Abstract
Background. Continuous veno-venous haemofiltration (CVVH) could be reasonable for attenuation of systemic complications in severe acute pancreatitis (SAP). The aim of the study was implementation and feasibility assessment of the CVVH in the treatment protocol of SAP. Patients and methods. CVVH was applied to 111 SAP patients during 2000–2005. APACHE II, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), serum lipase, C-reactive protein (CRP), complication rate and main outcomes were analysed comparing two periods. Results. Overall, 39 patients corresponded to Balthazar grade E SAP and 72 patients to necrotizing SAP (NSAP), with an average APACHE II score of 7 and 8.5, respectively, on admission. CVVH was started within 48 h in 82% of patients. Duration of CVVH was significantly augmented in NSAP patients during the routine period, comprising 92 h (p=0.006). The clinical presentation of SIRS and MODS was similar in both periods, with more initial pulmonary dysfunctions in NSAP (p=0.048). Peripancreatic infection decreased in the routine period; surgical interventions were performed in 34.8% vs 72.4% of patients. Hospital stay comprised on average 15.9 days for grade E SAP and 29.4 days for NSAP in the routine period, with overall mortality of 10.26% and 30.5%, respectively. Discussion. Application of CVVH in the treatment protocol of SAP is obscure due to relative invasiveness, a poorly understood mechanism of action and scarce clinical experience. We conclude that early pre-emptive application of CVVH is safe and feasible in the treatment of SAP. Duration of the procedure seems to be essential. Randomized clinical trials are justified. Our results are in favour of clinical application of CVVH in the treatment of SAP.
Keywords: acute pancreatitis, CVVH
Introduction
The clinical course of severe acute pancreatitis (SAP) is well studied and two phases of the systemic inflammatory response process are generally accepted as typical for this dangerous condition 1. Successful treatment of systemic inflammatory response syndrome (SIRS), prophylaxis of early organ dysfunction and prophylaxis of infection in the first phase of the disease form the basis for good prognosis and positive outcome 1,2,3. The second clinical phase, in its most severe forms – generally in cases with necrotizing sAP (NSAP) – depends almost entirely on the presence of infection. Effective treatment and prevention of infection in the first phase, even in NSAP, to a great extent is a guarantee of recovery and low rate of complications. On the other hand, presence of infection raises the risk of repeated development of SIRS and multiple organ dysfunction syndrome (MODS), the main causes of morbidity and mortality in the second phase. A conservative approach in the early phase of SAP rather than an aggressive surgical approach has proved to be more reasonable, due to effective treatment modalities of modern critical care 4. At the same time, positive bacteriological culture of the peripancreatic area with signs of repeated or continuing ascending SIRS and progressive MODS mandates surgical intervention mostly in the second phase of disease 1. Accordingly, effective initial treatment should be oriented to faster recovery of the organ perfusion and microcirculation, especially the visceral microcirculation. This is the most important task considering that good visceral perfusion is the main determinant of maintenance of safe bowel barrier function, prevention of bacterial translocation and prevention of peripancreatic infection 5. Rapid recovery of bowel function is mandatory for the initiation of early enteral feeding, another important treatment modality for down-regulation of SIRS and prevention of sepsis 6. Effective modulation of SIRS and organ support are the cornerstones of the conservative management of SAP. Prevention of septic complications also includes the principle of applying minimally invasive treatment modalities, to preserve other organism barriers such as respiratory and urinary tract mucosa and skin. The invasiveness, however minimal, together with the risk of bleeding, high costs and the not well-understood mechanism of functioning are the main arguments against very wide implementation of continuous veno-venous haemofiltration (CVVH) in the routine treatment protocol for SAP 7. Therefore the potential role of CVVH in the treatment plan of SAP is still a matter of debate. Positive results are available from experimental studies in animals 8. Scarce clinical experience has demonstrated positive treatment results after CVVH in SAP patients; however, no definite guidelines regarding the timing, duration and mode of CVVH are available for this category of patients 9,10. Since 1999, we have introduced clinical implementation of CVVH in the treatment protocol of SAP. At the present time, this procedure is routinely applied in our hospital. We have not found any published reports with similar experience in clinical application of CVVH for the conservative treatment of SAP patients. The aim of this feasibility study is to share our 6-year experience with implementation of the CVVH in the complex treatment protocol of SAP.
