Abstract
The self-assembly of helical ribbons is examined in a variety of multicomponent enantiomerically pure systems that contain a bile salt or a nonionic detergent, a phosphatidylcholine or a fatty acid, and a steroid analog of cholesterol. In almost all systems, two different pitch types of helical ribbons are observed: high pitch, with a pitch angle of 54 ± 2°, and low pitch, with a pitch angle of 11 ± 2°. Although the majority of these helices are right-handed, a small proportion of left-handed helices is observed. Additionally, a third type of helical ribbon, with a pitch angle in the range 30–47°, is occasionally found. These experimental findings suggest that the helical ribbons are crystalline rather than liquid crystal in nature and also suggest that molecular chirality may not be the determining factor in helix formation. The large yields of helices produced will permit a systematic investigation of their individual kinetic evolution and their elastic moduli.
Interest in molecular self-assembly of helical structures is driven by both technological and medical applications. Helices are often precursors in the growth of tubules (1–4), which can be used as a controlled release system for drug delivery in medicine and as templates for microelectronics and magnetic applications (5). The morphology of the tubules must be rationally optimized for each application. Therefore it is important to understand the role of the various constituent molecules in the formation of these structures.
Helical ribbons have been observed in a variety of systems composed of chiral amphiphiles. Although the diameters and lengths of the helical structures varied from system to system, the pitch angle observed in all systems was either 45° (6–8) or ≈60° (9–12). In almost all cases, helical ribbons in enantiomerically pure systems were either right- or left-handed (6, 8, 13). Recently, however, Thomas et al. studied an enantiomerically pure phosphonate analogue of 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine and related compounds, which self-assembled into a mixture of right- and left-handed helical ribbons (10, 11).
A biologically important system in which helical ribbons form is model bile, consisting of a mixture of three types of chiral molecules in water: a bile salt, a phosphatidylcholine, and cholesterol (4, 14–21). Helical ribbons are metastable intermediates in the process of cholesterol crystallization in bile (2, 19, 20), which precedes cholesterol gallstone formation (4, 18–23). In contrast to all other systems studied, two pitch types of helical ribbons are observed in bile: high pitch, with a pitch angle of 54 ± 2°, and low pitch, with a pitch angle of 11 ± 2°. To date, the production of these two helical pitch types has been thought to be a property unique to model biles. Indeed, previous work showed that all three components of model bile are required for helical ribbons to form. In the absence of the phosphatidylcholine, only needle-like crystals form (4, 21), whereas without the bile salt only stable plate-like crystals are produced (24). Konikoff et al. (4, 21) also argued that phosphatidylcholine was necessary only for the initial stages of helix formation, because its removal did not affect preformed helical structures. Helix formation was also unaffected by the specific identity of phosphatidylcholine and bile salt used, although the kinetics of helix formation was affected dramatically (4, 15, 17, 20, 21, 25). Various experimental techniques including density gradient centrifugation, synchrotron x-ray diffraction, and conventional x-ray diffraction (4) were consistent with the hypothesis that cholesterol is the major constituent of helical ribbons. Cholesterol was inferred to determine the bilayer structure; hence, the existence of helices of two pitch types was attributed exclusively to the structure of cholesterol (2). However, direct substitution of cholesterol by its analogs to confirm this hypothesis has never been performed.
Current explanations of the self-assembly of helices are based on theories of multilamellar liquid crystal bilayers or hexatic phases (1–3). These theories assume that high and low pitch helices correspond to two different molecular packings within the bilayer and further that molecular chirality is the driving force behind helix formation (1–3, 26, 27). However, the results of Thomas et al. (10, 11) suggest that chirality is not the only factor in helix formation. We discuss the implications of our results for current theories of liquid crystalline bilayers of chiral amphiphiles.
