Abstract
Ciliated epithelium represents the first line of host defense against lung infection. Most alcoholics smoke and are at high risk for developing lung infections. We reported that cigarette smoke activates protein kinase C (PKC) and alcohol desensitizes ciliary beat frequency (CBF) to β-agonists in bovine bronchial epithelial cells in vitro. The combined effect of smoke and alcohol exposure on mouse ciliated tracheal epithelium has not been studied in vivo. We hypothesized that previously observed in vitro effects of smoke and alcohol exposure could be replicated in vivo. Female C57BL/6 mice were exposed to whole body cigarette smoke only, 20% alcohol ad libitum in drinking water only, or the combination of cigarette smoke plus alcohol for 6 wk. Bronchoalveolar lavage (BAL) cell populations, CBF, and airway kinase activity were assessed. Total BAL cells were decreased in animals exposed to alcohol alone and increased in animals exposed to smoke alone. Mice receiving smoke and alcohol had cell levels similar to smoke alone. Baseline CBF was not affected in any group; however, isoproterenol stimulation of CBF was blunted by alcohol exposure and actually slowed below baseline in the smoke plus alcohol group. Isoproterenol-induced PKA activity was inhibited in mice receiving alcohol independent of smoke exposure. Smoke activated PKC independent of alcohol. The isoproterenol-induced slowing below baseline of CBF after combined smoke and alcohol exposure demonstrates a novel ciliary impairment likely related to the combination of alcohol-mediated PKA desensitization and smoke-stimulated PKC activation, possibly through acetaldehyde present in the vapor phase of cigarette smoke.
Keywords: cilia, cigarette smoke, alcohol, ethanol, cAMP
The lung is exposed to inhaled toxins and pathogens as a consequence of breathing in particle-laden air. The coordinated secretion of airway mucus along with synchronized ciliary beating provides a protective mucociliary barrier to such inhaled substances and microorganisms, with the ciliated bronchial-tracheal epithelium acting as the motor cell driving this primary host defense of the lung (1). The frequency of beating cilia can be changed in response to stress and during exercise (2), by temperature, pH, particle exposure or through the action of various ciliostimulatory compounds such as β-adrenergic receptor agonists (3). Cyclic AMP–dependent protein kinase (PKA) plays an important role in the up-regulation of ciliary beat frequency (CBF) during β-agonist stimulation (4). Conversely, protein kinase C (PKC) has been associated with an inhibitory effect on CBF (5). Impairment of ciliary motility may contribute to airway disease, since pathogens not properly cleared from the airways can stimulate a chronic inflammatory response (6).
CLINICAL RELEVANCE
The dual actions of alcohol desensitization on PKA-mediated ciliostimulatory pathways and cigarette smoke–mediated activation of PKC cilioinhibition combine to impair ciliary function more than either exposure alone.
Alcohol has been identified as an agent capable of impairing ciliary motility (7). Likewise, heavy alcohol consumption is associated with increased risk of pneumonia (8). Cigarette smoke has long been known to impair cilia beating (9). Because 80–95% of alcoholics smoke cigarettes (10), we think it is highly relevant to study the mechanisms of cilia dysfunction within the context of combined cigarette smoke and alcohol exposure to model the pathophysiology of alcohol in the lung. In organ systems such as the upper digestive tract (11), kidney (12), liver (13), and pancreas (14), the combination of cigarette smoke and alcohol exposure has produced unique effects not observed by either exposure alone. The lung represents a likely target for the effects of co-exposure, as the airways are directly exposed to inhaled cigarette smoke and exhaled alcohol.
Our previous in vitro studies have shown that cigarette smoke extract (CSE) increases PKC activity in human and bovine bronchial epithelial cells (15). While brief alcohol exposure can rapidly stimulate ciliary beating (16), via a nitric oxide and PKA-dependent pathway (17), prolonged alcohol exposure blocks β-agonist stimulation of PKA activity and ciliary beating in cell cultures (18). We obtained similar results using a rat model of smoke and alcohol co-exposure with Streptococcus pneumoniae infection (19). In this rat model, impairment of bacterial clearance correlated with decreased cilia beating and increased PKC activity (20). The purpose of the present study was to determine the impact of combined cigarette smoke and alcohol exposure on kinase-driven ciliary function and lung inflammatory cell accumulation. We hypothesized that combined smoke and alcohol treatment would have unique effects on ciliary motility and kinase activity in the tracheal epithelium different from either exposure alone. In this study we report that the dual actions of alcohol desensitization on PKA-mediated ciliostimulatory pathways and cigarette smoke–mediated activation of PKC cilioinhibition combine to impair ciliary function more than either exposure alone.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) at 7–8 wk of age and maintained at the Omaha VA Animal Research Facility (Omaha, NE) under standard housing conditions. Mice were acclimated to the facility for 1 wk before the start of exposure and received standard rodent chow ad libitum for the entire course of the study. Mice were monitored daily and weighed weekly. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Omaha Veterans Affairs Medical Center. All protocols conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Experimental Design
After a 1-wk acclimation period, mice were randomly assigned to one of five groups (Table 1).
TABLE 1.
TREATMENT GROUPS
| Group No. | Name | Sham (Air) | Cigarette Smoke | Water | 20% Ethanol in Water | BAC (mg/dl ± SEM) |
|---|---|---|---|---|---|---|
| 1 | Control | − | − | + | − | 0.3 ± 0.2 |
| 2 | Air | + | − | + | − | 0.7 ± 0.4 |
| 3 | Air + Alcohol | + | − | − | + | 133.4 ± 53.0 |
| 4 | Smoke | − | + | + | − | 1.4 ± 0.2 |
| 5 | Smoke + Alcohol | − | + | − | + | 148.9 ± 46.9 |
Definition of abbreviation: BAC, blood alcohol content.
Group 1: Control
To determine handling versus treatment effects, this group of mice received no treatment or handling of any kind aside from regular weighing and cage changing.
Group 2: Air
Mice in this group received exposure to air in an apparatus similar to the one used for smoke exposure (sham treatment) and were given water.
Group 3: Air + Alcohol
These mice received the sham treatment and were given 20% alcohol (wt/vol) in water.
Group 4: Smoke
Mice in this group were exposed to cigarette smoke and given water.
Group 5: Smoke + Alcohol
Mice in this group received both cigarette smoke exposure and 20% alcohol (wt/vol) in water. The number of cigarettes and concentration of alcohol were gradually increased over a 2-wk period, until the target treatments of 20 cigarettes/d and 20% alcohol (wt/vol) were reached (Table 2). Target treatment was performed for 6 wk. After that time, mice were killed and treatment affects on BAL cell numbers, ciliary beat and responsiveness to β-agonist stimulation, and kinase activities in the upper airway epithelium were assessed.
TABLE 2.
