Abstract
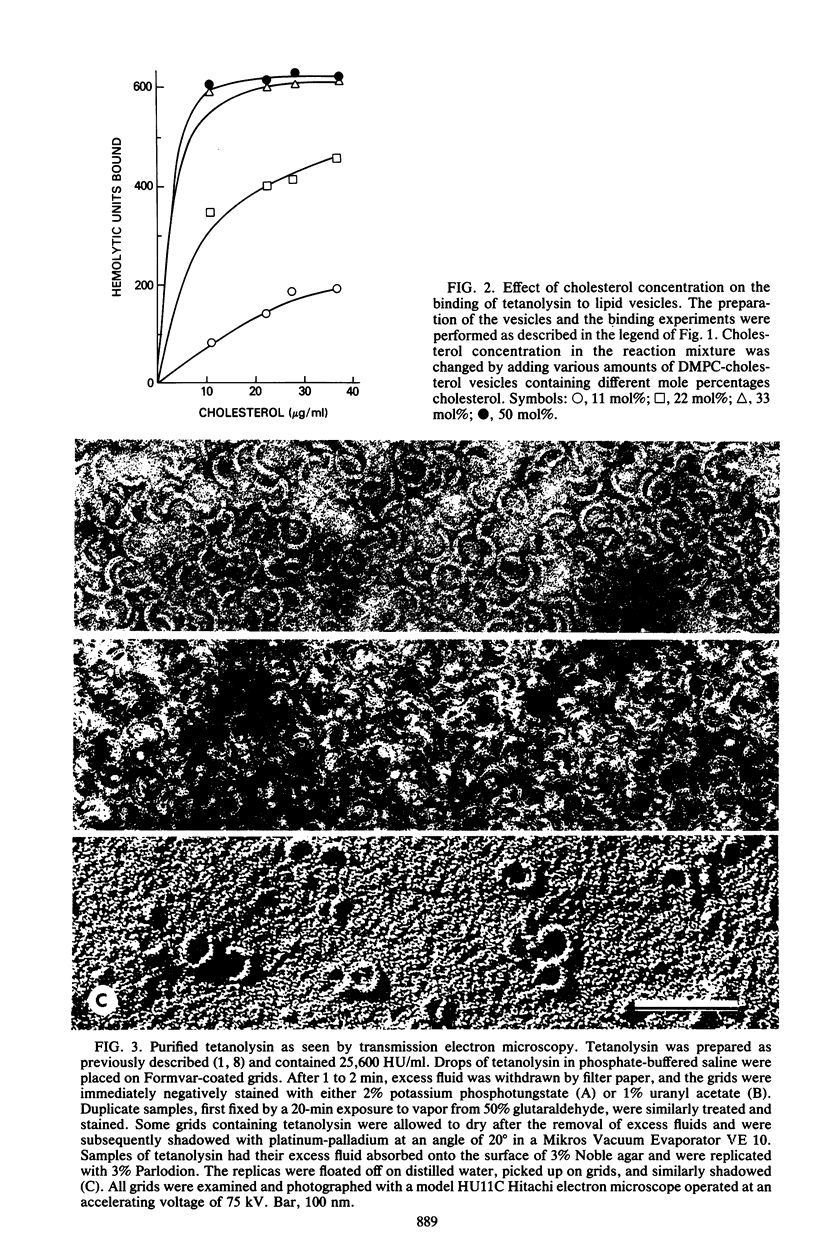
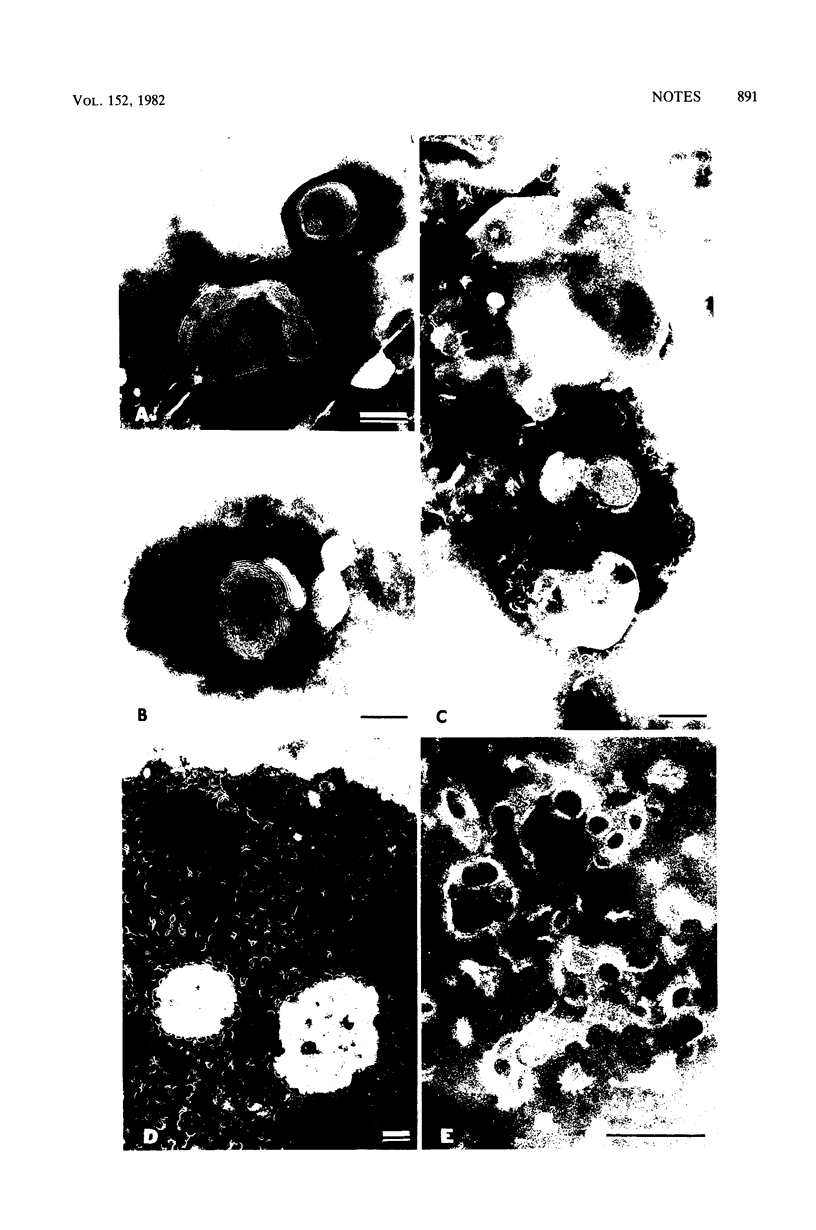
Tetanolysin binding to lipid vesicles was found to depend on the molar ratio of cholesterol to phospholipid, being low in vesicles containing up to 20 mol% cholesterol and high in vesicles containing more than 33 mol%. High concentrations of purified tetanolysin preparations formed arc- and ring-shaped structures. The structures were not readily detectable in diluted preparations unless incubated with lipid vesicles containing high molar ratios of cholesterol to phospholipid. It is suggested that the toxin is concentrated on the vesicles to local concentrations high enough to form the arcs and rings.
Full text
PDF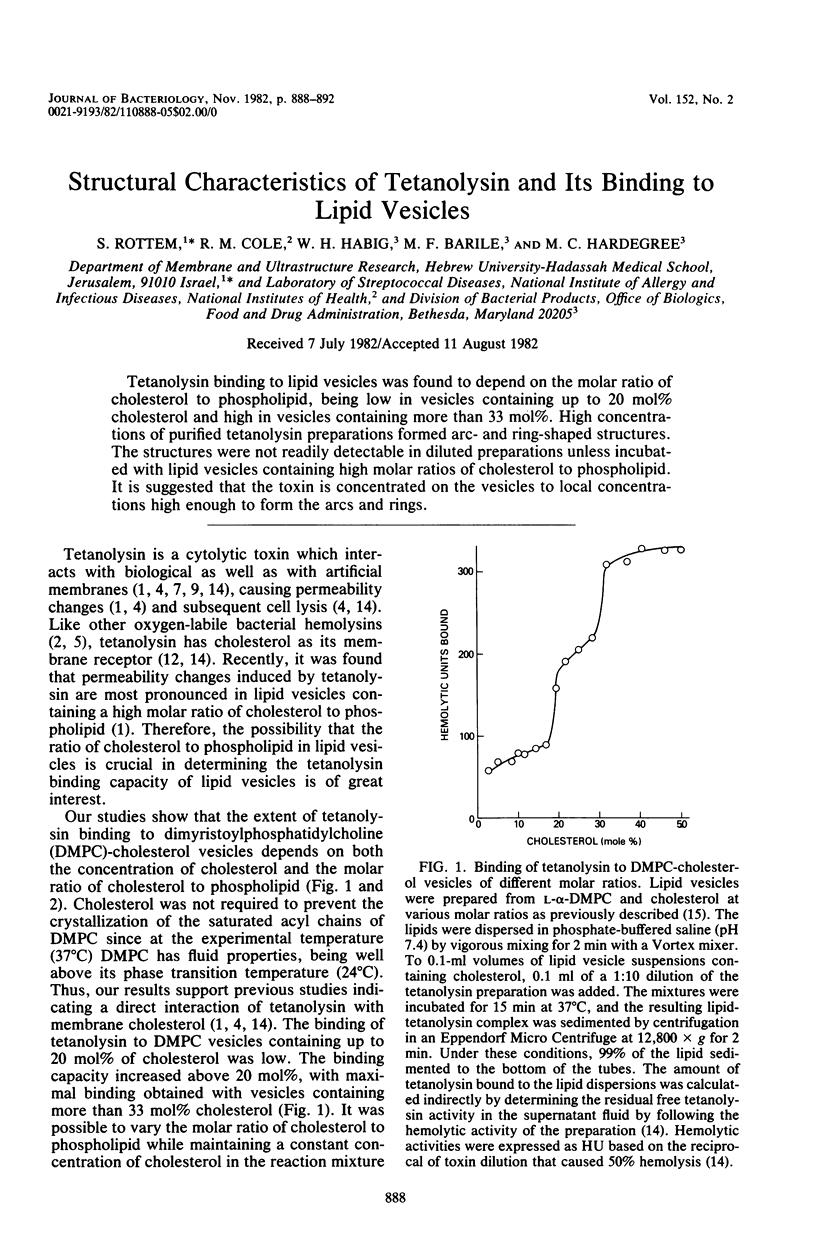


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Habig W. H., Urban K. A., Hardegree M. C. Cholesterol-dependent tetanolysin damage to liposomes. Biochim Biophys Acta. 1979 Feb 20;551(1):224–228. doi: 10.1016/0005-2736(79)90368-7. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Bernheimer A. W. Role of cholesterol in the action of cereolysin on membranes. Arch Biochem Biophys. 1978 Oct;190(2):603–610. doi: 10.1016/0003-9861(78)90316-8. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Kim K. S., Bernheimer A. W. Alteration by cereolysin of the structure of cholesterol-containing membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):230–241. doi: 10.1016/0005-2736(78)90419-4. [DOI] [PubMed] [Google Scholar]