In this report, we show that the formation of helical ribbons of at least two pitch types is a general phenomenon observed in a wide variety of multicomponent systems containing a sterol. The distinctive values of high and low pitch angles are also not unique to model biles, but are a general property of a whole range of multicomponent systems, which contain a micelle-forming surfactant, bilayer-forming amphiphiles, and a sterol. We describe our experiments on a variety of quaternary sterol systems (QSS), on a quaternary fatty acid system (QFAS), and on two lipid concentrate systems, as defined below. In addition to high and low pitch helical ribbons, almost all systems studied exhibited a third pitch type of helical ribbon with an intermediate pitch angle, whose value ranges between 30° and 47°. Although all the components that make up the systems studied were enantiomerically pure, specifically l-enantiomers, some systems produced both right- and left-handed helical ribbons. Left-handed helical ribbons, however, were not observed in QFAS or in lipid concentrate systems.
MATERIALS AND METHODS
All QSS were composed of the common bile salt sodium taurocholate (NaTC), one type of phosphatidylcholine, and a sterol in the molar ratio 97.5:0.8:1.7. NaTC was purchased from Sigma–Aldrich and was further purified by the method of Pope et al. (28). The following natural sterols were used: cholest-5-en-3β-ol (cholesterol), 5β-cholestan-3β-ol (coprostanol), 5β-cholestan-3α-ol (epicoprostanol), 5α-cholestan-3β-ol (cholestanol), cholesta-5,7-dien-3β-ol (7-dehydrocholesterol), ergosta-5,7,22-trien-3β-ol (ergosterol), 5α-lanosta-8,24-dien-3β-ol (lanosterol). Cholesterol (Nu Chek Prep, Elysian, MN) and other sterols (Sigma–Aldrich, St. Louis, MO) were used as received without further purification. Synthetic l-enantiomers of phosphatidylcholine used to prepare QSS were: 1,2-dioleoyl-glycero-3-phosphocholine (DOPC, sn-1–18:1-sn-2–18:1), 1,2-dielaidoyl-glycero-3-phosphocholine (DEPC, sn-1-trans18:1-sn-2-trans18:1), 1,2-dipalmitoyl-glycero-3-phosphocholine (DPPC, sn-1–16:0-sn-2–16:0), 1,2-dimyristoyl-glycero-3-phosphocholine (DMPC, sn-1–14:0-sn-2–14:0), 1,2-dilauroyl-glycero-3-phosphocholine (DLPC, sn-1–12:0-sn-2–12:0), dipalmitoleoyl phosphatidylcholine (DZPC, sn-1–16:1-sn-2–16:1), 1-palmitoyl-2-oleoyol-glycero-3-phosphocholine (POPC, sn-1–16:0-sn-2–18:1), 1-oleoyl-2-palmitoyl-glycero-3-phosphocholine (OPPC, sn-1–18:1-sn-2–16:0), 1-stearoyl-2-oleoyl-glycero-3-phosphocholine (SOPC, sn-1–18:0-sn-2–18:1), 1-oleoyl-2-stearoyl-glycero-3-phosphocholine (OSPC, sn-1–18:1-sn-2–18:0). All phosphatidylcholines and oleic acid were purchased from Avanti Polar Lipids. The QFAS was composed of NaTC, oleic acid, and cholesterol in the molar ratio 97.5:0.8:1.7. Phosphatidylcholines and oleic acid were used without further purification. Chemically defined lipid concentrate (CDLC) and lipid concentrate (LC) were purchased from GIBCO/BRL and observed without further manipulation. Table 1 summarizes the systems studied and their constituents.
Table 1.
The compositions of the systems studied
| System | Surfactant | Phospholipid or fatty acid | Sterol | Molar ratio % |
|---|---|---|---|---|
| QSS1 | NaTC | PC* | Cholesterol | 97.5:0.8:1.7 |
| QSS2 | NaTC | DOPC | St† | 97.5:0.8:1.7 |
| QFAS | NaTC | Oleic acid | Cholesterol | 97.5:0.8:1.7 |
| CDLC | TP‡ | FA1§ | Cholesterol | 94.2:1.9:3.9 |
| LC | TP | FA2¶ | Cholesterol | 75.5:6.4:18.1 |
One of the following phosphatidylcholines: DOPC, SOPC, DEPC, POPC, OSPC, OPPC, DZPC, DMPC, DPPC, DLPC.
