Abstract
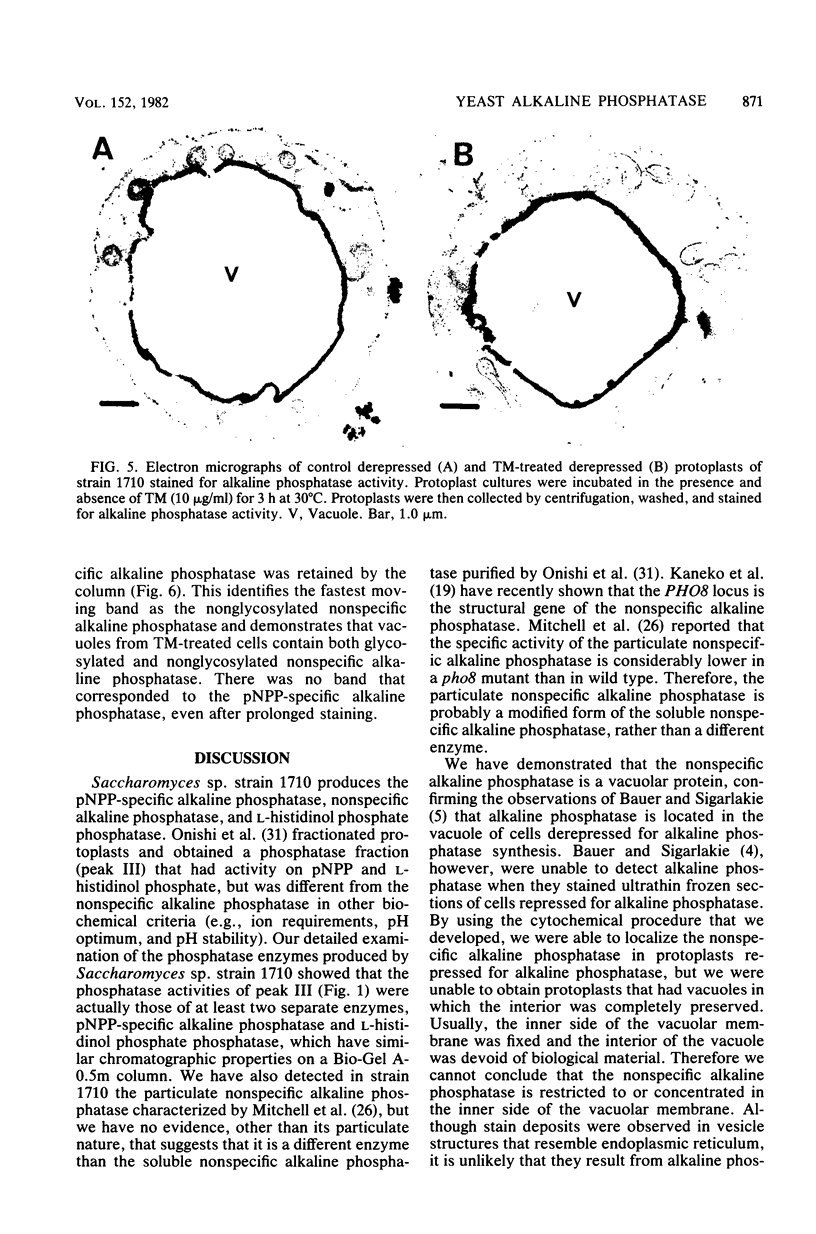
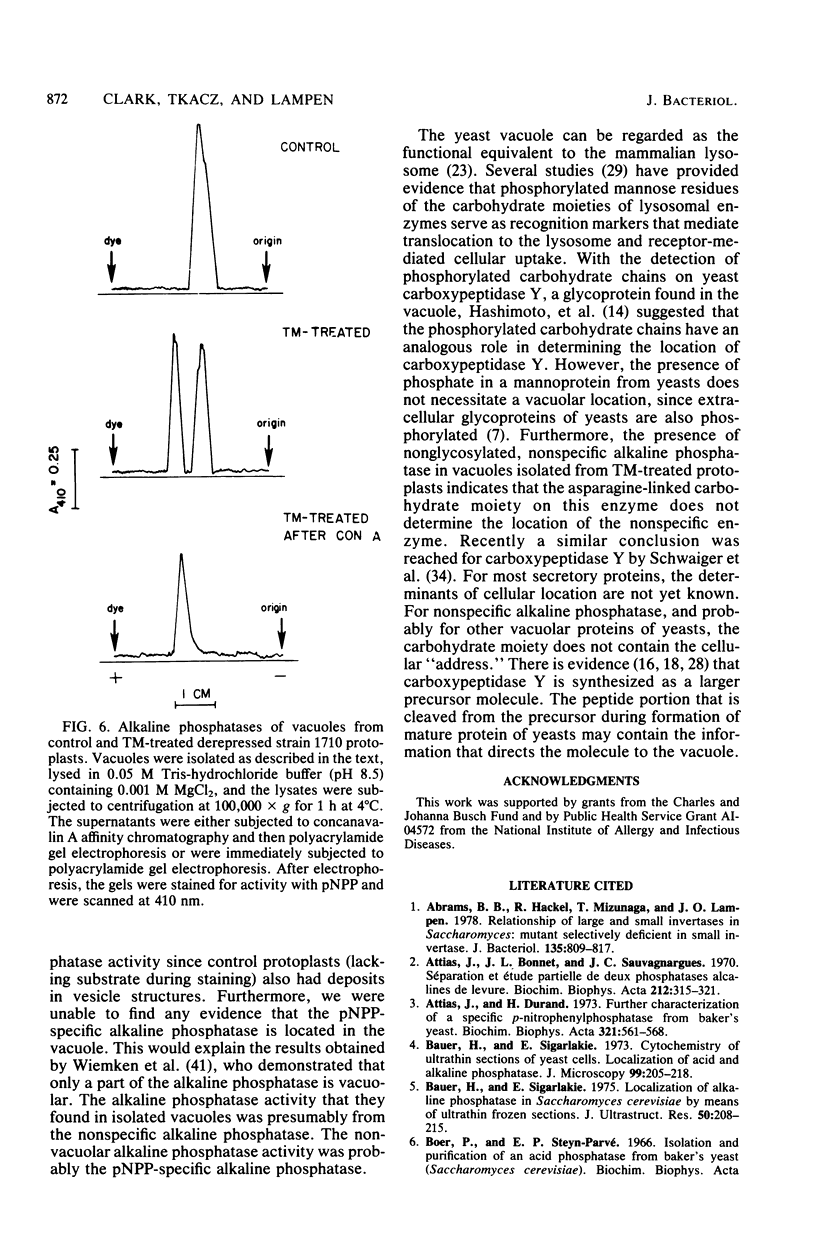
The nonspecific alkaline phosphatase of Saccharomyces sp. strain 1710 has been shown by phosphatase cytochemistry to be exclusively located in the vacuole, para-Nitrophenyl phosphate-specific alkaline phosphatase is not detected by this procedure because the activity of this enzyme is sensitive to the fixative agent, glutaraldehyde. To determine whether the oligosaccharide of nonspecific alkaline phosphatase is necessary to transport the enzyme into the vacuole, protoplasts were derepressed in the absence or in the presence of tunicamycin, an antibiotic which interferes with the glycosylation of asparagine residues in proteins. The location of the enzyme in the tunicamycin-treated protoplasts, as determined by electron microscopy and subcellular fractionation, was identical to its location in control protoplasts. In addition, carbohydrate-free alkaline phosphatase was found in vacuoles from tunicamycin-treated protoplasts. Our findings indicate that the asparagine-linked carbohydrate moiety does not determine the cellular location of the enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams B. B., Hackel R., Mizunaga T., Lampen J. O. Relationship of large and small invertases in Saccharomyces: mutant selectively deficient in small invertase. J Bacteriol. 1978 Sep;135(3):809–817. doi: 10.1128/jb.135.3.809-817.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias J., Bonnet J. L., Sauvagnargues J. C. Séparation et étude partielle de deux phosphatases alcalines de levure. Biochim Biophys Acta. 1970 Aug 15;212(2):315–321. doi: 10.1016/0005-2744(70)90212-3. [DOI] [PubMed] [Google Scholar]
- Attias J., Durand H. Further characterization of a specific p-nitrophenylphosphatase from baker's yeast. Biochim Biophys Acta. 1973 Oct 10;321(2):561–568. doi: 10.1016/0005-2744(73)90199-x. [DOI] [PubMed] [Google Scholar]