EXPERIMENTAL DESIGN FOR ALCOHOL AND CIGARETTE SMOKE EXPOSURE
| Week 1 | Week 2 | Weeks 3–8 | ||
|---|---|---|---|---|
| Time | 2 d | 5 d | 1 wk | 6 wk |
| Percentage of ethanol in water | 5 | 10 | 15 | 20 |
| Number of cigarettes | Gradual increase in number of cigarettes/day | 20 cigarettes/day | ||
Cigarette Smoke and Air Exposure
Mice were exposed to the smoke of 20 1R3F reference cigarettes (University of Kentucky, Lexington, KY) per day. Mice receiving cigarette smoke were gradually brought up to the target exposure in a period of 2 wk and treated 6 d/wk for 6 wk. Treatment was administered by placing mice in a plexiglass smoking chamber measuring 65 cm long, 50 cm wide, and 45 cm high. The smoking chamber was divided into three sections (1). The first section, 10 cm in width, contained two holes to the outside to hold the lit cigarettes and draw in mainstream smoke, an outer hood and vent to capture the sidestream smoke, and a fan to mix the smoke with air before it reached the middle chamber through a set of inner holes. The mice were placed in the middle chamber in separate cages, 3–4 to a cage, containing several 1.0-cm holes on all sides of the cages to ensure adequate and equal exposure. Cages were rotated within the chamber for each treatment. Smoke was pulled through the middle chamber into a third chamber before being drawn out of the exposure apparatus using a vacuum pump. Cigarettes were lit two at a time and burned for 6–7 min. This was followed by a cycle of filtered air of the same length. Treatment was repeated until all 20 cigarettes were burned (~ 1–1.5 h). Sham treatments were conducted in the same manner in a similar apparatus for the same periods of time, but mice were exposed to filtered room air only. To ensure adequate cigarette smoke exposure, blood carboxyhemoglobin (COHb) levels were measured using an IL-682 CO-Oximeter (Instrumentation Laboratories, Lexington, MA). COHb levels did not differ between water-and alcohol-receiving groups. Cigarette smoke–exposed mice had COHb levels of 10.14 ± 0.27%, whereas the air-exposed mice had COHb levels of 0.53 ± 0.07%.
Alcohol Feeding
Mice receiving alcohol were given increasing concentrations of ethanol in water over a 2-wk period until the target concentration of 20% was reached. Mice in the alcohol group were given 5% alcohol (wt/vol) to drink ad libitum (95% ethanol diluted with Milli-Q water) for 2 d, 10% ethanol (wt/vol) for 5 d, 15% ethanol (wt/vol) for 7 d, and 20% ethanol (wt/vol) for 6 wk, in a manner similar to that previously described (2). Mice in the matched control group were given water from the same source without ethanol. All durations of alcohol exposure indicated in the remaining text refer to the time spent on the final 20% alcohol concentration. To ensure adequate intake of alcohol, blood alcohol concentrations (BAC) were measured using a kit by Pointe Scientific (Canton, MI). Average BAC were 149 ± 47 mg/dl in the alcohol-fed groups with no significant average difference between sham and smoke exposure groups. BAC varied from mouse to mouse, but levels as high as 445.7 mg/dl were detected in the alcohol groups. The alcohol-exposed mice were observed to move normally around the cage at some times and to stumble around in a likely intoxicated state at a other times. Several of the alcohol-drinking mice appeared to “pass out,” which was coincident with very high BACs. Indeed, BACs recorded in “passed out” mice were as high as 446 mg/dl, which mirrors BACs recorded in heavy drinkers (21).
Bronchoalveolar Lavage
Mice were killed by pentobarbital overdose. Pentobarbital has been shown to have some effect on ciliary activity and mucociliary clearance (22, 23). The time between trachea harvest and ring cutting, as well as equilibration time after cutting, was sufficient to remove the anesthetic from the tissues, as normal responses and values were obtained for the control and air (sham)-exposed mice. The tracheas were exposed and a 24-gauge (3/4-in) cannula was inserted at the bottom of the larynx to perform the bronchoalveolar lavage (BAL). The proximal end of the trachea was tied off and 0.6 ml of sterile PBS (Gibco, Grand Island, NY) was gently introduced to the lungs and then aspirated. This was repeated three times for a total volume of 1.8 ml. Return volume varied by < 10% between samples. BAL fluid was centrifuged at 250 × g to collect cells. Cells were resuspended and cell populations were determined using cytospin preparations (Cytopro Cytocentrifuge; Wescor Inc, Logan, UT) stained with DiffQuik (Dade Behring, Newark, DE). Differential counts were made with ≥ 400 cells/sample and two slides per mouse.
Trachea Harvesting and Treatment
After BAL, tracheas were removed and maintained in a closed sterile 15-ml conical tube in serum-free M199 containing penicillin and streptomycin (100 units/100 µg per ml) (Gibco) and fungizone (2 µg/ml; Gibco) at room temperature until processing (30–60 min). Tracheal rings were cut (0.5-mm width) from the distal end of the trachea just above the carina. The rings were placed in Petri dishes containing serum-free M199 (Gibco) for CBF determinations. After baseline CBF determination, both the rings and the remaining tracheal tissue were stimulated with isoproterenol at a final concentration of 100 µM. The rings and tissue were incubated for 30 min at 37°C, 5% CO2 then allowed to equilibrate at 25°C for 10 min. The final CBF reading was taken from the tracheal rings. The remaining tracheal tissue was opened with a longitudinal cut to expose the ciliated epithelium and placed in a Petri dish containing serum-free M199. This tissue was removed from the media and the ciliated epithelium was gently scraped into cell lysis buffer as described (4). The epithelial lysate was then immediately flash-frozen in liquid nitrogen for kinase assays.
Ciliary Beat Frequency
The motion of the actively beating cilia on the tracheal ring was quantified using phase contrast microscopy and computerized frequency spectrum analysis. During CBF measurement, tracheal rings were maintained at a constant temperature (24 ± 0.5°C) by a thermostatically controlled heated stage, as the temperature gradient is known to affect CBF (3). All observations were recorded for analysis using a Kodak 310 digital video camera (Eastman Kodak Motion Analysis System Division, San Diego, CA). CBF was determined by whole-field analysis of video images sampled for 3 s at 85 frames/s and performing frequency spectrum analysis using the Sisson-Ammons Video analysis (SAVA) system (4).
PKA Activity
PKA activity was measured as described previously (5). Briefly, a portion of the epithelial cell lysates was sonicated and centrifuged at 10,000 × g for 30 min at 4°C. The supernatant fraction was collected and assayed. The assay employed is a modification of procedures previously described (6), with 130 µM PKA substrate heptapeptide (LRRASLG), 10 µM cAMP, 0.2 mM isobutylmethyxanthine, 20 magnesium-acetate, and 0.2 mM [γ-32P] adenosine triphosphate in a 40-mM Tris-HCl buffer (pH 7.5). Samples (20 µl) were added to 50 µl of the above reaction mixture and incubated for 15 min at 30°C. Incubations were halted by spotting 50 µl of each sample onto P-81 phosphocellulose papers (Whatman, Hillsboro, OR). Papers were then washed five times for 5 min each in phosphoric acid (75 mM), washed once in ethanol, dried, and counted in nonaqueous scintillant as previously described (7). Negative controls consisted of similar assay samples with or without the substrate peptide or cAMP. A positive control of 0.4 ng/ml purified catalytic subunit from type I bovine PKA (Promega, Madison, WI) was included as a sample. Kinase activity was expressed in relation to total cellular protein assayed and calculated in picomoles of phosphate incorporated per minutes per milligram.