One of the following sterols: cholesterol, coprostanol, epicoprostanol, cholestanol, 7-dehydrocholesterol, ergosterol, lanosterol.
A mixture of Tween 80 and Pluronic F-68.
Mixture of the following fatty acids: myristic, palmitic, stearic, palmitoleic, oleic, α-linoleic, linolenic, arachidonic.
Fatty acids in cod liver oil (29): myristic, palmitic, palmitoleic, oleic, stearic, α-linoleic, stearidonic, gadoleic, gondoic, 5,8,11,14,17-eicosapentaenoic, euricic, 7,10,13,16,19-docosapentaenoic, 4,7,10,13,16,19-docosahexanenoic.
Lipid films for each system were prepared as described by Konikoff et al. (4). Each lipid film was diluted in filtered (0.22-μm pore size filter) Milli-Q (Millipore) water at ambient temperature to a total lipid concentration of 70 mg/ml to obtain a system unsaturated with cholesterol. In previous work, Chung et al. (2) prepared model biles with 0.15 M NaCl to screen molecular charges and 3 mM NaN3 as an antibacterial agent. We did not add these salts to our systems, because we demonstrated helical ribbon production in their absence. To induce complete solubilization of cholesterol microcrystals, we incubated the unsaturated systems for 2 hr at 61°C. These systems were then filtered through a 0.22-μm Millex-GV4 filter (Millipore) and diluted 6-fold with Milli-Q water at ambient temperature to induce supersaturation. Supersaturated systems were incubated at 37°C, and 5-μl aliquots were removed daily to study structures formed. Frequent sampling did not affect the yield or variety of the structures formed.
The 5-μl aliquots were observed on glass slides by using a phase-contrast microscope (Diaphot-TMD, Nikon). Images were taken with a camera (Sony, DXC-970MD) and digitized with a built-in frame-grabber on a Power Macintosh computer. For image enhancement and measurements, we have used the public domain nih-image software package. Measurements of pitch angles were performed according to the method of Chung et al. (2). Because the apparent handedness of helices changed during a through-focus scan, the handedness of helical ribbons was unambiguously determined by positioning the microscope objective focal plane on the upper surface of the structures.
RESULTS
General Results.
To identify elements critical to the process of helix formation in various multicomponent systems (sterol, phosphatidylcholine/fatty acid, bile salt/nonionic surfactant), different components were systematically substituted. Various chiral constituents of multicomponent systems were substituted by nonchiral ones (Table 1): NaTC was substituted by a mixture of block copolymer Pluronic F-68 and nonionic surfactant Tween 80 (in CDLC and LC), various species of phosphatidylcholine were substituted by one or a mixture of fatty acids, and cholesterol was substituted by its chiral analogs. All the systems studied were chosen in such a way as to place them in the two-phase region of a phase diagram, where plate-like sterol crystals and saturated micelles coexist in an equilibrium state (19). Additionally, the concentrations of the components were such that the temporal crystallization sequences in all the systems studied would be the same (crystallization pathway A in ref. 19): filaments followed by helical wires that grew laterally to form helical ribbons, then tubules, and finally plate-like crystals.
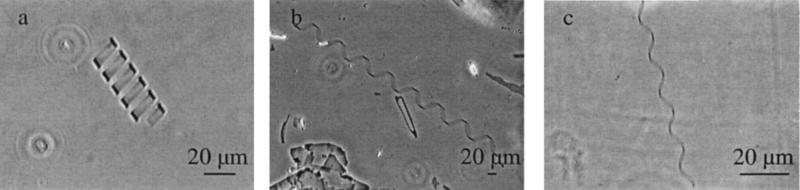
The majority of systems studied in our experiments produced high pitch and low pitch helices. The pitch angles of these helical ribbons were identical to those found by Chung et al. (2): 54 ± 2° for the high pitch and 11 ± 2° for the low pitch. Additionally, in almost all systems, we found small amounts of intermediate pitch helices. The value of the pitch angle of these helices depended on the surfactant, phosphatidylcholine (or fatty acid), or sterol species used and was in the range between 30° and 47°. Fig. 1 illustrates these three pitch types of helical ribbons formed in the CDLC system.