- Bauer H., Sigarlakie E. Localization of alkaline phosphatase in Saccharomyces cerevisiae by means of ultrathin frozen sections. J Ultrastruct Res. 1975 Feb;50(2):208–215. doi: 10.1016/s0022-5320(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Boer P., Steyn-Parvé E. P. Isolation and purification of an acid phosphatase from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1966 Nov 15;128(2):400–402. doi: 10.1016/0926-6593(66)90189-5. [DOI] [PubMed] [Google Scholar]
- Colonna W. J., Cano F. R., Lampen J. O. Microheterogeneity in yeast invertase. Biochim Biophys Acta. 1975 Mar 28;386(1):293–300. doi: 10.1016/0005-2795(75)90271-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Rodriguez L., Villanueva J. R., Sentandreu R. Regulation of acid phosphatase synthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Nov 21;521(1):342–351. doi: 10.1016/0005-2787(78)90276-9. [DOI] [PubMed] [Google Scholar]
- Gorman J. A., Hu A. S. The separation and partial characterization of L-histidinol phosphatase and an alkaline phosphatase of Saccharomyces cerevisiae. J Biol Chem. 1969 Mar 25;244(6):1645–1650. [PubMed] [Google Scholar]
- Hashimoto C., Cohen R. E., Zhang W. J., Ballou C. E. Carbohydrate chains on yeast carboxypeptidase Y are phosphorylated. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2244–2248. doi: 10.1073/pnas.78.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Biosynthesis of the vacuolar yeast glycoprotein carboxypeptidase Y. Conversion of precursor into the enzyme. Eur J Biochem. 1978 Apr 17;85(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12275.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Inhibition of the apparent rate of synthesis on the vacuolar glycoprotein carboxypeptidase Y and its protein antigen by turicamycin in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1976 Sep;10(3):402–410. doi: 10.1128/aac.10.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Toh-e A., Oshima Y. Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):127–137. doi: 10.1128/mcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Osmotic regulation of invertase formation and secretion by protoplasts of Saccharomyces. J Bacteriol. 1971 Apr;106(1):183–191. doi: 10.1128/jb.106.1.183-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A. The vacuole as the lysosome of the yeast cell. Arch Mikrobiol. 1967 Feb 20;56(2):148–155. doi: 10.1007/BF00408765. [DOI] [PubMed] [Google Scholar]