Patients and methods
SAP patients with Balthazar CTSI grade E and/or necrotizing SAP in whom CVVH was applied as an integral part of the treatment protocol were prospectively enrolled between January 2000 and December 2005. SAP was classified according to Atlanta 1992 11. NSAP diagnosis was based on the clinical picture supported by radiological evidence of pancreatic necrosis and/or involvement of the peripancreatic tissue in contrast-enhanced CT scan or proved during surgical intervention. An additional criterion for this group was maximal C-reactive protein (CRP) level >200 mg/L. All patients were treated in the intensive care unit (ICU). APACHE II scores were calculated at admission and were used as initial prognostic criteria. Symptoms of SIRS, organ dysfunction and incidence of MODS, dynamics of serum lipase and CRP were recorded. The incidence of septic complications was analysed considering the primary and secondary types of peripancreatic infection. Primary infection was considered when application of CVVH and conservative treatment failed to prevent peripancreatic septic complications and the source of infection was mainly the gastrointestinal tract. Secondary infection was considered when contamination by nosocomial flora was observed after early surgical intervention. Sepsis was considered when positive blood culture was obtained.
Haemofiltration was performed using Diapact CRRT from B. Braun Co. or Fresenius Medical Care Multifiltrate Machines, Germany. Synthetic high volume membrane filters were used (surface area 1.5–2.2 m2). Filter change was performed routinely once every 24 h or when filter clotting occurred. Vascular access was obtained by one double or triple line catheter inserted into the femoral or jugular vein. Non-fractionated heparin was used for anticoagulation, and the adequate dosage was individually adjusted to APTL. The procedure was provided without heparin or with low heparin dose whenever possible. The substitution fluid was infused at the rate of 1000 ml/h in pre-diluted or post-diluted manner, comprising 24–35 L of total substitute in 24 h. The blood flow ranged within 50–200 ml/min. Ultrafiltration rate was controlled depending on diuresis and fluid balance. Haemodiafiltration was provided in accordance with individual indications.
The main indications for application of CVVH were progression of SIRS, abdominal compartment syndrome (IAP >25 cm/H2O) and MODS despite complex initial intensive therapy within 24–48 h.
Organ failure was defined according to the recommendations of the Consensus Conference of American College of Chest Physicians/Society of Critical Care Medicine in 1991 12,13. SIRS was diagnosed if two or more of the following conditions were detected: (a) temperature above 38°C or below 36°C, (b) heart rate above 90 beats/min, (c) respiratory rate above 20 breaths/min, or arterial carbon dioxide tension below 32 mmHg, and (d) white blood cell count above 12 000/mm3 or below 4000/mm3, or immature (band) forms accounting for more than 10% of neutrophils present 14. MODS was diagnosed if dysfunction of more than one organ was detected, requiring intervention to maintain homeostasis. SOFA score was calculated for daily assessment of the dynamics of organ dysfunction 13. Radiological evidence of basal atelectasis, pneumonia or pleural effusion was recorded as pulmonary complications. Pulmonary dysfunction was classified according to SOFA score data. Acidosis was diagnosed when pH in the peripheral blood was <7.2. Complication rate, ICU, hospital stay and main outcomes were recorded and analysed in the learning curve period and the routine period (the first and the second 3-year periods). CVVH procedure for SAP patients was accepted by the hospital administration and the local ethics commission, and patients were informed about the procedure and the associated risks and complications.
Statistical comparison was performed with paired samples t test. All clinical data were expressed as average±standard deviation. Correlation data were calculated using Pearson's and Spearman's correlation test. Data analysis was performed with SPSS software version 8.0 (SPSS Inc.).
