Abstract
Background and aim: Resection of colorectal liver metastases has become a standard of care, although the value of this procedure in non-colorectal non-neuroendocrine (NCRNNE) metastases remains controversial and is still a matter of debate. The aim of the study was to determine the utility of liver resection in the long-term outcome of patients with NCRNNE metastases. Material and methods: The records of 106 patients who underwent liver resection for NCRNNE metastases in the period 1989 to 2006 at 5 HPB Centers in Argentina were analyzed. Patient demographics, tumor characteristics, type of resection, long-term outcome and prognostic factors were analyzed. Depending on primary tumor sites, a comparative analysis of survival was performed. Results: Mean age was 54 (17–76). Hepatic metastases were solitary in 62.3% and unilateral in 85.6%. Primary tumor sites: Urogenital (37.7%), sarcomas (21.7%), breast (17.9%), gastrointestinal (6.6%), melanoma (5.7%), and others (10.4%). Fifty-one major hepatectomies and 55 minor resections were performed. Twenty patients underwent synchronous resections. An R0 resection could be achieved in 89.6%. Perioperative mortality was 1.8%. Overall, 1-year, 3-year, and 5-year survival rates were 67%, 34%, and 19%, respectively. Survival was significantly longer for metastases of urogenital (p=0.0001) and breast (p=0.003) origin. Curative resections (p=0.04) and metachronous disease (p=0.0001) were predictors of better survival. Conclusions: Liver resection is an effective treatment for NCRNNE liver metastases; it gives satisfactory long-term survival especially in metachronous disease, in patients with metastases from urogenital and breast tumors and when R0 procedures can be performed.
Introduction
Resection of colorectal liver metastases has become a standard of care, with 5-year survival rates from 42% to 71% reported in solitary metastases 1. A benefit from resection has also been demonstrated in patients with liver metastases from neuroendocrine tumors, with a reduced incidence of disease-related symptoms and an extended median patient survival from 61% to 76% at 5 years 2. However, the efficacy of resection in patients with non-colorectal non-neuroendocrine (NCRNNE) liver metastases remains unclear. Main factors are: heterogeneity of the primary tumor types and its biological behavior, the limited number of patients reported in each study, the frequent inclusion of patients with neuroendocrine or unknown primary tumors, and the absence of prospective studies.
Our study was designed to determine the utility of liver resection in the long-term outcome of patients with NCRNNE liver metastases treated at five hepatopancreatobiliary (HPB) centers in Argentina.
Material and methods
The records of 106 patients who underwent liver resection for NCRNNE liver metastases from 1989 to 2006 at 5 HPB centers in Argentina were analyzed. Patients with direct invasion of the liver by the primary tumor or by peritoneal implants, and patients with liver metastases from unknown primary tumors were excluded. Liver metastases diagnosed along with the primary tumor were defined as synchronous, and the time frame to define a metachronous metastase was at least 2 months after completion of treatment of the primary tumor. Major hepatic resection was defined as resection of three or more segments, while a curative resection (R0) was defined as complete removal of any clinically evident tumor lesion(s) with negative pathological margins. Any infiltration of the resection margin with tumor cells in the histological specimen was defined as R1 resection. Operative mortality included any death attributed to liver resection and all deaths within 30 days of partial hepatectomy. Patient demographics, tumor characteristics, type of resection, long-term outcome, and prognostic factors were analyzed. For statistical analysis, and depending on the primary tumor origin, patients were grouped within the following categories: genitourinary, gastrointestinal, breast, sarcomas, melanoma and others.
The following factors were assessed specifically as prognostic factors: age, disease-free interval from resection of the primary tumor to discovery of liver metastases, primary tumor origin, synchronous vs metachronous presentation, number of liver metastases, intrahepatic distribution (unilobar vs bilobar), type of liver resection (minor vs major), and resection margin (R0 vs R1).
An analysis was performed in accordance with the risk model for patients with NCRNNE liver metastases developed by Adam et al. 3.
Statistical analysis
Qualitative variables expressed as frequencies and percentages were compared using the chi-square test. For quantitative variables expressed as mean values and standard deviation, comparison was done by applying Student's t-test. The Kaplan-Meier method was used to calculate actuarial survival rates, and intergroup comparisons were performed by means of the log-rank test. Variables found to be significant by univariate analysis were assessed by multivariate analysis using a Cox regression. Statistical significance was considered to exist when p<0.05. Statistical analyses were performed using the SPSS software v. 12.0 (SPSS Inc., Chicago, Ill., USA).