- Cox C. B., Hardegree C., Fornwald R. Effect of tetanolysin on platelets and lysosomes. Infect Immun. 1974 Apr;9(4):696–701. doi: 10.1128/iai.9.4.696-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershfeld N. L. Equilibrium studies of lecithin-cholesterol interactions I. Stoichiometry of lecithin-cholesterol complexes in bulk systems. Biophys J. 1978 Jun;22(3):469–488. doi: 10.1016/S0006-3495(78)85500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEGREE M. C. SEPARATION OF NEUROTOXIN AND HEMOLYSIN OF CLOSTRIDIUM TETANI. Proc Soc Exp Biol Med. 1965 Jun;119:405–408. doi: 10.3181/00379727-119-30195. [DOI] [PubMed] [Google Scholar]
- Haberkorn R. A., Griffin R. G., Meadows M. D., Oldfield E. Deuterium nuclear magnetic resonance investigation of the dipalmitoyl lecithin-cholesterol-water system. J Am Chem Soc. 1977 Oct 26;99(22):7353–7355. doi: 10.1021/ja00464a043. [DOI] [PubMed] [Google Scholar]
- Hardegree M. C., Palmer A. E., Duffin N. Tetanolysin: in-vivo effects in animals. J Infect Dis. 1971 Jan;123(1):51–60. doi: 10.1093/infdis/123.1.51. [DOI] [PubMed] [Google Scholar]