Results
Patient characteristics and severity of disease
A total of 246 patients with SAP were treated in our hospital during the last 6 years. CVVH was applied in 111 SAP patients as an integral part of the treatment protocol. The male to female ratio was 89:22. Alcohol and gallstones were the major aetiological factors. Of all patients, 39 suffered acute oedematous pancreatitis with multiple localizations of the inflammatory exudates complying with Balthazar CTSI grade E criteria. Radiological or intraoperative evidence of pancreatic necrosis and/or involvement of the peripancreatic tissue in parallel with CRP >200 mg/L were observed in 72 patients who were classified as NSAP. All patients received initial conservative treatment including organ support, active recovery of the tissue perfusion and reduction of the fluid sequestration in the third space by means of isovolaemic haemodilution with adequate colloid/crystalloid infusions.
The average APACHE II score at admission was 7±5.4 points for grade E SAP and 8.5±5.3 points for NSAP. Relatively low APACHE II scores at admission were partially due to the prevalence of young male alcohol abusers. The clinical course of SAP was characterized by a similar incidence of SIRS and MODS in both clinical groups in the first phase of the disease, when evaluating the whole period of the study. However, the overall incidence of pulmonary dysfunction/pulmonary complications and metabolic acidosis was significantly higher in NSAP (Table I).
Table I. Clinical presentation of SAP during all study periods.
| Parameter | Grade E SAP | NSAP | p value |
|---|---|---|---|
| Patients (n) | 39 | 72 | – |
| APACHE II (average points) | 7 | 8.49 | NS |
| SIRS (no. of cases) | 35 | 69 | NS |
| MODS (no. of cases) | 32 | 70 | NS |
| Metabolic acidosis (no. of cases) | 2 | 13 | 0.032 |
| Renal dysfunction (no. of cases) | 15 | 31 | NS |
| Pulmonary dysfunction (no. of cases) | 15 | 51 | 0.048 |
| Atelectasis (no. of cases) | 0 | 8 | 0.006 |
| Pneumonia (no. of cases) | 2 | 24 | 0.006 |
A more detailed comparison of the clinical data in the learning period and the routine period did not show significant differences in either clinical group except more frequent development of encephalopathies among NSAP patients in the routine period (p=0.047) (Table II).
Table II. Clinical course of SAP and duration of the CVVH.
| NSAP |
Grade E SAP |
|||
|---|---|---|---|---|
| Clinical group | Learning period | Routine period | Learning period | Routine period |
| Patients (n) | 29 | 43 | 10 | 29 |
| APACHE II (points) | 7,9 | 9 | 6 | 7.55 |
| MODS (no. of cases) | 28 | 42 | 8 | 24 |
| Pulmonary dysfunction (no. of cases) | 18 | 33 | 1 | 14 |
| Pulmonary complications (no. of cases) | 27 | 48 | 1 | 17 |
| Sepsis (no. of cases) | 2 | 4 | 0 | 0 |
| Encephalopathy (no. of cases) | 6 | 20* | 3 | 12 |
| Duration of CVVH (h) | 51.9 | 92.0** | 42.5 | 70.9 |
| Duration of CVVH overall (h) | 75.8 | 63.6 | ||
*p=0.047; **p=0.006.
Timing of CVVH
An overall increase in the number of patients who received CVVH was characteristic of the routine period for both clinical groups. Commencement of the CVVH procedure was started within 48 h from admission in 82% of patients. Duration of the procedure was optimized during the learning period approaching roughly 48 h and more. Longer procedures were applied in NSAP patients when the whole study period was evaluated; however, this did not reach statistical significance. The average duration of CVVH was prolonged in both clinical groups, as experience with the procedure grew. Significant increase in the duration of CVVH was evident in NSAP patients when comparing the learning and the routine periods, comprising an average of 51.9±25.3 and 92±70.4 h, respectively (p=0.006) (Table II).