Figure 1.
Typical helical structures in CDLC system. (a) Low pitch helical ribbon with a pitch angle ψ = 11 ± 2°. (b) High pitch helical ribbons with a pitch angle ψ = 54 ± 2°. (c) Intermediate pitch helical ribbons with a pitch angle ψ = 40.8 ± 3.8°.
The temporal sequence of the appearance of helical structures was the same in all systems studied. High pitch helices typically formed on the first day of incubation and were stable for 2–5 d. Within 5–8 d of incubation, intermediate pitch helical ribbons formed and remained in solution for 1–3 d. These were followed by the independent formation of low-pitch helical ribbons that were stable in solution for up to 3 wk, depending on the species of constituents used. In agreement with Chung et al. (2), we never observed a direct transition from helical ribbons of one pitch to helical ribbons of another pitch in any of the systems studied. The diameters of helices observed in all systems were in the range between 3 μm and 70 μm.
We further consider the specific behavior of the QSS, the QFAS, and the LC systems.
QSS.
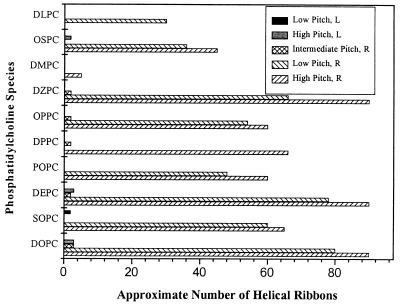
Varying the phosphatidylcholine and sterol species (Table 1) affected the number of helical structures formed, the kinetics of their formation, and their handedness. Fig. 2 displays the approximate number of each pitch type of helix observed in a typical microscopic field of view (×60) at the time of maximum yield for QSS with various phosphatidylcholines. As can be seen in this figure, almost all QSS formed both right-handed high and low pitch helices. The two exceptions were QSS with DMPC and DLPC, in which helical ribbons of only high or low pitch, respectively, were found. Additionally, QSS with DOPC, DEPC, DPPC, OPPC, and DZPC formed right-handed intermediate pitch structures. We also observed left-handed helical ribbons in QSS with DOPC, SOPC, DEPC, and OSPC. Consistent with the observations of Chung et al. (2), all helical ribbons, without exception, were right-handed in QSS with POPC. Contrary to the results of Konikoff et al. (18), our experimental technique did yield helical ribbons in quaternary sterol system with DLPC. We also found that QSS with DPPC produced low quantities of helical ribbons in the first 5 d of formation.
Figure 2.
Approximate number of helical ribbons per typical field of view (×60, i.e., a field of view of approximately 0.071 mm2) at the time of maximum yield in QSS with cholesterol and various phosphatidylcholines (see Table 1). Striped columns represent right-handed helices; solid columns represent left-handed helices.
The sterols in the QSS were chosen to investigate the effects of their structure on helix formation. To study the influence of a planar sterol nucleus, sterols with a double bond at the 5-position (cholesterol, 7-dehydrocholesterol, ergosterol) and sterols with either 5α (cholestanol, lanosterol) or 5β (coprostanol, epicoprostanol) hydrogens were chosen. We also investigated the influence of the 3β-hydroxy group (cholesterol, coprostanol, cholestanol, 7-dehydrocholesterol, ergosterol, lanosterol) as compared with the 3α-hydroxy group (epicoprostanol). The effects of the sterol side chains on formation of helical ribbons were also studied by comparing sterols with saturated side chains (cholesterol, coprostanol, epicoprostanol, cholestanol, and 7-dehydrosterol) and sterols with unsaturated side chains (ergosterol and lanosterol). For these QSS, DOPC was used, because this phosphatidylcholine species produced the largest long-lived yields of both high and low pitch helical ribbons in QSS with cholesterol (Table 1).