PKC Activity
The remaining epithelial lysate was used to determine PKC activity. Both the supernatant and particulate fractions were assayed. Activated PKC was found in the particulate fraction. The assay employed was a modification of procedures previously described (8) using 900 µM PKC substrate peptide (Peninsula, Belmont, CA), 12 mM calcium-acetate, 8 µM phosphatidyl-L-serine, 24 µg/ml phorbol 12-myristate 13-acetate, 30 mM dithiothreitol, 150 µM ATP, 45 mM magnesium acetate, and 10 µCi/ml [γ-32P] adenosine triphosphate (MP Biomedicals, Irvine, CA) in a Tris-HCl buffer (pH 7.5). Samples (20 µl) were added to 40 µl of the above reaction mixture and incubated for 15 min at 30°C. Spotting 50 µl of each sample onto P-81 phosphocellulose papers (Whatman, Clifton, NJ) halted incubations. Papers were then washed five times for 5 min each in phosphoric acid (75 mM), washed once in ethanol, dried, and counted in nonaqueous scintillant as previously described (7). Kinase activity was expressed in relation to total cellular protein assayed and calculated in picomoles of phosphate incorporated per minutes per milligram.
Statistical Analysis
Results are expressed as the mean ± SEM of the indicated number of animals in each group. Statistical differences between the various group means were determined using the Mann-Whitney test and one-way ANOVA with Tukey’s multiple comparison as a post test (Graphpad Prism, San Diego, CA). A probability of less than 0.05 was accepted as significant.
RESULTS
Weight Gain
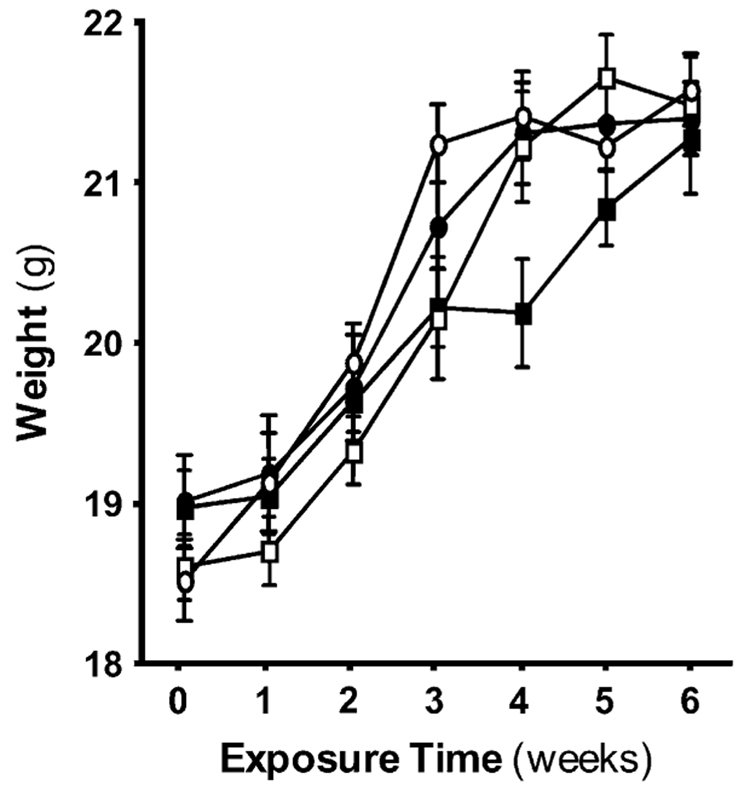
To determine if the combined treatment with cigarette smoke and alcohol would affect weight gain, mice were weighed weekly. The rate of weight gain was slightly decreased in the treatment groups receiving alcohol, smoke, or both compared with the air and control groups (Figure 1). However, by 6 wk, there was no significant difference in weight between the groups. Weight gain and condition of the mice was similar between the Control and Air groups (data not shown). Data in all other parameters measured throughout this study were also similar between the Control and Air groups. Because we found no significant effect of handling, data for the Control group have been omitted for brevity.
Figure 1.
Overall weight gain among the mice exposure groups is comparable. The vertical axis represents the mean (± SEM) of the weights of the mice within each exposure group. The horizontal axis represents the exposure to time in weeks (see Table 2 for details of the exposure protocol). Weight gain was roughly comparable among the four groups over the 6-wk experiment with no significant difference in weight between the groups. Open circles, air; open squares, air + alcohol; solid circles, smoke; solid squares, smoke + alcohol.
BAL Cell Populations
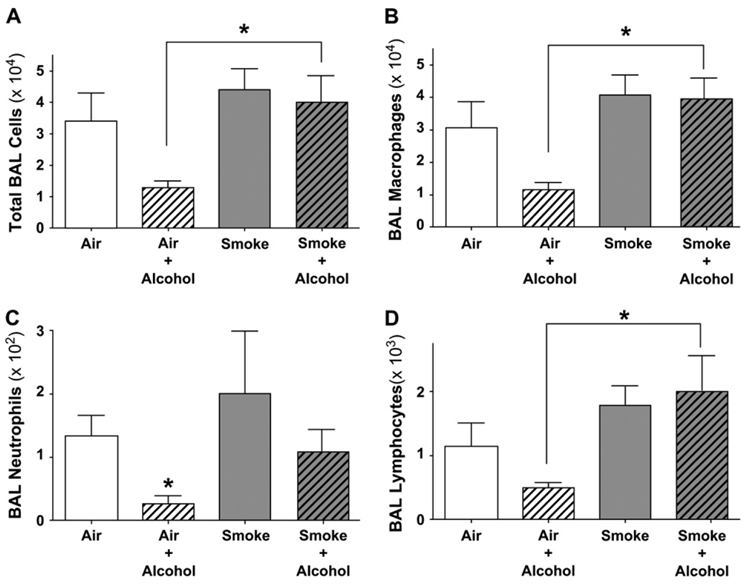
Exposure to cigarette smoke is known to induce an inflammatory response in the lung (24). Chronic exposure to alcohol decreases inflammatory cell function and recruitment (25, 26). We hypothesized that chronic alcohol exposure reduces the numbers of lung inflammatory cells recovered from BAL independent of smoke exposure. To test this hypothesis, we measured BAL cells to determine the effect combined cigarette smoke and alcohol exposure would have on inflammatory cell recruitment to the lung. The number of total cells recovered in the BAL from cigarette smoke–exposed animals was increased slightly over that of air-exposed animals, but this change was not statistically significant (Figure 2A). In contrast, mice in the Air + Alcohol group had 62% fewer total BAL cells compared with Air group. This smaller number of total BAL cells observed in the Air + Alcohol group was not observed when smoke was combined with alcohol (P < 0.05). Further examination of BAL differential cell counts revealed a similar pattern of lower total numbers of macrophages, neutrophils, and lymphocytes that only occurred in the presence of alcohol alone and not when alcohol was combined with smoke exposure (Figures 2B–2D).