Efficacy of CVVH
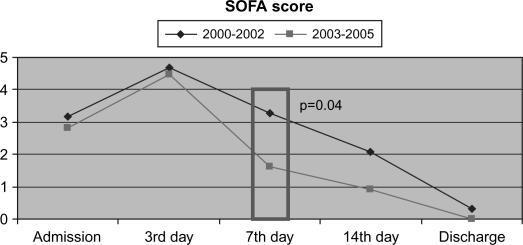
More effective recovery of the organ function in all surviving patients was observed in the routine period when the CVVH procedure lasted longer compared with the learning period. A statistically significant difference in SOFA score was evident on day 7 after admission (p=0.04) (Figure 1).
Figure 1. .
Regression of the organ dysfunction comparing the learning curve and the routine period in all surviving patients.
The duration of CVVH was ≤48 h in 49 patients and >48 hours in 62 patients. The longer CVVH procedure (>48 h) was provided in patients with a significantly higher number of initial pulmonary dysfunctions and pulmonary complications. At the same time, longer procedures were associated with lower mortality rate (p=0.038) (Table III).
Table III. Clinical course in relation to the duration of the CVVH procedure.
| Parameter | CVVH <48 h | CVVH >48 h | p value |
|---|---|---|---|
| Patients (n) | 49 | 62 | NS |
| CVVH application period (h) | 30,3 | 103,8 | <0.05 |
| APACHE II (points) | 8,77 | 7,54 | NS |
| SIRS (no. of cases) | 45 | 59 | NS |
| MODS (no. of cases) | 42 | 60 | NS |
| Pulmonary dysfunction (no. of cases) | 23 | 43 | 0.006 |
| Pleural effusion (no. of cases) | 16 | 41 | 0.001 |
| Renal dysfunction (no. of cases) | 17 | 29 | NS |
| Death (n) | 16 | 10 | 0.038 |
Laboratory data
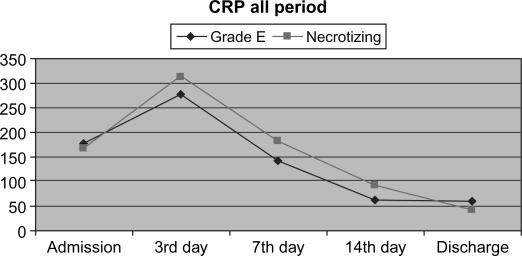
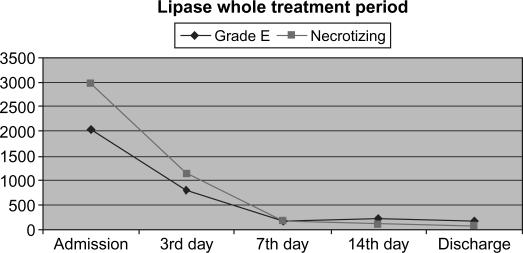
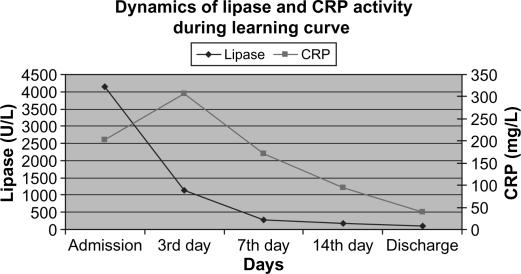
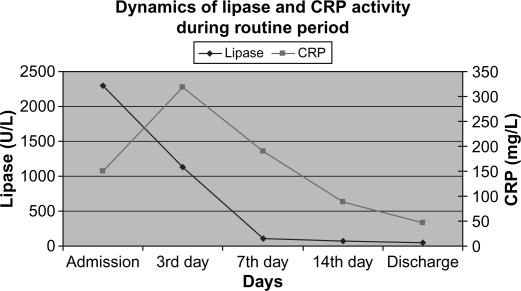
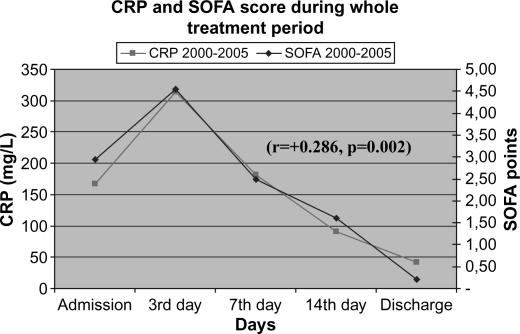
Laboratory data were in concordance with the clinical course and the signs of systemic inflammatory response. Overall dynamics of CRP and serum lipase activity showed similar patterns in both clinical groups (Figures 2 and 3). Furthermore, a very similar pattern of CRP and serum lipase activity after application of CVVH was observed in the learning and the routine period, with a strong tendency towards normalization on average at day 7 from admission (Figures 4 and 5). A decrease in CRP activity positively interrelated with regression of organ dysfunction (r= + 0.286, p=0.002) (Figure 6).