Results
A total of 106 patients were identified as undergoing hepatic resection for NCRNNE metastases. The median age was 54 years (range 17 to 76 years), with a male:female ratio of 1:1.3 (45:61). Twenty-three percent of the patients presented with synchronous lesions, whereas 77% had metachronous lesions. The median disease-free interval after primary treatment in all patients was 29 months, and in the 83 patients presenting with metachronous disease this was 40 months. The primary tumor origins are described in Table I. Most common sites in the genitourinary group were renal and ovarian. Stomach and pancreas were most important in the gastrointestinal group and other relevant primary sites were breast and sarcomas. The melanoma group included 4 patients with choroid melanoma and 2 with cutaneous melanoma. Depending on primary tumor origin, the median disease-free interval after primary treatment was 14 months in gastrointestinal tumors, 29 months in sarcoma, 37 months in melanoma, 38 months in genitourinary, 46 months in others, and 75 months in breast tumors. After treatment of the primary tumor, 47 patients (44%) received chemotherapy and/or radiotherapy (84% in breast tumors and 35% in genitourinary tumors).
Table I. Primary tumor origin in 106 patients with NCRNNE metastases.
| Genitourinary | 40 (37.7%) | |
| Renal | 21 | |
| Ovarian | 14 | |
| Testicular | 3 | |
| Uterine | 1 | |
| Bladder | 1 | |
| Sarcomas | 23 (21.7%) | |
| Breast | 19 (17.9%) | |
| Gastrointestinal | 7 (6.6%) | |
| Stomach | 3 | |
| Pancreas | 3 | |
| Duodenum | 1 | |
| Melanoma | 6 (5.7%) | |
| Others | 11 (10.4%) | |
| Pulmonary | 3 | |
| Neck | 2 | |
| Adrenal | 2 | |
| Miscellaneous | 4 |
Diagnostic imaging was not standardized over the study period because of the duration of the study and the evolution of imaging modalities. However, ultrasonography and computed tomography were used in 95% of the patients. Other imaging modalities were magnetic resonance imaging in 14% and positron emission tomography (PET) scanning in 5.7%. A percutaneous biopsy was performed in 8 patients (7.5%).
At the time of hepatic resection, 66 (62.3%) patients had a single lesion and 91 (85.8%) unilateral liver involvement (right or left hemiliver). Major hepatic resection was required in 51 (48.1%). A synchronous resection of the liver and the primary tumor was performed in 20 (18.8%) patients (8 sarcomas, 6 genitourinary, 3 gastrointestinal, 2 melanomas and 1 suprarenal). Ninety-five (89.6%) patients underwent complete (R0) resection of the tumor and 11 (10.3%) had microscopic residual disease (R1). A second liver resection was performed in 5 patients (4.7%) and the other 6 were treated by chemotherapy because of unresectable disease. Perioperative mortality occurred in two (1.8%) patients: sepsis (1) and liver failure (1) were the main causes.
Outcome
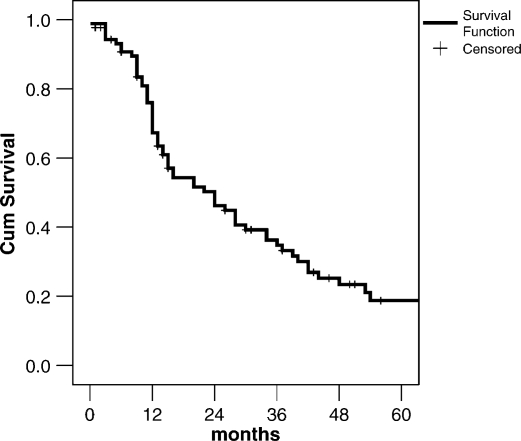
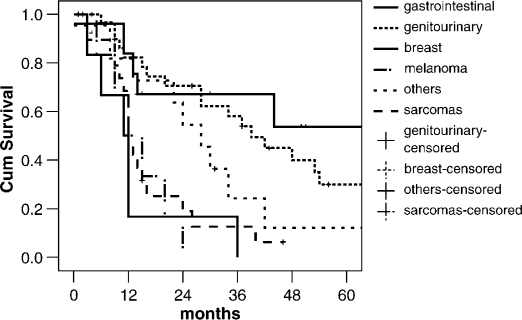
The median follow-up time was 28 months and 5 patients were lost to follow-up. Overall, 1, 3, and 5-year survival rates were 67%, 34%, and 19%, respectively, with a median overall survival of 27 months (Figure 1). Depending on primary tumor origin, 5-year survival was 53% for breast origin, 30% for genitourinary, and 12% for other tumors, i.e. principally related to neck sites (larynx, submaxilar). In the sarcoma group, only 1 patient is alive at 46 months and there were no 5-year survivors from the gastrointestinal and melanoma groups (Figure 2).