Figure 2.
Total and differential BAL cell populations are reduced in alcohol-fed mice. The vertical axis represents the mean (± SEM) of the total or differential BAL cell counts within each exposure group. The horizontal axis represents the exposure (* P < 0.05 by ANOVA). (A) BAL total cell counts. Mice in the Air + Alcohol group had a mean decrease in total BAL cells compared with all other groups. Combined exposure to smoke and alcohol did not result in total BAL cell numbers significantly different from that of mice receiving smoke alone. (B) BAL total macrophage cell counts: The number of BAL macrophages was similarly decreased in the Air + Alcohol group compared with the other groups. Combined exposure to smoke and alcohol did not result in BAL macrophage cell numbers significantly different from that of mice receiving smoke alone. (C) BAL total PMN cell counts. The number of neutrophils in BAL fluid was significantly decreased in the Air + Alcohol group compared with the Air group. Smoke-exposed mice had a variable increase in the number of neutrophils. Total neutrophil counts from mice exposed to Smoke + Alcohol were comparable to those of the Air group. (D) BAL total lymphocyte cell counts. BAL lymphocytes were decreased in the Air + Alcohol group. This decrease not present when alcohol was combined with smoke exposure.
Ciliary Beat Frequency
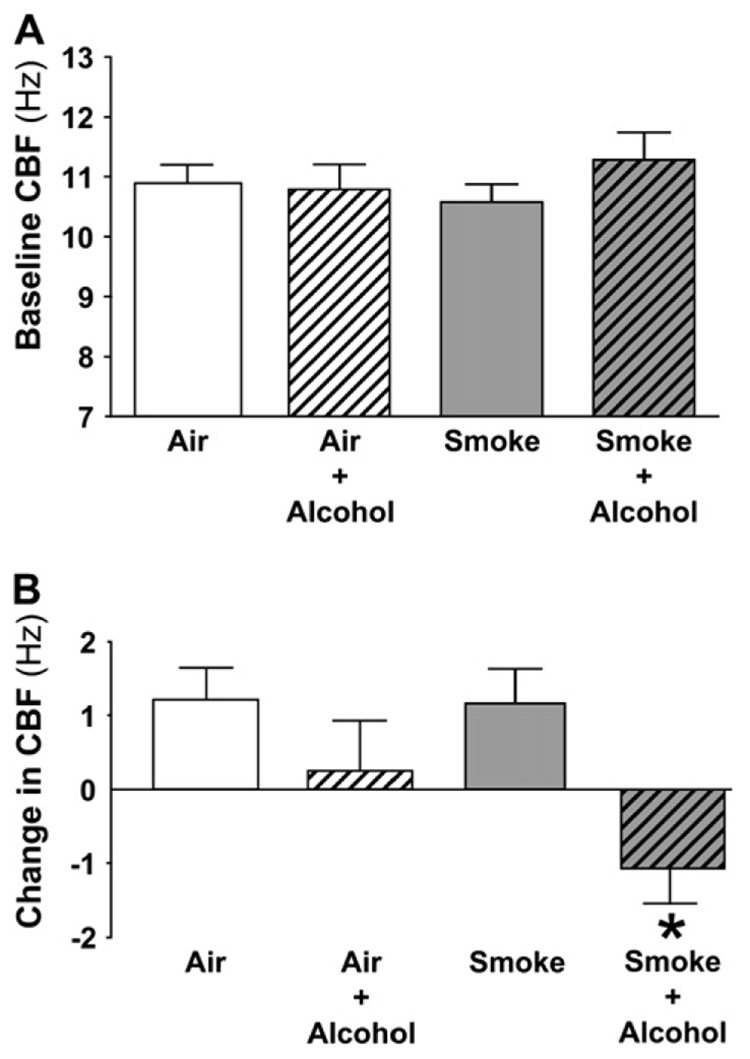
We have observed that chronic alcohol exposure, both in vitro in bovine airway cells and in vivo in alcohol-fed rats, desensitizes airway cilia to stimulation by β-agonists (18, 19). We hypothesized that chronic alcohol exposure would also desensitize airway cilia in alcohol-fed mice and that cilia desensitization would occur independent of smoke exposure. To test these hypotheses, baseline and isoproterenol-stimulated cilia beating were measured after alcohol and/or smoke exposure. Baseline (unstimulated) CBF was not different among the four groups (Figure 3A). When stimulated CBF was measured after treatment with isoproterenol for 40 min, a predictable increase in CBF in the Air + Smoke groups of > 1 Hz was observed. In contrast, the isoproterenol response was blunted in the Air + Alcohol group compared with Air group (Figure 3B). Surprisingly, exposure to isoproterenol actually slowed CBF to > 1 Hz below baseline in the group exposed to the combination of smoke and alcohol (P < 0.05). While an increase in goblet cell metaplasia was detected in response to cigarette smoke as expected, alcohol exposure resulted in no observed histologic differences in airway cell populations between the smoke exposure groups (data not shown).
Figure 3.
Alcohol desensitizes stimulated, but not baseline CBF. (A) Baseline CBF. The vertical axis represents the mean (± SEM) of the CBF of tracheal rings within each exposure group. The horizontal axis represents the exposure. Baseline (unstimulated) CBF is not altered by exposure to alcohol, smoke, or a combination of both. (B) β-agonist–stimulated CBF. The vertical axis represents the mean (± SEM) of the change in CBF in Hz after a 40-min treatment of tracheal rings with isoproterenol (100 µM) for each exposure group. The horizontal axis represents the exposure. Stimulation with isoproterenol induced a normal (> 1 Hz) increase in the Air and Smoke groups. The response to isoproterenol stimulation was blunted in the alcohol group and resulted in a significant decrease from baseline CBF in the group with Smoke + Alcohol exposure (* P < 0.05 by ANOVA).
PKA Activity
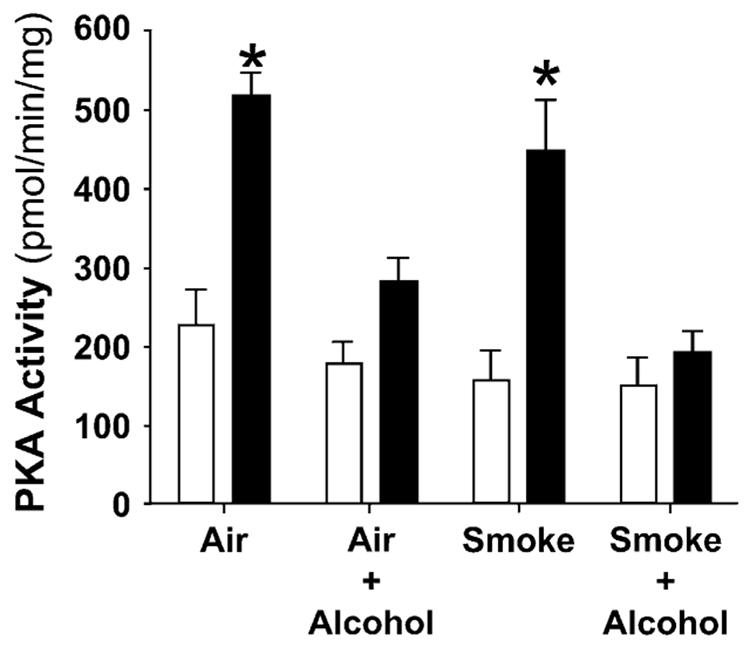
We have demonstrated that β-agonist stimulation of CBF is tightly linked to activation of PKA in ciliated epithelial cells in vitro and in vivo (4, 19). The effect of combined cigarette smoke and alcohol treatment on PKA activity in mice, however, is not known. We hypothesized that alcohol would desensitize airway PKA activity independent of smoke exposure. To test this hypothesis, PKA activity was measured in the ciliated epithelium of tracheas from alcohol- and/or smoke-exposed mice. Treatment with isoproterenol induced a significant increase in PKA activity (Figure 4) in the ciliated epithelium of the Air and Smoke groups (P < 0.05). Alcohol exposure, independent of smoke exposure, blunted the isoproterenol induced increase in PKA activity in the ciliated tracheal epithelium consistent with PKA down-regulation.