Figure 2. .
Dynamics of CRP in clinical groups in whole treatment period.
Figure 3. .
Activity of serum lipase in clinical groups.
Figure 4. .
CRP and lipase activity in learning curve.
Figure 5. .
CRP and lipase activity in routine period.
Figure 6. .
Positive interrelation of CRP and SOFA score.
Infection, surgical interventions and outcomes
The overall incidence of peripancreatic infection decreased from 37.9% during the learning curve to 23.3% in the routine period. This was achieved by a more conservative approach, and consequently the number of surgical interventions dropped from 72.4% to 34.8%. All surgical interventions, except one, were done in NSAP patients. Primary peripancreatic infection was the indication for surgical intervention in 10 NSAP patients after the second week from admission, on average 17.6±11.9 days after the commencement of CVVH.
Secondary peripancreatic infection was observed similarly in 10 patients who were operated early in the course, mostly due to obscure diagnosis or uncertain progression of MODS, on average 2.3±3.5 days after application of CVVH. All secondarily infected patients received CVVH before and after the surgical intervention. Overall mortality in infected patients was 25% (five cases). Positive bacteriological blood cultures were observed in only six NSAP patients, with similar incidence regarding the duration of CVVH. The growing experience with the application of CVVH procedure in the treatment protocol of SAP resulted in shorter ICU and hospital stays, comparing the learning and the routine periods; however, this did not reach a statistically significant difference (Table IV).
Table IV. Septic complications, surgical interventions and outcomes.
| Grade E SAP (n=39) |
NSAP (n=72) |
||||
|---|---|---|---|---|---|
| Parameter | Learning period | Routine period | Learning period | Routine period | p value |
| Positive blood culture (no. of cases) | 0 | 0 | 3 | 3 | 0.023 |
| ICU stay (days) | 6.1 | 7.34 | 18.6 | 13.3 | NS |
| Hospital stay (days) | 18 | 15.9 | 38.7 | 29.4 | NS |
| Mortality (n/%) | 2/20 | 1/6.9 | 10/34.5 | 12/27.9 | NS |
| Overall mortality | 10.26% (4 cases) | 30.5% (22 cases) | 0.01 | ||
| Overall hospital stay (days) | 16.4 | 33.5 | <0.05 | ||
| Overall ICU stay (days) | 7 | 15.46 | <0.05 | ||
The average length of ICU stay for the whole study period was 7±4.7 days for grade E SAP and 15.46±12.1 days for NSAP patients. Correspondingly, hospital stay comprised 16.4±10.5 and 33.5±27.2 days, respectively. Finally, routine application of CVVH was associated with 10.26% (4 cases in grade E SAP) and 30.5% (22 cases in NSAP) mortality rate (p=0.01). Application of CVVH for >48 h resulted in a significantly lower mortality rate, compared with a shorter application of the procedure (p=0.038).
