Abstract
Background and aims. Recognized prognostic factors for resected pancreatic ductal adenocarcinoma (PDAC) include tumour size, differentiation, resection margin involvement and lymph node metastases. A further prognostic factor of less certain significance is lymphocyte count. The aim of this study was to investigate whether preoperative lymphocyte count is a prognostic indicator in patients with PDAC. Material and methods. Patients who had undergone a potentially curative pancreaticoduodenectomy (PD) for PDAC between 1998 and 2005 were analysed. Standard prognostic factors, preoperative lymphocyte count, preoperative neutrophil count and survival data were collected. Results. Of the 44 patients studied, univariate analysis identified predictors of a poor survival as lymph node status (node positive (+ve) 10.3 [5.4–20.9] months versus node negative (−ve) 14.2 [10.9–31.4] months; p=0.038), posterior resection margin invasion (margin +ve 7.0 [5.1–15.0] months versus margin −ve 13.1 [10.0–28.3] months; p=0.025) and lymphocyte count below the reference range (<1.5×109/litre 8.8 [7.0–13.1] months versus ≥1.5×109/litre 14.3 [7.0–28.3] months; p=0.029). Low preoperative lymphocyte count (p=0.027) and posterior margin invasion (p=0.023) retained significance on multivariate analysis. Preoperative neutrophil to lymphocyte ratio was not a significant prognostic factor. Conclusion. Preoperative lymphocyte count is a significant prognostic factor in patients with PDAC.
Keywords: Lymphocyte count, pancreatic cancer, prognostic factors
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a disease with a poor prognosis and a median survival of 12–18 months when resected with curative intent 1,2,3,4,5,6,7,8,9,10,11. However, there are few actual 5-year survivors, with even fewer patients truly cured of the disease 10. In a study of over 100,000 patients diagnosed with pancreatic cancer, only 9% were eligible for potentially curative resection 12. The majority of pancreatic cancers are in the head of the gland, i.e. necessitating pancreaticoduodenectomy (PD). This is a major operative intervention normally associated with a high mortality and morbidity, although large series with no in-hospital or 30-day mortality have been reported from specialist centers 13.
Well-established prognostic factors include tumour size, differentiation, resection margin involvement and lymph node metastases 1,3,8,9,10,11,14,15. A further prognostic factor of less certain significance is lymphocyte count, which has previously been shown to be associated with shorter survival 16,17. Lymphocytopenia has been reported in various cancers, but is particularly marked in patients with PDAC 18,19,20. It has been suggested that a low lymphocyte count indicates a state of immunosuppression, which is thought to be present at a systemic 21 and local level in patients with PDAC 21,22,23,24,25. In these studies, quantitative and functional deficiencies of lymphocytes were identified and it is therefore hypothesized that this may contribute to the poor prognosis of PDAC with a reduced host response against tumour cells.
The aim of this study was to investigate whether preoperative lymphocyte count is a prognostic indicator in patients with PDAC.
Methods
Patients who had undergone a potentially curative PD for pancreatic cancer between 1998 and 2005 were identified. Only those with PDAC confirmed on pathological examination were included in the analysis. Data were collected from a prospectively collected database and supplemented by retrospective case note review and cross-referenced against the laboratory computerized results system.
Standard histopathological prognostic factors including lymph node involvement, resection margin involvement, tumour size and differentiation were collected to ensure lymphocyte counts were independent of these. Demographic data including age at operation, sex, and survival (date of operation to date of death) were collected. In addition, lymphocyte and neutrophil counts on routine blood tests taken in the week prior to surgery were recorded. The median lymphocyte and neutrophil counts were calculated. Patients were grouped according to whether the lymphocyte count was above or below the lower limit of the normal reference range (1.5×109/litre). Furthermore, the preoperative neutrophil to lymphocyte count ratio was calculated. Patients were grouped according to whether their neutrophil to lymphocyte ratio (NLR) was less than or greater than 5.
Statview version 5.0.1 software was used for statistical analysis. Continuous data are presented as median (interquartile range). Actual survival was assessed by the Kaplan-Meier method and comparisons were made using the log rank test. Multivariate analysis was performed to determine the significance of prognostic variables using the Cox proportional hazards method. A p-value of <0.05 was considered significant.