Figure 1. .
Overall survival of patients who underwent liver resection for NCRNNE metastases (n=106).
Figure 2. .
Comparison of survival depending on primary tumor origin.
The univariate and multivariate analyses of prognostic factors demonstrated that the origin of the primary tumor, the metachronous metastases presentation, and the curative resections (R0) were important predictors of long-term survival (Table II). Age, disease-free interval, number of metastases, unilateral versus bilateral disease, and type of liver resection did not affect prognosis.
Table II. Analysis of prognostic factors for survival after resection for NCRNNE liver metastases.
| Prognostic factor | Univariate (p) | Multivariate (p) |
|---|---|---|
| Age <30 year, >30 year, >60 year | NS | NS |
| Presentation (metachronous vs synchronous) | 0.0001 | 0.001 |
| Disease-free interval <24 m, >24 m | NS | NS |
| Origin of primary tumor | ||
| Breast | 0.003 | 0.03 |
| Genitourinary | 0.0001 | 0.001 |
| No. of metastases (solitary vs multiple) | NS | NS |
| Distribution (unilateral vs bilateral) | NS | NS |
| Type of liver resection (minor vs major) | NS | NS |
| Curative resection (R0) | 0.04 | 0.01 |
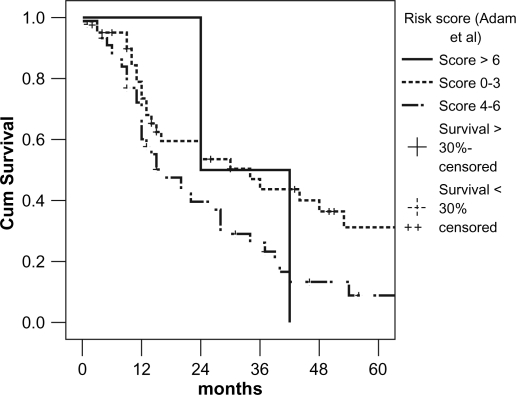
In accordance with the risk model for patients with NCRNNE liver metastases developed by Adam et al., patients were grouped into three categories (low-risk 0–3 points, mid-risk 4–6 points, and high risk >6 points) 3. Our study showed no 5-year survival in the high-risk patients, only 8% in the mid-risk, and 31% in the low-risk group (Figure 3).
Figure 3. .
Comparison of survival according to risk score described by Adam et al. 3.
Discussion
The benefits of surgical resection have been well documented in patients with metastases from colorectal and neuroendocrine tumors 2. However, its role in NCRNNE metastases remains controversial. Previous studies have reported single-center experiences with a wide variety of primary tumor types distributed in a small number of patients 4,5,6,7. In order to overcome the limitations of single-center reports we designed a study to analyze data from five referral HPB centers from Argentina.
Average age in the fifth decade and predominance of the female population in our study were common features to those in previous reports (Table III). Only 3 single centers and 1 multicenter study had already reported data from more than 100 patients with resection of NCRNNE metastases. In general, the time intervals of the different studies ranged from 10 to 23 years 5,8. In respect of exclusion criteria, although 7 out of 11 previous studies included patients with metastases from unknown primary tumors, we excluded these studies in order to avoid bias in the outcome analysis of the miscellaneous groups 2,3,4,8,9,10,11,12. Furthermore, as already demonstrated, metastases from neuroendocrine tumors should be analyzed separately considering this is a unique group with better prognosis 3.
Table III. Major studies on results of hepatectomy for NCRNNE liver metastases.