Discussion
The clinical course of SAP with ascending SIRS and development of organ dysfunction is recognized and described with delineation of two clinical phases 1. According to the current recommendations, successful treatment is related to a prompt recognition of the most severe forms, with emphasis on necrotizing pancreatitis. A general agreement exists that early adequate fluid and electrolyte restitution, organ support and infection prophylaxis are the cornerstones of the conservative treatment in the first phase of the disease. Proven presence of the peripancreatic infection mandates surgical intervention most frequently in the second phase of the disease, mainly in NSAP patients 1,2,3,4. Effective intravascular and extravascular fluid balance is crucial for recovery of the organ perfusion and microcirculation, being severely impaired in SAP. The magnitude of the impaired visceral perfusion is of utmost importance for further prognosis, as it is the key factor for bowel barrier integrity and the main condition for prevention of bacterial translocation 15. Early enteral feeding, another important treatment modality for down- regulation of SIRS and prevention of sepsis 6, is not applicable when bowel function is severely impaired. Increase of the intra-abdominal pressure (IAP) and development of the abdominal compartment syndrome as a result of the huge extravascular fluid loss and visceral oedema further deteriorates visceral perfusion. This scenario is more often seen in obese patients and/or in cases when initial large volume fluid replacement was provided with crystalloids only. Then fluid retention in the extravascular compartment, or the so-called ‘third space’, is inevitable due to increased vascular permeability as a consequence of SIRS. Dyspnoea and oligoanuria are the most characteristic clinical signs in this condition. It was often the case when our patients with SAP rapidly deteriorated after initial 48 h therapy. Transfers to the ICU frequently were too late for prevention and successful treatment of MODS. Unfortunately, clinical deterioration, especially in necrotizing forms, mandated surgeons to intervene earlier than generally recommended, even after commencement of the organ support therapy and improvement of the microcirculation. At the same time, even when it was possible to prolong conservative management into the second week, the rate of peripancreatic infection was remarkably high in this category of patients. Severity of the disease in SAP patients is not directly related solely to the extent of necrosis, but rather to individual systemic inflammatory response. This individual systemic inflammatory response could also be marked in patients with grade E SAP and associated with early development of organ dysfunction and abdominal compartment syndrome.
In 1999 we reconsidered our treatment strategy. The main breakthrough was upgrade of the duty staff regarding admission criteria for early recognition of SAP. All patients with suspected SAP were consulted by an intensive care specialist and directly admitted to the ICU according to indications. Implementation of the early enteral feeding directed our attention towards the problem of elevated IAP and the finding that enteral feeding is not possible when IAP approaches or exceeds 25 cmH2O 16. Unfortunately at that time conservative treatment was not effective enough for prompt reduction of the sustained increase of the IAP. Therapy results improved considerably when the Department of Renal Replacement Therapy came forward with the recommendation to try CVVH in the treatment of SAP. The first successful series with CVVH prompted formulation of more definite indications for the application of the procedure. The valuable observation that an early application of CVVH was effective in reduction of the severely increased IAP pushed us to continue this strategy and follow a more conservative approach. The comparison of two patient groups was done to compare the overall outcomes in association with application of the CVVH in two different periods. After the learning period, certain experience was collected. The important finding was a very similar pattern of down-regulation of SIRS and recovery of the organ function in both groups when improvement was achieved. Another important finding was a similar decrease in the number of occurrences of surgical sepsis when experience was collected. We did not compare CVVH with other treatment modalities, although our experience demonstrates that CVVH can be a safe additional treatment option for a basic SAP management protocol and results are improving with more experience in the application of the procedure. The marginal difference in CRP on day 7 proves a uniform pattern of the decrease of the systemic inflammatory reaction regardless of whether the patient suffered from necrotizing or grade E SAP. This is evident in all study periods and proves the safety of CVVH. We do not state that this is only due to successful application of the CVVH; however, certainly this is in association with this procedure.