Results
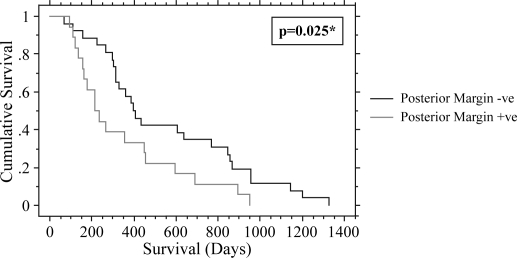
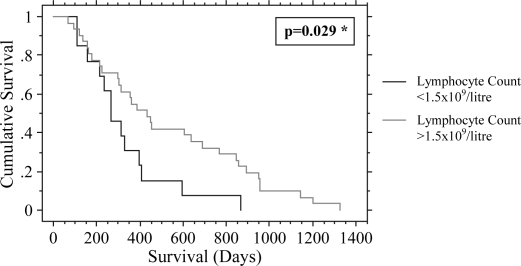
Forty-four patients with a median age of 65 (59–71) years were analysed. The median survival was 11.7 (6.4–17.0) months. Potential prognostic factors are given in Table I. On univariate analysis, lymph node status (node positive (+ve) 10.3 [5.4–20.9] months versus node negative (−ve) 14.2 [10.9–31.4] months; p=0.038), posterior resection margin involvement (margin +ve 7.0 [5.1–15.0] months versus margin −ve 13.1 [10.0–28.3] months; p=0.025) and lymphocyte count (<1.5×109/litre 8.8 [5.3–13.3] months versus ≥1.5×109/litre 15.0 [10.0–28.3] months; p=0.0087) were shown to be predictors of a poor survival. Low lymphocyte count (p=0.027) and posterior margin invasion (p=0.023) remained significant on multivariate analysis (Figures 1 and 2). Tumour size, resection margin involvement (any margin), differentiation and preoperative NLR were found not to be significant prognostic factors.
Table I. Prognostic factors in patients with PDAC.
| Variable | n | Median survival (IQR) (months) | p-value univariate analysis | p-value multivariate analysis | |
|---|---|---|---|---|---|
| Lymph nodes involved | +ve | 34 | 10.3 (5.4–20.9) | p=0.038 | p=0.20 |
| −ve | 10 | 14.2 (10.9–31.4) | |||
| Posterior resection margin | +ve | 16 | 7.0 (5.1–15.0) | p=0.025 | p=0.023 |
| −ve | 28 | 13.1 (10.0–28.3) | |||
| Resection margin | +ve | 21 | 8.8 (5.4–15.0) | p=0.10 | – |
| −ve | 23 | 14.3 (9.9–28.3) | |||
| Tumour size ≤2 cm | ≤2 cm | 6 | 20.9 (19.9–31.4) | p=0.18 | – |
| >2 cm | 38 | 10.3 (7.0–19.6) | |||
| Poorly differentiated | Yes | 20 | 8.7 (5.1–12.7) | p=0.44 | – |
| No | 24 | 14.8 (8.8–27.9) | |||
| Neutrophil to lymphocyte ratio ≥5 | Yes | 4 | 8.9 (5.3–13.3) | p=0.16 | – |
| No | 40 | 10.5 (7.1–21.2) | |||
| Lymphocyte count <1.5×109/litre | Yes | 20 | 8.8 (5.3–13.3) | p=0.0087 | p=0.027 |
| No | 24 | 15.0 (10.0–28.3) |
Figure 1. .
Effect of posterior margin invasion on survival *univariate analysis.
Figure 2. .
Effect of lymphocyte count on survival *univariate analysis.
Discussion
Pancreatic cancer is a disease with a poor prognosis. Of the prognostic variables analysed, low preoperative lymphocyte count and posterior resection margin were found to be significant on multivariate analysis (Figures 1 and 2).
Although invasion of the posterior resection margin was identified as a significant prognostic factor, involvement of the resection margin (any margin) was not a significant prognostic variable (p=0.10). Residual tumour is often found at the posterior resection margin with complete resection sometimes difficult due to the immediate proximity of major vascular structures. Invasion specifically of the posterior resection margin has previously been identified to be associated with an adverse prognosis 5,6,14,26.
Preoperative lymphocyte count less than the lower limit of the reference range (1.5×109/litre) was also found to be a predictor of shorter survival on multivariate analysis (p=0.027). Preoperative lymphocyte count has previously been shown to be predictive of survival in patients with potentially resectable PDAC 16,17; however, the numbers of subjects in these studies were small. The current study consisted only of patients who underwent pancreaticoduodenectomy for PDAC (as confirmed by pathological analysis) representing a very select group, removing the potentially confounding variable of tumour type but confirming the previous findings.