Figure 4.
Alcohol exposure, independent of smoke exposure, blunts β-agonist–induced PKA enzyme activity in ciliated tracheal epithelium. The vertical axis represents the mean (± SEM) of the PKA activity in pmol/min/mg of ciliated tracheal epithelial tissue within each exposure group. The horizontal axis represents the exposure. The open bars indicate the baseline (unstimulated) PKA activity. The solid bars indicate the isoproterenol (stimulated) PKA activity after treatment with 100 µM isoproterenol for 40 min. Isoproterenol induced a significant (2-fold) increase in PKA activity in the Air and Smoke groups. In contrast, mice exposed to alcohol, independent of smoke exposure, had either a blunted or no response to isoproterenol consistent with PKA desensitization (*P < 0.05 for + iso compared with baseline by paired Student’s t test).
PKC Activity
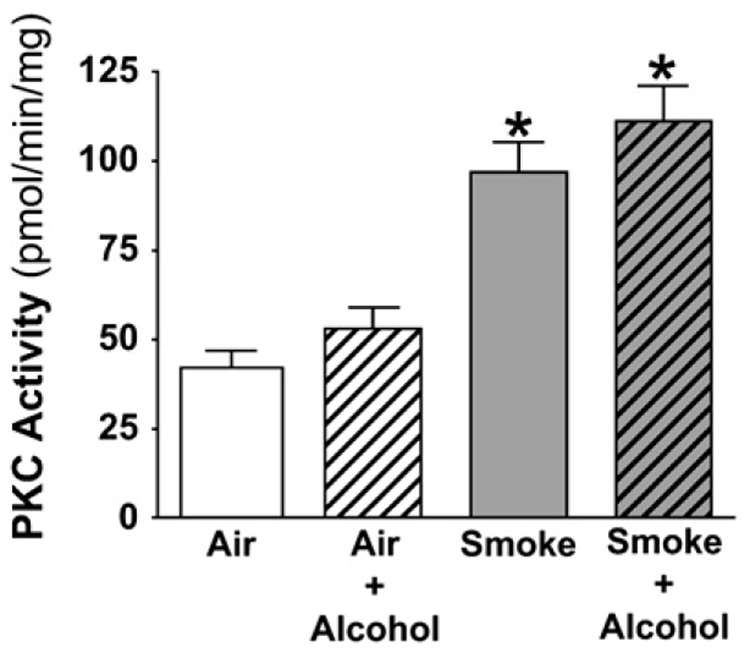
Exposure to CSE induces an increase in PKC activity in bronchial epithelial cells in vitro and in vivo in rats exposed to cigarette smoke (15, 20). We hypothesized that smoke would stimulate PKC activity in mice exposed to smoke independent of alcohol exposure. Prolonged exposure to alcohol had no effect on PKC activity in alcohol-fed mice compared with Air controls (Figure 5). Exposure to cigarette smoke, however, significantly increased the activity of PKC in the ciliated tracheal epithelium. Concurrent exposure to alcohol did not alter the smoke-induced increase in PKC activity.
Figure 5.
Cigarette smoke, independent of alcohol exposure, activates baseline PKC activity in the ciliated tracheal epithelium. The vertical axis represents the mean (± SEM) of the PKC activity in pmol/min/mg of ciliated tracheal epithelial tissue within each exposure group. The horizontal axis represents the exposure. Cigarette smoke induced a significant (2-fold) increase PKC activity in the ciliated tracheal epithelium regardless of alcohol exposure (*P < 0.05 by ANOVA).
DISCUSSION
Excessive alcohol intake compromises the host lung defense mechanisms from the nasopharynx to the alveolus, resulting in severe upper and lower respiratory tract infections. Recent studies of the mechanical, innate, and adaptive immune systems have begun to explore the mechanisms by which alcohol exposure increases host susceptibility to lung infection (27). While the innate cellular immunity, represented by neutrophils and resident macrophages, or the adaptive immunity orchestrated through cellular (T cells) and humoral (B cells) events are the mediators of the pathologic response to chronic infection, it is the cellular mechanistic events orchestrating mucociliary clearance that govern initial protection against such an infection. Because cilia drive airway clearance, we have examined the impact of alcohol on cilia in combination with cigarette smoke co-exposure.
Studying the impact of combined smoke and alcohol exposure on lung defenses is important because increasing health care costs incurred by patients who smoke and drink alcohol represent a significant public health burden. Most heavy drinkers smoke cigarettes (10) and ~ 30% of all smokers are heavy alcohol drinkers (28). Therefore, studies that consider the impact of cigarette smoke or alcohol alone on the lung may miss important synergistic or masking effects observed only under conditions of co-exposure. To our knowledge this study represents the first report of a cigarette smoke and alcohol co-exposure in mice to evaluate effects on the upper airways and BAL cell recruitment in the lung. The reasons for this study were twofold: (1) to determine if the combined exposure of alcohol and cigarette smoke would produce differential effects in the lung compared with either treatment alone; and (2) to characterize the combined exposure in C57BL/6 mice to use in future studies as a large percentage of knockout mice are bred onto this background. Importantly, this exposure model likely mimics the combined exposure observed in heavy drinkers who smoke. Blood alcohol concentrations (BAC) in this model varied from mouse to mouse, with levels averaging 133–148 mg/dl (Table 1) in the Air + Alcohol and Smoke + Alcohol groups. This range of BAC is commonly documented in individuals arrested for driving while intoxicated (21), with levels between 200 and 400 mg/dl often observed in conscious individuals. In the current model alcohol in the water was provided ad libitum so that the mice could drink any time they wanted, which mirrors drinking behavior of humans who consume alcohol heavily. The alcohol-exposed mice were observed to move normally around the cage at some times and to stumble around in a likely intoxicated state at a other times. Several of the alcohol-drinking mice appeared to “pass out,” which was coincident with very high BACs. Indeed, BACs recorded in “passed out” mice were as high as 446 mg/dl, paralleling BACs recorded in heavy drinkers. We also measured daily liquid volume consumption (water ± alcohol) by the mice. Although they drank approximately the same volume from one 24-h period to the next, it is likely that they varied their alcohol intake during any 24-h period. Therefore, this model approximates exposure seen in heavy drinkers.