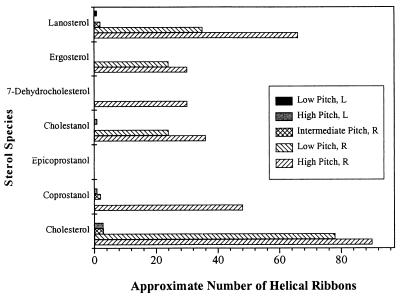
Fig. 3 displays the approximate number of helical ribbons as a function of sterol species in a typical field of view. With the exception of epicoprostanol, all sterol systems formed right-handed helical ribbons of at least one pitch type, either of high or low pitch angle. Right-handed intermediate pitch helical ribbons were observed in QSS with cholesterol, coprostanol, and lanosterol. Left-handed helical ribbons were observed only in QSS with cholesterol, coprostanol, cholestanol, and lanosterol. The only metastable structures formed in the sterol system with epicoprostanol, however, were short straight nonflexible filaments 3–5 μm long (this is in contrast to long flexible filaments formed in all other systems, which were 80 μm long).
Figure 3.
Approximate number of helical ribbons per typical field of view (×60) in QSS with DOPC and various sterols. Striped columns represent the number of right-handed helical structures; solid columns represent the number of left-handed helices.
In addition, QSS with cholesterol, coprostanol, and lanosterol also formed intermediate pitch helices, whose pitch angle depended on the particular sterol species used. Fig. 3 indicates that, in addition to right-handed helical structures, we also found small quantities (one to two structures per typical field of view) of left-handed helices in QSS with cholesterol, coprostanol, cholestanol, and lanosterol.
The Lipid Concentrate Systems (CDLC and LC) and QFAS.
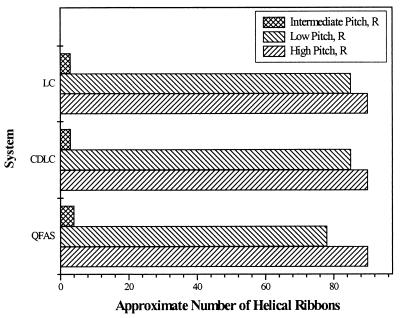
We discovered a new class of systems, lipid concentrates, that also form helical ribbons of high, low, and intermediate pitch angles. We studied two concentrated lipid emulsions, CDLC and LC. Though CDLC and LC are multicomponent systems, they are analogous to the QSS in that they are mixtures of three groups of components, surfactants (which serve a role of the bile salt), fatty acids (which serve a role of a phosphatidylcholine), and cholesterol (Table 1). Despite the fact that cholesterol is the only chiral component in CDLC and LC, we observed the formation and evolution of the same structures seen in the QSS: helical ribbons of high, low, and intermediate pitch angles. The approximate numbers of helices of high and low pitch angles are displayed in Fig. 4. All helical ribbons in CDLC and LC were right-handed, without exception.
Figure 4.
Approximate number of right-handed helical ribbons of high, low, and intermediate pitch angle per typical field of view (×60) in the QFAS and in the lipid concentrate systems, CDLC and LC.
We also prepared a simpler system, the QFAS. This system is a mixture of NaTC, oleic acid, and cholesterol in water. The molar ratio of the components in the QFAS is identical to that of the components in the QSS (Table 1). In this case, both cholesterol and bile salt are chiral species in the fatty acid system, whereas oleic acid is achiral. As displayed in Fig. 4, we observed the formation and evolution of helical ribbons with the same values of pitch angles, high, low, and intermediate. All of the helical ribbons found in this system were right-handed, identical to the lipid concentrate systems. The yields of the structures were very high, as in the quaternary sterol system with DOPC and cholesterol (Fig. 4).