| First author | Year | n | Median age | UPT | NE | Major resection | Perioperative mortality% | R0 | Overall 5-year survival% |
|---|---|---|---|---|---|---|---|---|---|
| Elias 10 | 1998 | 147 | 56 | 3 | 18% | – | 2 | 75% | 36 |
| Hemming 4 | 2000 | 37 | 56 | 3 | – | 62% | 0 | 89% | 45 |
| Benevento 5 | 2000 | 18 | 54 | – | 22% | 17% | 0 | – | 21 |
| Laurent 7 | 2001 | 39 | 55 | – | – | 51% | 0 | – | 35 |
| Yedibela 8 | 2005 | 162 | 60 | 10 | 9% | 38% | 1 | 72% | 26 |
| Ercolani 14 | 2005 | 83 | 54 | – | – | 41% | 0 | – | 34 |
| Cordera 11 | 2005 | 64 | 56 | 3 | – | 59% | 1.5 | 88% | 30 |
| Weitz 12 | 2005 | 141 | 55 | 5 | – | 48% | 0 | 96% | 57 (3-year) |
| Adam 3 | 2006 | 1452 | 53 | 29 | – | 55% | 2.3 | 83% | 36 |
| Earle 20 | 2006 | 95 | 58 | – | 19% | 41% | 2.1 | 88% | 35 |
| Reddy 2 | 2006 | 82 | 52 | 2 | 9% | 52% | 4 | 79% | 37 |
| Lendoire | 2007 | 106 | 54 | – | – | 48% | 1.8 | 90% | 19 |
UPT = number of patients with unknown primary tumors; NE = number of patients with neuroendocrine tumors.
In the past decade, liver resection has become a fairly common therapeutic option for patients with NCRNNE metastases because mortality with this procedure has declined as a result of better preoperative imaging, patient selection, anesthetic, and critical care management and understanding of liver anatomy. Analyzing the present series along with 11 other series reported in the literature indicates that major resections were generally performed from 17% to 67% and perioperative mortality ranged from 0 to 4% 2,4,5.
The decision to proceed with liver resection in a patient with NCRNNE metastases must come after thorough evaluation. PET will have a progressively critical role in the selection of patients for surgery improving the preoperative staging of the disease 2. Staging laparoscopy could also be a useful tool in patient selection. In a study by D'Angelica et al., 20% of the patients with potentially resectable NCRNNE metastases, and who underwent a staging laparoscopy, were spared a non-therapeutic laparotomy, and two-thirds of them with unresectable disease were identified in this procedure 13.
Patients with NCRNNE metastases conform with a heterogeneous group with different anatomic factors and distinct primary tumor biology. As an example, liver metastasis in non-gastrointestinal cancer, by definition, indicates systemic tumor spread. Selection of patients with favorable tumor biology is the key point in defining which patients will benefit most from liver resection. Several studies have demonstrated that long-term survival can be achieved in a subset of patients with NCRNNE metastases (Table III). It is important to identify the prognostic factors predictive of more favorable outcomes after liver resection for attempted cure. In our data, primary tumor origin, metachronous metastases, and curative resections were independent prognostic factors of long-term survival. Primary tumor type is the most common prognostic factor described, and favorable survivals are generally reported for genitourinary, breast, and soft tissue sites 3,7,12,14. In the present study, genitourinary origin demonstrated a 30% 5-year survival compatible with previous reports 7,12,14,15. Weitz et al. have recently shown a better free relapse survival for reproductive versus non-reproductive tract tumors 12. Breast tumors, too, have been considered a favorable group for resection of liver metastases, with 5-year survival rates ranging from 27% to 51% 16. In our series, the highest survival for breast tumors (53% at 5 years) could be explained by the long median disease-free interval (78 months) presented in this group of patients compared to other primary tumor origins. However, no consensus has been demonstrated in this topic in previous studies 17,18. As far as sarcoma and other soft tissue tumors are concerned, better outcomes have been presented by other authors 3,9,10. This could be explained in our data by the high number of patients who underwent synchronous resection of the primary tumor and the metastases in the sarcoma group 8/23 (34.7%) – a parameter that was associated with poorer long-term survival. The worst outcome was generally reported for metastases from gastrointestinal tumors 6,14,15,19,20.
The disease-free interval between treatment of the primary tumor and development of liver metastases is viewed as a marker for tumor biology. The notion of a longer disease-free interval possibly being associated with less aggressive tumor biology is supported by studies demonstrating longer survival in patients with disease-free intervals more than 12 or 24 months 2,7,9,11,12,20. Metastases with short disease-free intervals could be associated with advance stages of the disease and worse prognosis. Our study did not show significant differences and this is probably related to the discrepancy between the disease-free intervals in the groups analyzed (14 months in gastrointestinal tumors to 75 months in breast tumors). As shown previously by others, only patients with synchronous presentation of the primary tumor and the metastases demonstrated a significant reduction in overall survival 11,20.
The third relevant prognostic factor in our study was the likelihood of performing a microscopically complete tumor resection, which was achieved in 90% of the patients. Other studies have reported R0 resections in the range 72% to 96% 8,9 (Table III).