Although lymphocyte count was a significant prognostic factor, the NLR was not. In the present study, patients were grouped according to whether their NLR was less than or greater then 5, as previous studies have shown that a preoperative NLR of >5 has a prognostic significance in patients undergoing resection of colorectal cancer and colorectal cancer liver metastases 28,29. In the present study, only 4 patients had a neutrophil to lymphocyte count >5, and therefore the results of the present study are difficult to interpret with respect to the prognostic value of the NLR. However, given that the majority of patients in this study had a normal neutrophil count, the observed lymphocytopenia of patients included in this study is less likely to be due to biliary sepsis, which would be associated with an increased neutrophil count and reduced lymphocyte count 27.
The prognostic value of lymphocyte count in irresectable PDAC has not been reported in the litreature. However, leukocytosis has previously been shown to be associated with poor prognosis in patients with irresectable PDAC 30. It was suggested that this may be explained by the significant morbidity and mortality associated with cholangitis with which a high white cell count would be expected.
Lymphocytopenia is present in many types of cancers and is thought to reflect a generalized state of depressed immune function 18. Depressed immune function may influence survival adversely due to reduced host response to the tumour cells. A study comparing patients with pancreatic, gastric and colorectal carcinoma found that patients with PDAC had more marked lymphocytopenia preoperatively and postoperatively 20.
In patients with PDAC, increasing T-stage has been shown to correlate with decreasing CD3-, CD4- and CD8- lymphocyte counts 18. Furthermore, part of the host defence against PDAC is thought to involve local tumour infiltration with lymphocytes 21,22,23. It has been shown that tumour infiltration with a large number of CD4+ and CD8+ lymphocytes in PDAC is associated with improved prognosis 22.
Immunological tumour escape mechanisms involving lymphocytes have also been identified in PDAC, which may potentially adversely influence survival. The Fas system, which comprises the Fas receptor and its ligand, Fas ligand (FasL), is a central mediator of apoptosis under physiological and pathological conditions 31. Abnormal expression of functional FasL has been shown to occur in PDAC cells and is thought to induce apoptosis of tumour infiltrating lymphocytes which have high expression of the Fas receptor 24,25. Impairment of T cell function is also thought to occur in PDAC due to high circulating levels of the inhibitory cytokines interleukin-10 and transforming growth factor-β1/2 with inactivation of these cells being reflected by loss of the CD3κ chain 21. In the majority of PDAC specimens, lymphocytes are “trapped” in fibrous peritumoral tissue and prevented from infiltrating the cancer, a further possible tumour escape mechanism 21.
Another simple blood test, which has been identified to have prognostic significance in patients with PDAC, is C-reactive protein (CRP) 32,33,34,35,36,37. This has been shown to correlate inversely with survival and was used in these studies as an index measurement of the systemic inflammatory response which is thought to exist in PDAC patients. A raised CRP level has also been shown to be related to the degree of cancer cachexia 32,33,38. Furthermore, encouraging results have been achieved using combined adjuvant chemoradiotherapy and immunotherapy with a reported 5-year survival of 55% 39. Taken together, these findings suggest that there may be an immunological basis to this disease.
The well-established prognostic variables in resected PDAC are pathological features, but few discriminating preoperative variables have been identified as having prognostic significance in patients with potentially resectable PDAC. The results of this study suggest that lymphocyte count may be useful in determining prognosis in patients with PDAC. This study also adds to the body of evidence suggesting that immunological mechanisms may contribute to the poor prognosis of PDAC. If the results of this study are borne out in a larger study, preoperative lymphocyte count could potentially be included in a prognostic nomogram for PDAC similar to that previously constructed by Brennan et al. 11.
