PubMed] [Google Scholar]
- 22.Atsma DE, Bastiaanse EM, Jerzewski A, Van der Valk LJ, Van der Laarse A. Role of calcium-activated neutral protease (calpain) in cell death in cultured neonatal rat cardiomyocytes during metabolic inhibition. Circ Res. 1995;76:1071–8. doi: 10.1161/01.res.76.6.1071. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki T, Kikuchi T, Yumoto N, Yoshimura N, Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984;259:12489–94. [PubMed] [Google Scholar]
- 24.Muramatu M, Itoh T, Takei M, Endo K. Tryptase in rat mast cells: properties and inhibition by various inhibitors in comparison with chymase. Biol Chem Hoppe Seyler. 1988;369:617–25. doi: 10.1515/bchm3.1988.369.2.617. [DOI] [PubMed] [Google Scholar]
- 25.Kunugi S, Fukuda M, Hayashi R. Action of serine carboxypeptidases on endopeptidase substrates, peptide-4-methyl-coumaryl-7-amides. Eur J Biochem. 1985;153:37–40. doi: 10.1111/j.1432-1033.1985.tb09263.x. [DOI] [PubMed] [Google Scholar]
- 26.Sawada H, Yokosawa H, Hoshi M, Ishii S. Ascidian sperm chymotrypsin-like enzyme; participation in fertilization. Experientia. 1983;39:377–8. doi: 10.1007/BF01963132. [DOI] [PubMed] [Google Scholar]
- 27.Hames BD. Gel electrophoresis of proteins: a practical approach. Oxford University Press; Oxford ; New York: 1998. [Google Scholar]