In a recently published multicenter study, Adam et al. analyzed 1452 patients with hepatic resection for NCRNNE liver metastases and developed a prognostic model 3. The prognostic factors considered in the risk model were based on patient, tumor, and hepatectomy characteristics. Application of the risk model in our study showed 5-year survival within the range demonstrated by Adam et al. 3. Only the mid-risk group presented a lower survival.
In conclusion, this report indicates that liver resection is an effective treatment for NCRNNE liver metastasis. Long-term survival after liver resection is satisfactory, especially in patients with metachronous disease, in patients with metastases from genitourinary and breast tumors, and when a curative resection can be performed. Analysis of our data using the risk model developed by Adam et al. 3 demonstrated a clear benefit of resection in the low-risk group of patients. Improvement in preoperative staging and progressive application of development of new multimodality treatments will be the key to improved survival in this disease.
References
- 1.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–6. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 2.Reddy SK, Barbas AS, Marroquin CE, Morse MA, Kuo PC, Clary BM. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–82. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemming AW, Sielaff TD, Gallinger S, Cattral MS, Taylor BR, Greig PD, et al. Hepatic resection of noncolorectal nonneuroendocrine metastases. Liver Transpl. 2000;6:97–101. doi: 10.1002/lt.500060114. [DOI] [PubMed] [Google Scholar]
- 5.Benevento A, Boni L, Frediani L, Ferrari A, Dionigi R. Result of liver resection as treatment for metastases from noncolorectal cancer. J Surg Oncol. 2000;74:24–9. doi: 10.1002/1096-9098(200005)74:1<24::aid-jso6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Takada Y, Otsuka M, Seino K, Taniguchi H, Koike N, Kawamoto T, et al. Hepatic resection for metastatic tumors from noncolorectal carcinoma. Hepatogastroenterology. 2001;48:83–6. [PubMed] [Google Scholar]
- 7.Laurent C, Rullier E, Feyler A, Masson B, Saric J. Resection of noncolorectal and nonneuroendocrine liver metastases: late metastases are the only chance of cure. World J Surg. 2001;25:1532–6. doi: 10.1007/s00268-001-0164-7. [DOI] [PubMed] [Google Scholar]
- 8.Yedibela S, Gohl J, Graz V, Pfaffenberger MK, Merkel S, Hohenberger W, et al. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: a single-institutional review. Ann Surg Oncol. 2005;12:778–85. doi: 10.1245/ASO.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–32. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 10.Elias D, Cavalcanti de AA, Eggenspieler P, Plaud B, Ducreux M, Spielmann M, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–93. doi: 10.1016/s1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 11.Cordera F, Rea DJ, Rodriguez-Davalos M, Hoskin TL, Nagorney DM, Que FG. Hepatic resection for noncolorectal, nonneuroendocrine metastases. J Gastrointest Surg. 2005;9:1361–70. doi: 10.1016/j.gassur.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D'Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Angelica M, Jarnagin W, Dematteo R, Conlon K, Blumgart LH, Fong Y. Staging laparoscopy for potentially resectable noncolorectal, nonneuroendocrine liver metastases. Ann Surg Oncol. 2002;9:204–9. doi: 10.1007/BF02557375. [DOI] [PubMed] [Google Scholar]
- 14.Ercolani G, Grazi GL, Ravaioli M, Ramacciato G, Cescon M, Varotti G, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–66. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Detry O, Warzee F, Polus M, De RA, Meurisse M, Honore P. Liver resection for noncolorectal, nonneuroendocrine metastases. Acta Chir Belg. 2003;103:458–62. doi: 10.1080/00015458.2003.11679467. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe MS, Mullin EJ, Maddern GJ. Hepatectomy for metastatic noncolorectal gastrointestinal, breast and testicular tumours. ANZ J Surg. 2006;76:246–50. doi: 10.1111/j.1445-2197.2006.03689.x. [DOI] [PubMed] [Google Scholar]
- 17.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ. Hepatic resection for breast cancer metastases: results and prognosis (65 cases)] Ann Chir. 2001;126:413–20. doi: 10.1016/s0003-3944(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Maisonnette F, Druet-Cabanac M, Ouellet JF, Guinebretiere JM, Spielmann M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158–64. doi: 10.1016/s0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- 19.Zacherl J, Zacherl M, Scheuba C, Steininger R, Wenzl E, Muhlbacher F, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg. 2002;6:682–9. doi: 10.1016/s1091-255x(01)00075-0. [DOI] [PubMed] [Google Scholar]
- 20.Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS, Franceschi D, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006;203:436–46. doi: 10.1016/j.jamcollsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]