- Melchior D. L., Scavitto F. J., Steim J. M. Dilatometry of dipalmitoyllecithin-cholesterol bilayers. Biochemistry. 1980 Oct 14;19(21):4828–4834. doi: 10.1021/bi00562a018. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Sekiya T., Okamura S., Nozawa Y., Hase J. Ring formation of perfringolysin O as revealed by negative stain electron microscopy. Biochim Biophys Acta. 1979 Dec 12;558(3):307–313. doi: 10.1016/0005-2736(79)90265-7. [DOI] [PubMed] [Google Scholar]
- Mitsui N., Mitsui K., Hase J. Purification and some properties of tetanolysin. Microbiol Immunol. 1980;24(7):575–584. doi: 10.1111/j.1348-0421.1980.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Rosenqvist E., Michaelsen T. E., Vistnes A. I. Effect of streptolysin O and digitonin on egg lecithin/cholesterol vesicles. Biochim Biophys Acta. 1980 Jul 16;600(1):91–102. doi: 10.1016/0005-2736(80)90414-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hardegree M. C., Grabowski M. W., Fornwald R., Barile M. F. Interaction between tetanolysin and Mycoplasma cell membrane. Biochim Biophys Acta. 1976 Dec 14;455(3):876–888. doi: 10.1016/0005-2736(76)90057-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Shinar D., Bittman R. Symmetrical distribution and rapid transbilayer movement of cholesterol in Mycoplasma gallisepticum membranes. Biochim Biophys Acta. 1981 Dec 21;649(3):572–580. doi: 10.1016/0005-2736(81)90161-9. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]