DISCUSSION
The formation of helical structures exhibiting two distinct pitch angles has previously been regarded as a phenomenon unique to model bile systems. The present work, on the other hand, reveals that helical ribbons of two distinct pitch angles can be formed in a wide variety of multicomponent systems containing alternative sterols, including QSS, QFAS, and a set of lipid concentrate systems (CDLC, LC). It is important to recognize that with the current improved experimental techniques, we have achieved very high yields of self-assembled helical ribbons. The concentration of helices in the majority of systems studied, some of which are readily available, is typically a factor of 10 greater than that found previously in the model bile system. This high concentration greatly facilitates the detection, observation, and isolation of individual helical ribbons.
The distinctive values of 54 ± 2° for the high pitch and 11 ± 2° for the low pitch helical ribbons were similarly regarded as a special property of model biles. However, we find that helical ribbons of precisely these pitch angles form in almost all the systems studied regardless of surfactant, phosphatidylcholine (fatty acid), or sterol component. We also observe intermediate pitch helices with pitch angle in the range between 30° and 47° in almost all of the above systems. However, these helices represent less than 10% of the total helical ribbon concentration.
To explain the observed values of the pitch angles, current theories model the helices as multilamellar liquid crystal bilayers, in which the constituent molecules have orientational but not positional order (1–3). The existence of two (or more) helical pitch types with different pitch angles was thought to result from different molecular packings within a bilayer of each helical pitch type. We have found that all the diverse helix-forming systems produce exactly the same high and low pitch angles. It is very unlikely that the same two (or more) packings occur for each of the diverse molecular species in the various systems investigated.
Our work also raises questions about the role of molecular chirality in the formation of helices. Current theories (1, 26, 27) hold that the handedness of the macroscopic ribbon or tubule is a reflection of the chirality of the underlying constituent molecules. However, we find that in seven of the sixteen QSS studied, in which all the components are enantiomerically pure, both right- and left-handed helices are formed. This is consistent with the work of Thomas et al. (10, 11), who find both right- and left-handed helices in an enantiomerically pure system of phosphonate analogue of 1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine. It is to be noted that the left-handed helices in our systems typically represent less than 5% of the total helical ribbons observed (Figs. 2 and 3). In QFAS, CDLC, and LC, the only chiral component is cholesterol. Interestingly, we find only right-handed helices in these systems.
The experiments of Konikoff et al. (4, 21) in model bile show that filaments, the precursors of helical ribbons, are a polymorph of cholesterol monohydrate and anhydrous cholesterol covered by a monolayer of phosphatidylcholine. Furthermore, the helical ribbons themselves are precursors to stable cholesterol monohydrate crystals. This suggests that helical ribbons may actually have a crystalline, positionally ordered, molecular structure. If we model the helices as thin anisotropic crystalline ribbons, then it can be shown that in general there are two preferred directions for bending (30). If such thin anisotropic crystalline ribbons have a propensity to bend, helices of two pitches are predicted to form. In this picture, both the high and low pitch helices could emerge from a single underlying molecular structure.
Finally, we found that neither the specific structure of phosphatidylcholines and fatty acids nor the presence of charged groups on surfactant molecules affects helix formation. However, the differences in the structures of the sterols do affect formation of helices. Our results suggest that if both a nonplanar sterol nucleus and a 3α-hydroxy group are present, then helices will not form (epicoprostanol). Further experiments need to be performed to investigate whether a 3β-hydroxy group is critical for the formation of helical ribbons (i.e., a sterol possessing a planar sterol nucleus and a 3α-hydroxy group, as in epicholestanol). On the other hand, the sterol side chain does not seem to affect the formation of helices. If indeed helical ribbons are crystalline in nature, the crystal structures of the sterols for which helices are formed should be similar. Furthermore, any change in the sterol structure that affects crystal formation should affect helix formation. The only crystallographic data available, to our knowledge, is that for anhydrous cholesterol, cholesterol monohydrate, and cholestanol dihydrate (ref. 31 and references therein). All of these crystals are triclinic in nature (space group P1). It is also known that cholesterol crystals (both anhydrous and monohydrate) are of characteristic bilayer nature, with a molecular arrangement similar to that of cholesterol in biological membranes (32). Such data are, however, not available for comparison for any of the other sterols used in our experiments. It would be interesting to have the crystallographic data for all sterols in our experiments to see whether and how their crystallographic space groups and the molecular arrangement within crystals affect helix formation.