In the present study, we observed a unique β-agonist–mediated cilia slowing in smoke and alcohol co-exposed mice that is not present after exposure to either smoke or alcohol alone. In addition, a difference was observed on total BAL cells in the combination model, with a lower number of total BAL cells observed in the alcohol-exposed mice. To the best of our knowledge, the combined effect of alcohol + smoke on BAL cell populations in mice has not been published. Both exposures are known to separately alter lung immune cell functions. Previous studies have shown that exposure to either alcohol or cigarette smoke alone alters BAL cell population and numbers recovered from the lungs (24, 28, 29). The number of cells recovered in the BAL from cigarette smoke–exposed animals was increased over that of air-exposed animals, but not significantly so. Similar findings were obtained by Higashimoto and coworkers in a study to evaluate the alteration of alveolar macrophage function occurring with CS exposure (30). In addition, a study by Gairola showed the 6-wk time point to be the point at which the BAL cell numbers begin to increase in long-term smoke exposure in mice (24). In contrast, BAL cell numbers were significantly lower in our study in alcohol-exposed mice. This finding is consistent with previous reports (29). Interestingly, the reduced number of BAL cells present after alcohol exposure alone was absent under conditions of smoke and alcohol co-exposure, although the number of neutrophils remained lower than in smoke-only–exposed mice. Quinton and colleagues (31) suggest that the neutrophil remains “reluctant” under conditions of alcohol exposure, and our findings support that this occurs even when alcohol exposure is combined with cigarette smoking.
Changes in lung inflammatory cells are relevant to ciliary motility because cytokines released from lung cells are known to alter ciliary function. For example, we have demonstrated that TNF-α and IL-1β both stimulate nitric oxide–dependent stimulation of CBF (32). In contrast, we have also demonstrated that IL-8, a proinflammatory cytokine released from lung inflammatory cells, desensitizes CBF to subsequent β-agonist stimulation (33). While we cannot draw direct links between changes in CBF and changes in BAL cell populations, we think these findings are relevant to combined alcohol and smoke exposure in this mouse model.
Although in the present study, baseline CBF was not affected by combined or individual treatments of alcohol or cigarette smoke, β-agonist–induced changes in CBF were. In our previous work, β-agonist stimulation of tracheal ciliated epithelial cells leads to increased cilia beat (18). In our study, alcohol and smoke, alone or in combination, did not alter baseline CBF. This is consistent with our findings in the rat model using the Lieber-DeCarli alcohol diet (19). The cilia of a live cell are never static, suggesting that the regulation of baseline cilia motion in vivo is not significantly altered by cigarette smoke or alcohol consumption under the conditions of our model. However, under stress response conditions, as modeled by β-agonist stimulation (34), CBF increases do occur and provide a mechanism for enhanced clearance. Although the physiological impact of cilia slowing was not explored in this study, CBF slowing has been correlated with reduced particle clearance rates (35). While our findings in mice reproduced those of rats exposed to alcohol with regard to blocking or desensitization of β-agonist–induced increases in CBF (19), the combination of smoke and alcohol resulted in a paradoxical slowing of the cilia to a frequency below that of baseline. For example, the β-agonist used in this study, isoproterenol, is a synthetic catecholamine with a chemical structure and action similar to that of epinephrine (i.e., stimulation of both β1- and β2-adrenergic receptors [βARs]) (36). Isoproterenol stimulation is a well-established maneuver that allows assessment of the acute stress response. This stress response is critical during normal physiologic situations such as exercise, when high minute ventilation equates to high particle deposition in the airways. Another highly relevant stress context in the alcoholic is acute aspiration of oral or stomach contents. Our previous studies indicate the blunting of this “fight-or-flight” response in animals caused by heavy alcohol intake is sufficient to impair bacterial clearance from the lung (20), which leads to airway colonization and pneumonia. For these reasons we assert that a loss of the CBF stimulatory response represents biologically relevant impairment of lung defenses.
The biological significance of our observation is not known in humans. However, in recent studies we have published, using a rat model of S. pneumoniae lung clearance, we demonstrated significantly impaired bacterial lung clearance in rats exposed to alcohol and/or smoke (20). Impaired clearance was present in all animals with modest or heavy alcohol intake and correlated to CBF changes of 0.5–1.5 Hz. This link between modest reductions CBF and impaired clearance suggests that small decreases in CBF may be biologically very important.
The response of cilia from animals exposed to combined alcohol and cigarette smoke is counterintuitive and the precise mechanism(s) unknown. Studies have revealed that ciliary activity is controlled by phosphorylation of axonemal proteins with direct involvement of PKA and PKC (37). The increased activity of PKA and PKC is associated with increases and decreases in cilia beat, respectively (4). PKA and PKC have been shown to be co-localized on the scaffolding protein gravin, associated with the βAR in a human epidermal cell line (38), and are likely co-localized in ciliated epithelial cells as well (39). Given the dynamic nature of regulatory kinases in the cell, we propose three possible mechanisms. (1) PKA and PKC are both associated with PKA anchoring proteins (AKAPs), which constitute multi-enzyme signaling complexes involving cell signaling. The combined effect of alcohol and smoke may have a direct action on kinase interactions within the AKAP. (2) Another possibility is that translocation of activated kinase to its target is disrupted so that PKA and PKC do not phosphorylate substrate properly. Altered localization or activity of PKA and/or PKC would result in altered signaling events. (3) Finally, there is ample evidence that cigarette smoke increases PKC activity (15, 20, 40). Sustained high levels of PKC activity may affect βAR signaling or expression in the ciliated epithelium. There is evidence that increased PKC activity has been associated with changes in βAR expression in BEAS-2B cells (41). In addition, increased levels of PKC activity are correlated with desensitization of βAR-mediated adenylyl cyclase stimulation in the mammalian heart (42). In contrast to smoke, alcohol affects both PKA and PKC activation and localization in a number of cell types (19). Exactly how localization and targeting of PKA and PKC, in general, or which subtypes, in particular, are altered in the ciliated epithelium with smoke and alcohol exposure is unknown. Determining mechanistic links between changes in airway PKC and PKA activities and the dysregulation of ciliary motility will be critical to defining how the combination of alcohol and smoke impairs mucociliary clearance.
These in vivo findings of combined smoke and alcohol exposure suggest potential mechanisms based on our earlier published work. For example, we have firmly established that long-term alcohol exposure blocks the cilia stimulatory pathway by down-regulating ciliary PKA at the level of the cilia axoneme kinase and/or its substrate (18, 43). We also have established that cigarette smoke activates PKC in airway cells due in large part due to the release of acetaldehyde into the vapor phase of smoke and possibly linked to the release and/or production of airway oxidants (40). The findings of the current study not only recapitulate these observations in mice but also demonstrate a novel effect of combined alcohol and smoke on β-agonist stimulation of CBF.