According to the available reports from the literature, application of CVVH is not well recognized in patients with SAP, although it is recommended for the treatment of severe sepsis 17,18. Disturbances of the microcirculation, increased vascular permeability and insufficient perfusion/oxygenation of the end organ are claimed to be the central pathophysiologic event in the sepsis-like systemic inflammatory response, characteristic also for SAP 19,20. The concept of the application of CVVH is based on the hypothesis that elimination of numerous proinflammatory cytokines in SIRS and anti-inflammatory cytokines in counter anti-inflammatory response syndrome (CARS) can provide equilibrated immune response. This concept is known as the hypothesis of ‘cutting the peaks’ 18. However, the question remains whether elimination of the cytokines from the bloodstream is the main impacting factor for the modulation of SIRS, because changes in the interstitial and tissue compartment are equally clinically important. According to the Honoré concept, removal of the mediators and pro-mediators at the interstitial and tissue levels secondary to removal from the blood compartment could block basic pathways and stop further tissue damage and organ injury 21,22. It seems that early application of CVVH can modulate SIRS and consequently diminish incidence of systemic complications. Our data are not sufficient to draw firm conclusions regarding the true mechanisms of the action underlying the positive effect of CVVH application in SAP patients. Initially, treatment of the renal dysfunction was the basic concern. Afterwards, reduction of the third space and state of hyperhydration were identified as additional indications. Effective decrease and normalization of the IAP, reduction of the peripancreatic exudation in parallel with recovery of the pulmonary, gastrointestinal and kidney function after application of CVVH validated this approach 23. A pre-emptive strategy was favoured for MODS control. Early commencement of CVVH and prolongation of the procedure over a 48 h period resulted in significantly reduced mortality. At the same time, the degree of the renal dysfunction corresponded to ‘R’ (risk of dysfunction) and ‘I’ (injury) according to RIFLE criteria 24 in the majority of patients.
Our data comply with the reported existence of the so-called ‘therapeutic window’ within 48–72 h from the onset of SAP when acute inflammatory response reaches maximum due to peak output of cytokines 25. However, according to our observations, the point of reference could be the start of adequate treatment in most cases, rather than the time of the onset of disease. Average highest values of the SOFA score and CRP in our patients were observed around the third day from admission and then declined. This was not in parallel with the time from the onset of the disease. Considering the fact that the majority of patients received CVVH within 48 h from admission, presumably effective early application of the CVVH can give an additional chance to patients with >48 h anamnesis. It seems that an important condition is an appropriate duration of the procedure rather than the high volume mode recommended by some authors in severe sepsis 26,27, if it is applied in a pre-emptive way. We did not observe serious procedure-associated complications, which is in parallel with the results from other studies 28. Despite some invasiveness of the procedure, the overall rate of infection decreased in common with the number of surgical interventions, and final outcomes are in the range of internationally reported data 29,30,31.
Conclusion
Early pre-emptive application of CVVH is a safe and feasible therapeutic modality in the treatment protocol of SAP. Adequate duration of the procedure seems to be essential. Further randomized clinical trials are justified for better understanding of the mechanism of action and efficacy of the CVVH, comparing to other conservative treatment modalities.
Acknowledgements and disclosures
No disclosures.