References
- 1.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993;165:68–72; discussion 72–63. [DOI] [PubMed] [Google Scholar]
- 2.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 1991;161:120–4; discussion 124–5. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621–33; discussion 633–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagakawa T, Konishi I, Ueno K, Ohta T, Kayahara M, Miyazaki I. Extended radical pancreatectomy for carcinoma of the head of the pancreas. Hepatogastroenterology. 1998;45:849–54. [PubMed] [Google Scholar]
- 6.Nagakawa T, Nagamori M, Futakami F, Tsukioka Y, Kayahara M, Ohta T, et al. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996;77:640–5. [PubMed] [Google Scholar]
- 7.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721–31; discussion 731–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas – 616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 9.Millikan KW, Deziel DJ, Silverstein JC, Kanjo TM, Christein JD, Doolas A, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999;65:618–23; discussion 614–23. [PubMed] [Google Scholar]
- 10.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–8. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 13.Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217:430–5; discussion 435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo CJ, Cameron JL. Prognostic factors in ductal pancreatic cancer. Langenbecks Arch Surg. 1998;383:129–33. doi: 10.1007/s004230050104. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Noshiro H, Shimizu S, Morisaki T, Chijiiwa K, Tanaka M. Long-term and short-term survivors after pancreatectomy for pancreatic cancer. Int Surg. 2000;85:71–6. [PubMed] [Google Scholar]
- 17.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 18.Wenger FA, Jacobi CA, Zieren J, Docke W, Volk HD, Muller JM. Tumour size and lymph-node status in pancreatic carcinoma – is there a correlation to the preoperative immune function? Langenbecks Arch Surg. 1999;384:473–8. doi: 10.1007/s004230050233. [DOI] [PubMed] [Google Scholar]
- 19.Maltoni M, Pirovano M, Nanni O, Marinari M, Indelli M, Gramazio A, et al. Biological indices predictive of survival in 519 Italian terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1997;13:1–9. doi: 10.1016/s0885-3924(96)00265-5. [DOI] [PubMed] [Google Scholar]
- 20.Romano F, Uggeri F, Crippa S, Di Stefano G, Scotti M, Scaini A, et al. Immunodeficiency in different histotypes of radically operable gastrointestinal cancers. J Exp Clin Cancer Res. 2004;23:195–200. [PubMed] [Google Scholar]
- 21.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res 2001;7(3 Suppl):925s–32s. [PubMed] [Google Scholar]
- 22.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumour-infiltrating lymphocytes together with CD4+ tumour-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Abe M, Kondo S, Hirano S, Ambo Y, Tanaka E, Morikawa T, et al. Long-term survival after radical resection of advanced pancreatic cancer: a case report with special reference to CD8+ T-cell infiltration. Int J Gastrointest Cancer. 2003;33:107–10. doi: 10.1385/IJGC:33:2-3:107. [DOI] [PubMed] [Google Scholar]
- 24.von Bernstorff W, Spanjaard RA, Chan AK, Lockhart DC, Sadanaga N, Wood I, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO–1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999;125:73–84. doi: 10.1067/msy.2099.93570. [DOI] [PubMed] [Google Scholar]
- 25.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, et al. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;58:1741–9. [PubMed] [Google Scholar]
- 26.Luttges J, Vogel I, Menke M, Henne-Bruns D, Kremer B, Kloppel G. The retroperitoneal resection margin and vessel involvement are important factors determining survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Virchows Arch. 1998;433:237–42. doi: 10.1007/s004280050242. [DOI] [PubMed] [Google Scholar]
- 27.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 2007. [DOI] [PubMed] [Google Scholar]
- 28.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 29.Wyllie DH, Bowler IC, Peto TE. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004;57:950–5. doi: 10.1136/jcp.2004.017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelken FJ, Bettschart V, Rahman MQ, Parks RW, Garden OJ. Prognostic factors in the palliation of pancreatic cancer. Eur J Surg Oncol. 2003;29:368–73. doi: 10.1053/ejso.2002.1405. [DOI] [PubMed] [Google Scholar]
- 31.Bohm I, Schild H. Apoptosis: the complex scenario for a silent cell death. Mol Imaging Biol. 2003;5:2–14. doi: 10.1016/s1536-1632(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 32.Barber MD, Powell JJ, Lynch SF, Fearon KC, Ross JA. A polymorphism of the interleukin-1 beta gene influences survival in pancreatic cancer. Br J Cancer. 2000;83:1443–7. doi: 10.1054/bjoc.2000.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–0. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 34.Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–82. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson NB, Glen P, McMillan DC, McKay CJ, Foulis AK, Carter R, et al. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer. 2005;92:21–3. doi: 10.1038/sj.bjc.6602305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno H, Okada S, Okusaka T, Ikeda M. Prognostic factors in patients with metastatic pancreatic adenocarcinoma receiving systemic chemotherapy. Oncology. 2000;59:296–301. doi: 10.1159/000012186. [DOI] [PubMed] [Google Scholar]
- 37.Wigmore SJ, Todorov PT, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Characteristics of patients with pancreatic cancer expressing a novel cancer cachectic factor. Br J Surg. 2000;87:53–8. doi: 10.1046/j.1365-2168.2000.01317.x. [DOI] [PubMed] [Google Scholar]
- 38.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–4. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–80. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]