Generally, it is thought that cilia stimulation is mediated via the action of nitric oxide/cGMP/PKG and cAMP/PKA signaling (44–48). Chronic alcohol exposure desensitizes ciliated epithelium to β-agonist–stimulated increases in PKA activity in in vitro cell culture models (18). In the mouse model, we observed that PKA was also desensitized by alcohol independent of smoke. This replicated our rat in vivo findings of PKA desensitization in response to alcohol (19) showing that the link of CBF desensitization to blocking stimulated PKA activation is consistent and reproducible in both animal and cell models. The consequence of such an alcohol-induced desensitization response would be to block the signaling pathway that initiates a ciliary frequency increase in response to pathogens or toxins leading to increased infection or exposure. Our findings showed that cigarette smoke exposure had no effect on PKA activity, but rather resulted in significant PKC activation in tracheal epithelial cells.
PKA and PKC may represent opposite regulators of ciliary motility. Unlike PKA, agents that activate PKC have also been reported to decrease or inhibit ciliary beating (5, 49, 50). In our study, PKC was activated by smoke independent of alcohol. Smoke alone did not alter β-agonist–stimulated CBF and had no effect on PKA activity. These findings mirrored our rat in vivo data; however, in vitro studies show that cigarette smoke exposure of ciliated epithelium results in a significant decrease in CBF (51, 52). Our observation that the combination of cigarette smoke and alcohol produces a significant CBF decrease in response to β-agonists may reflect the dual activation of PKC and suppression of PKA pathways. It will be important to determine the roles and locations of specific PKC isoforms on the ciliary axoneme to better understand the interplay between PKA- and PKC-modulated cilia function. The combination of alcohol and cigarette smoke has been shown to produce unique disruptions of cellular processes such as those occurring in squamous-cell carcinoma of the head and neck (53). The effect of combined smoke and alcohol exposure on mucociliary clearance in the lungs is not known. Decreased ciliary motility may be a contributing factor to the immunomodulatory effects of alcohol and contribute to increased susceptibility to pulmonary infection. Cigarette smoke exposure may act as an additional insult, augmenting alcohol’s effect on the primary defense function of the tracheal epithelium. Elucidating the mechanisms behind the effects of the combined exposure will allow the development of treatments directed at the disrupted signaling in ciliated cells of the upper airway during infection in persons who drink heavily and smoke. Treatments of this nature may work in concert with antibiotics to speed recovery and reduce morbidity and mortality from airway infection.
Acknowledgments
Support for this research was provided by R01AA008769 (J.H.S.), VA Merit Review (T.A.W.) and T32AA007582 (M.K.E.). Dr. Wyatt is an ALA Career Investigator.
Footnotes
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Olseni L, Wollmer P. Mucociliary clearance in healthy men at rest and during exercise. Clin Physiol. 1990;10:381–387. doi: 10.1111/j.1475-097x.1990.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande VR, Jadhav JH. Specific blockade of spasmogens by beta-receptor stimulation with nylidrin and isoprenaline. J Pharm Pharmacol. 1970;22:101–103. doi: 10.1111/j.2042-7158.1970.tb08400.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–L835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- 5.Salathe M, Pratt MM, Wanner A. Protein kinase C-dependent phosphorylation of a ciliary membrane protein and inhibition of ciliary beating. J Cell Sci. 1993;106:1211–1220. doi: 10.1242/jcs.106.4.1211. [DOI] [PubMed] [Google Scholar]
- 6.Pavia D, Agnew JE, Lopez-Vidriero MT, Clarke SW. General review of tracheobronchial clearance. Eur J Respir Dis Suppl. 1987;153:123–129. [PubMed] [Google Scholar]
- 7.Leitch GJ, Frid LH, Phoenix D. The effects of ethanol on mucociliary clearance. Alcohol Clin Exp Res. 1985;9:277–280. doi: 10.1111/j.1530-0277.1985.tb05749.x. [DOI] [PubMed] [Google Scholar]
- 8.Winterbauer RH, Bedon GA, Ball WC., Jr Recurrent pneumonia: predisposing illness and clinical patterns in 158 patients. Ann Intern Med. 1969;70:689–700. doi: 10.7326/0003-4819-70-4-689. [DOI] [PubMed] [Google Scholar]
- 9.Ballenger JJ. Experimental effect of cigarette smoke on human respiratory cilia. N Engl J Med. 1960;263:832–835. doi: 10.1056/NEJM196010272631704. [DOI] [PubMed] [Google Scholar]
- 10.Patten CA, Martin JE, Owen N. Can psychiatric and chemical dependency treatment units be smoke free? J Subst Abuse Treat. 1996;13:107–118. doi: 10.1016/0740-5472(96)00040-2. [DOI] [PubMed] [Google Scholar]
- 11.Izzotti A, Balansky RM, Blagoeva PM, Mircheva ZI, Tulimiero L, Cartiglia C, De Flora S. DNA alterations in rat organs after chronic exposure to cigarette smoke and/or ethanol ingestion. FASEB J. 1998;12:753–758. doi: 10.1096/fasebj.12.9.753. [DOI] [PubMed] [Google Scholar]
- 12.Cigremis Y, Turkoz Y, Akgoz M, Sozmen M. The effects of chronic exposure to ethanol and cigarette smoke on the level of reduced glutathione and malondialdehyde in rat kidney. Urol Res. 2004;32:213–218. doi: 10.1007/s00240-004-0406-x. [DOI] [PubMed] [Google Scholar]
- 13.Eke BC, Vural N, Iscan M. Combined effects of ethanol and cigarette smoke on hepatic and pulmonary xenobiotic metabolizing enzymes in rats. Chem Biol Interact. 1996;102:155–167. doi: 10.1016/s0009-2797(96)03742-8. [DOI] [PubMed] [Google Scholar]
- 14.Hartwig W, Werner J, Ryschich E, Mayer H, Schmidt J, Gebhard MM, Herfarth C, Klar E. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21:272–278. doi: 10.1097/00006676-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt TA, Heires AJ, Sanderson SD, Floreani AA. Protein kinase C activation is required for cigarette smoke-enhanced C5a-mediated release of interleukin-8 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;21:283–288. doi: 10.1165/ajrcmb.21.2.3636. [DOI] [PubMed] [Google Scholar]
- 16.Maurer DR, Liebman J. Effects of ethanol on in vitro ciliary motility. J Appl Physiol. 1988;65:1617–1620. doi: 10.1152/jappl.1988.65.4.1617. [DOI] [PubMed] [Google Scholar]
- 17.Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23:1528–1533. [PubMed] [Google Scholar]