References
- 1.Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426–36. doi: 10.1136/gut.2003.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Working Party on Acute Pancreatitis. UK guidelines for the management of acute pancreatitis. Gut 2005;54(Suppl 3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda K, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:42–7. doi: 10.1007/s00534-005-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathens AB, Curtis JR, Beale RJ, Cook DJ, Moreno RP, Romand JA, et al. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524–36. doi: 10.1097/01.ccm.0000148222.09869.92. [DOI] [PubMed] [Google Scholar]
- 5.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–35. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 6.Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431–5. doi: 10.1136/gut.42.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao EQ, Tang YQ, Zhang SD. Effects of time interval for hemofiltration on the prognosis of severe acute pancreatitis. World J Gastroenterol. 2003;9:373–6. doi: 10.3748/wjg.v9.i2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan XW, Li WQ, Wang H, Zhang ZH, Li N, Li JS. Effects of high-volume continuous hemofiltration on experimental pancreatitis associated lung injury in pigs. Int J Artif Organs. 2006;29:293–302. doi: 10.1177/039139880602900307. [DOI] [PubMed] [Google Scholar]
- 9.Jiang HL, Xue WJ, Li DQ, Yin AP, Xin X, Li CM, et al. Influence of continuous veno-venous hemofiltration on the course of acute pancreatitis. World J Gastroenterol. 2005;11:4815–21. doi: 10.3748/wjg.v11.i31.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie H, Ji D, Gong D, Liu Y, Xu B, Zhou H, et al. Continuous veno venous hemofiltration in treatment of acute necrotizing pancreatitis. Chin Med J (Engl) 2003;116:549–53. [PubMed] [Google Scholar]
- 11.Bradley EL, 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, , September11through13, 1992. Arch Surg1993;128:586–90. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23. [PubMed] [Google Scholar]
- 15.Dervenis Ch, Smailis D, Hatzitheoklitos E.Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg 2003;10:415–18. [DOI] [PubMed] [Google Scholar]
- 16.Pupelis G, Plaudis H, Snippe K, Rudakovska M. Increased intra-abdominal pressure: is it of any consequence in severe acute pancreatitis? J Hepatobiliary Pancreat Surg. 2006;8:227–32. doi: 10.1080/13651820500540956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard SH, Irene EK. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27:792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson C. Buchler MW, Uhl W, Friess H, Malfertheiner P. Blackwell Wissenschafts-Verlag; Berlin: 1999. Role of cytokines and their antagonists, Acute pancreatitis. Novel concepts in biology and therapy; pp. 71–5. [Google Scholar]
- 20.Pezzilli R, Ceciliato R, Barakat B, Corinaldesi R. Immune-manipulation of the inflammatory response in acute pancreatitis. What can be expected? JOP. 2004;5:115–21. [PubMed] [Google Scholar]
- 21.Di Carlo JV, Alexander SR. Hemofiltration for cytokine-driven illness: the mediator delivery hypothesis. Int J Artif Organs. 2005;28:777–86. doi: 10.1177/039139880502800803. [DOI] [PubMed] [Google Scholar]
- 22.Joannes-Boyau O, Honore PM, Boer W. Hemofiltration: the case for removal of sepsis mediators from where they do harm. Crit Care Med. 2006;34:2244–6. doi: 10.1097/01.CCM.0000227650.77064.5F. [DOI] [PubMed] [Google Scholar]
- 23.Pupelis G, Austrums E, Snippe K, Berzins M. Clinical significance of increased intra-abdominal pressure in severe acute pancreatitis. Acta Chir Belg. 2002;102:71–4. doi: 10.1080/00015458.2002.11679269. [DOI] [PubMed] [Google Scholar]
- 24.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–17. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 25.Pezzilli R, Fantini L, Morselli-Labate AM. New approaches for the treatment of acute pancreatitis. JOP. 2006;7:79–91. [PubMed] [Google Scholar]
- 26.Piccinni P, Dan M, Barbacini S, Carraro R, Lieta E, Marafon S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32:80–6. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R, Honore PM, Matson J, Ronco C, Winchester J. Extracorporeal blood treatment (EBT) methods in SIRS/sepsis. Int J Artif Organs. 2005;28:450–8. doi: 10.1177/039139880502800505. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Li WQ, Zhou W, Li N, Li JS. Clinical effects of continuous high volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. World J Gastroenterol. 2003;9:2096–9. doi: 10.3748/wjg.v9.i9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint R, Windsor J, Bonham M. Trends in the management of severe acute pancreatitis: interventions and outcome. ANZ J Surg. 2004;74:335–42. doi: 10.1111/j.1445-1433.2004.02940.x. [DOI] [PubMed] [Google Scholar]
- 30.Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay CJ, Imrie CW. The continuing challenge of early mortality in acute pancreatitis. Br J Surg. 2004;91:1243–4. doi: 10.1002/bjs.4750. [DOI] [PubMed] [Google Scholar]