Our experimental finding that a variety of readily available multicomponent systems consisting of a surfactant, a phosphatidylcholine or a fatty acid, and a sterol can produce large yields of high and low pitch helices will greatly facilitate further investigations. These include the isolation, removal, and observation of the growth and dissolution of individual ribbons in an environment in which the concentrations of individual solute species and temperature can be controlled. Furthermore, direct investigations of the elastic properties of these helices could be performed by tethering them, applying well-defined stresses, and measuring the associated strain. When the elastic properties of these microsprings are known, they may well prove to be useful micromechanical devices. Such devices could be used for the measurement of the forces and energies associated with interacting biological macromolecules such as molecular motors and bioreceptors. Conceivably, these structures may also prove useful for the quantitative characterization of the elastic properties of biological structures such as one-dimensional chains and two-dimensional membranes.
Acknowledgments
We thank Dr. A. L. Biere of Amgen Corporation for drawing our attention to the presence of helices in the CDLC system. We also thank Prof. M. C. Carey, Dr. D. S. Chung, Dr. G. M. Thurston, and Mr. E. Kangas for very helpful discussions. Special thanks are sent to Ms. A. Jackson for her able assistance in sample preparation. We gratefully acknowledge the support of the Office of Naval Research under grant N00014-95-1-0871 and Veterans Affairs Research Funding.
ABBREVIATIONS
- CDLC
chemically defined lipid concentrate
- LC
lipid concentrate
- QSS
quaternary sterol systems
- QFAS
quaternary fatty acid system
- NaTC
sodium taurocholate
- DOPC
1,2-dioleoyl-glycero-3-phosphocholine
- DEPC
1,2-dielaidoyl-glycero-3-phosphocholine
- DPPC
1,2-dipalmitoyl-glycero-3-phosphocholine
- DMPC
1,2-dimyristoyl-glycero-3-phosphocholine
- DLPC
1,2-dilauroyl-glycero-3-phos-phocholine
- DZPC
dipalmitoleoyl phosphatidylcholine
- POPC
1-palmitoyl-2-oleoyol-glycero-3-phosphocholine
- OPPC
1-oleoyl-2-palmitoyl-glycero-3-phosphocholine
- SOPC
1-stearoyl-2-oleoyl-glycero-3-phosphocholine
- OSPC
1-oleoyl-2-stearoyl-glycero-3-phosphocholine
References
- 1.Selinger J V, MacKintosh F C, Schnur J M. Phys Rev E. 1996;53:3804–3818. doi: 10.1103/physreve.53.3804. [DOI] [PubMed] [Google Scholar]
- 2.Chung D S, Benedek G B, Konikoff F M, Donovan J M. Proc Natl Acad Sci USA. 1993;90:11341–11345. doi: 10.1073/pnas.90.23.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komura S, Zhong-can O Y. Phys Rev Lett. 1998;81:473–476. [Google Scholar]
- 4.Konikoff F M, Chung D S, Donovan J M, Small D M, Carey M C. J Clin Invest. 1992;90:1155–1160. doi: 10.1172/JCI115935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnur J M. Science. 1993;262:1669–1675. doi: 10.1126/science.262.5140.1669. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima N, Asakuma S, Kunitake T. J Am Chem Soc. 1985;107:509–510. [Google Scholar]