- 18.Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L575–L581. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vander Top EA, Wyatt TA, Gentry-Nielsen MJ. Smoke exposure exacerbates an ethanol-induced defect in mucociliary clearance of Streptococcus pneumoniae. Alcohol Clin Exp Res. 2005;29:882–887. doi: 10.1097/01.alc.0000164364.35682.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCartt AT, Northrup VS. Effects of enhanced sanctions for high-BAC DWI offenders on case dispositions and rates of recidivism. Traffic Inj Prev. 2004;5:270–277. doi: 10.1080/15389580490467821. [DOI] [PubMed] [Google Scholar]
- 22.Patrick G, Stirling C. Measurement of mucociliary clearance from the trachea of conscious and anesthetized rats. J Appl Physiol. 1977;42:451–455. doi: 10.1152/jappl.1977.42.3.451. [DOI] [PubMed] [Google Scholar]
- 23.Iravani VJ, Melville GN. Arzneimittelforschung. 1975;25:415–417. (Effect of terodiline on bronchial muscles and on tracheobronchial clearance in the rat) [PubMed] [Google Scholar]
- 24.Gairola CG. Free lung cell response of mice and rats to mainstream cigarette smoke exposure. Toxicol Appl Pharmacol. 1986;84:567–575. doi: 10.1016/0041-008x(86)90262-0. [DOI] [PubMed] [Google Scholar]
- 25.Pickrell KL. The effect of alcoholic intoxication and ether anesthesia on resistance to pneumococcal infection. Johns Hopkins Med J. 1938;63:3826. [Google Scholar]
- 26.Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- 27.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 28.Miller NS, Gold MS. Comorbid cigarette and alcohol addiction: epidemiology and treatment. J Addict Dis. 1998;17:55–66. doi: 10.1300/J069v17n01_06. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg SS, Xie J, Ouyang J, Zhao X. Ethanol metabolism is not required for inhibition of LPS-stimulated transcription of inducible nitric oxide synthase. Alcohol. 1999;17:203–213. doi: 10.1016/s0741-8329(98)00048-2. [DOI] [PubMed] [Google Scholar]
- 30.Higashimoto Y, Fukuchi Y, Ishida K, Shimada Y, Ohata M, Funasako M, Shu C, Teramoto S, Matsuse T, Sudo E, et al. Effect of chronic tobacco smoke exposure on the function of alveolar macrophages in mice. Respiration (Herrlisheim) 1994;61:23–27. doi: 10.1159/000196298. [DOI] [PubMed] [Google Scholar]
- 31.Quinton LJ, Nelson S, Zhang P, Happel KI, Gamble L, Bagby GJ. Effects of systemic and local CXC chemokine administration on the ethanol-induced suppression of pulmonary neutrophil recruitment. Alcohol Clin Exp Res. 2005;29:1198–1205. doi: 10.1097/01.alc.0000171927.66130.aa. [DOI] [PubMed] [Google Scholar]
- 32.Jain B, Rubinstein I, Robbins RA, Sisson JH. TNF-alpha and IL-1 beta upregulate nitric oxide-dependent ciliary motility in bovine airway epithelium. Am J Physiol. 1995;268:L911–L917. doi: 10.1152/ajplung.1995.268.6.L911. [DOI] [PubMed] [Google Scholar]
- 33.Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J Aerosol Med. 2004;17:107–115. doi: 10.1089/0894268041457138. [DOI] [PubMed] [Google Scholar]
- 34.Verdugo P, Johnson NT, Tam PY. beta-adrenergic stimulation of respiratory ciliary activity. J Appl Physiol. 1980;48:868–871. doi: 10.1152/jappl.1980.48.5.868. [DOI] [PubMed] [Google Scholar]
- 35.Kurosawa H, Wang CG, Dandurand RJ, King M, Eidelman DH. Mucociliary function in the mouse measured in explanted lung tissue. J Appl Physiol. 1995;79:41–46. doi: 10.1152/jappl.1995.79.1.41. [DOI] [PubMed] [Google Scholar]
- 36.Hyman AL, Higashida RT, Spannhake EW, Kadowitz PJ. Pulmonary vasoconstrictor responses to graded decreases in precapillary blood PO2 in intact-chest cat. J Appl Physiol. 1981;51:1009–1016. doi: 10.1152/jappl.1981.51.4.1009. [DOI] [PubMed] [Google Scholar]
- 37.Gertsberg I, Hellman V, Fainshtein M, Weil S, Silberberg SD, Danilenko M, Priel Z. Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway. J Gen Physiol. 2004;124:527–540. doi: 10.1085/jgp.200409153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 39.Edwards AS, Scott JD. A-kinase anchoring proteins: protein kinase A and beyond. Curr Opin Cell Biol. 2000;12:217–221. doi: 10.1016/s0955-0674(99)00085-x. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt TA, Schmidt SC, Rennard SI, Sisson JH. Acetaldehyde-stimulated PKC activity in airway epithelial cells treated with smoke extract from normal and smokeless cigarettes. Proc Soc Exp Biol Med. 2000;225:91–97. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- 41.Bin W, Aksoy MO, Yang Y, Kelsen SG. IL-1beta enhances beta2-adrenergic receptor expression in human airway epithelial cells by activating PKC. Am J Physiol Lung Cell Mol Physiol. 2001;280:L675–L679. doi: 10.1152/ajplung.2001.280.4.L675. [DOI] [PubMed] [Google Scholar]
- 42.Guimond J, Mamarbachi AM, Allen BG, Rindt H, Hebert TE. Role of specific protein kinase C isoforms in modulation of beta1- and beta2-adrenergic receptors. Cell Signal. 2005;17:49–58. doi: 10.1016/j.cellsig.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163:1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain B, Rubinstein I, Robbins RA, Leise KL, Sisson JH. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem Biophys Res Commun. 1993;191:83–88. doi: 10.1006/bbrc.1993.1187. [DOI] [PubMed] [Google Scholar]
- 45.Yang B, Schlosser RJ, McCaffrey TV. Dual signal transduction mechanisms modulate ciliary beat frequency in upper airway epithelium. Am J Physiol. 1996;270:L745–L751. doi: 10.1152/ajplung.1996.270.5.L745. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23:175–181. doi: 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- 47.Zagoory O, Braiman A, Priel Z. The mechanism of ciliary stimulation by acetylcholine: roles of calcium, PKA, and PKG. J Gen Physiol. 2002;119:329–339. doi: 10.1085/jgp.20028519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyatt TA, Forget MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L546–L551. doi: 10.1152/ajplung.00107.2004. [DOI] [PubMed] [Google Scholar]
- 49.Wong LB, Park CL, Yeates DB. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via nPKC, independently of PKA. Am J Physiol. 1998;275:C440–C448. doi: 10.1152/ajpcell.1998.275.2.C440. [DOI] [PubMed] [Google Scholar]
- 50.Mwimbi XK, Muimo R, Green M, Mehta A. Protein kinase C regulates the flow rate-dependent decline in human nasal ciliary beat frequency in vitro. J Aerosol Med. 2000;13:273–279. doi: 10.1089/jam.2000.13.273. [DOI] [PubMed] [Google Scholar]
- 51.Sisson JH, Papi A, Beckmann JD, Leise KL, Wisecarver J, Brodersen BW, Kelling CL, Spurzem JR, Rennard SI. Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am J Respir Crit Care Med. 1994;149:205–213. doi: 10.1164/ajrccm.149.1.8111584. [DOI] [PubMed] [Google Scholar]
- 52.Knoll M, Shaoulian R, Magers T, Talbot P. Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod. 1995;53:29–37. doi: 10.1095/biolreprod53.1.29. [DOI] [PubMed] [Google Scholar]
- 53.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]