- Millay R. H., Jr, Houston L. L. Purification and properties of yeast histidinol phosphate phosphatase. Biochemistry. 1973 Jul 3;12(14):2591–2596. doi: 10.1021/bi00738a007. [DOI] [PubMed] [Google Scholar]
- Mitchell J. K., Fonzi W. A., Wilkerson J., Opheim D. J. A particulate form of alkaline phosphatase in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1981 Feb 13;657(2):482–494. doi: 10.1016/0005-2744(81)90333-8. [DOI] [PubMed] [Google Scholar]
- Montenecourt B. S., Kuo S. C., Lampen J. O. Saccharomyces mutants with invertase formation resistant to repression by hexoses. J Bacteriol. 1973 Apr;114(1):233–238. doi: 10.1128/jb.114.1.233-238.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Müller H. Synthesis and processing of in vitro and in vivo precursors of the vacuolar yeast enzyme carboxypeptidase Y. J Biol Chem. 1981 Dec 10;256(23):11962–11965. [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. The glycoprotein structure of yeast invertase. Biochemistry. 1969 Sep;8(9):3552–3556. doi: 10.1021/bi00837a010. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Onishi H. R., Tkacz J. S., Lampen J. O. Glycoprotein nature of yeast alkaline phosphatase. Formation of active enzyme in the presence of tunicamycin. J Biol Chem. 1979 Dec 10;254(23):11943–11952. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Schwaiger H., Hasilik A., von Figura K., Wiemken A., Tanner W. Carbohydrate-free carboxypeptidase Y is transferred into the lysosome-like yeast vacuole. Biochem Biophys Res Commun. 1982 Feb 11;104(3):950–956. doi: 10.1016/0006-291x(82)91341-9. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Nakamura H., Oshima Y. A gene controlling the synthesis of non specific alkaline phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1976 Mar 25;428(1):182–192. doi: 10.1016/0304-4165(76)90119-7. [DOI] [PubMed] [Google Scholar]