- 7.Georger J H, Singh A, Price R R, Schnur J M, Yager P, Schoen P E. J Am Chem Soc. 1987;109:6169–6175. [Google Scholar]
- 8.Fuhrhop J-H, Schnieder P, Boekema E, Helfrich W. J Am Chem Soc. 1988;110:2861–2867. [Google Scholar]
- 9.Yager P, Schoen P E. Mol Cryst Liq Cryst. 1984;106:371–381. [Google Scholar]
- 10.Thomas B N, Corcoran R C, Cotant C L, Lindemann C M, Kirsch J E, Persichini P J. J Am Chem Soc. 1998;120:12178–12186. doi: 10.1021/ja012137a. [DOI] [PubMed] [Google Scholar]
- 11.Thomas B N, Lindemann C M, Clark N M. Phys Rev E. 1999;59:3040–3047. [Google Scholar]
- 12.Servuss R M. Chem Phys Lipids. 1988;46:37–41. [Google Scholar]
- 13.Singh A, Burke T G, Calvert J M, Georger J H, Herendeen B, Price R R, Schoen P E, Yager P. Chem Phys Lipids. 1988;47:135–148. [Google Scholar]
- 14.Kaplun A, Talmon Y, Konikoff F M, Rubin M, Eitan A, Tadmor M, Lichtenberg D. FEBS Lett. 1994;340:78–82. doi: 10.1016/0014-5793(94)80176-2. [DOI] [PubMed] [Google Scholar]
- 15.Ochi H, Tazuma S, Kajiyama G. Biochem J. 1996;318:139–144. doi: 10.1042/bj3180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao S, Tazuma S, Kajiyama G. Biochim Biophys Acta. 1993;1167:142–146. doi: 10.1016/0005-2760(93)90154-2. [DOI] [PubMed] [Google Scholar]
- 17.Tazuma S, Ochi H, Teramen K, Yamashita Y, Horikawa K, Miura H, Hirano N, Sasaki M, Aihara N, Hatsushika S, et al. Biochim Biophys Acta. 1994;1215:74–78. doi: 10.1016/0005-2760(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 18.Konikoff F M, Cohen D E, Carey M C. J Lipid Res. 1994;35:60–70. [PubMed] [Google Scholar]
- 19.Wang D Q-H, Carey M C. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 20.Ringel Y, Somjen G J, Konikoff F M, Rosenberg R, Gilat T. Biochim Biophys Acta. 1998;1390:293–300. doi: 10.1016/s0005-2760(97)00192-6. [DOI] [PubMed] [Google Scholar]
- 21.Konikoff F M, Cohen D E, Carey M C. Mol Cryst Liq Cryst A. 1994;248:291–296. [Google Scholar]
- 22.Cabral D J, Small D M. Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts. Bethesda, MD: Am. Physiol. Soc.; 1989. , Section 6, pp. 621–662. [Google Scholar]
- 23.Carey M C. In: Bile Acids in Health and Disease. Northfield T C, Jazrawi R P, Zentler-Munro P L, editors. Dordrecht, the Netherlands: Kluwer; 1988. pp. 61–82. [Google Scholar]
- 24.Collins J J, Phillips M C. J Lipid Res. 1982;23:291–298. [PubMed] [Google Scholar]
- 25. Jungst D, Lang T, Huber P, Lange V, Paumgartner G. J Lipid Res. 1993;34:1457–1464. [PubMed] [Google Scholar]
- 26.Nandi N, Bagchi B. J Am Chem Soc. 1996;118:11208–11216. [Google Scholar]
- 27.Nandi N, Bagchi B. J Phys Chem A. 1997;101:1343–1351. [Google Scholar]
- 28.Pope J L. J Lipid Res. 1967;8:146–147. [PubMed] [Google Scholar]
- 29.Gunstone F. Fatty Acid and Lipid Chemistry. 1st Ed. Glasgow, Scotland: Blackie Academic and Professional; 1996. pp. 61–86. [Google Scholar]
- 30.Landau L D, Lifshitz E M. Theory of Elasticity. 3rd Ed. Vol. 7. Oxford: Pergamon; 1986. pp. 38–87. [Google Scholar]
- 31.Goad L J, Akishia T. Analysis of Sterols. 1st Ed. Glasgow, Scotland: Blackie Academic and Professional; 1997. pp. 277–282. [Google Scholar]
- 32.Shieh H-S, Hoard L G, Nordman C E. Acta Crystallogr B. 1981;37:1538–1543. [Google Scholar]