Abstract
If begun early in life, exercise effectively reduces the development of cognitive deficits in transgenic mouse models of Alzheimer's disease (AD). However, the effectiveness of exercise, once the cognitive impairments are established, is not as clear. In terms of translating research in animal models to treatments involving exercise in Alzheimer's disease patients, it is critical to evaluate exercise intervention at time points that address not only prevention, but also treatment of cognitive decline. We provided exercise wheels to Tg2576 (TG) (n=12) and C57BL6 (WT) (n=17) mice at 17-19 months of age for three weeks. At this age animals have significant cognitive impairment and neuropathology consistent with AD. Age matched sedentary TG (n=13) and WT (n=12) mice were also included, as well as groups provided access to an immobile wheel (TG n=9, WT n=12). After three weeks, animals were evaluated in a radial arm water maze. Significant impairments were observed in the sedentary TG mice compared to WT in reference/long-term and working/short-term memory, as well as in probe trials. Exercised TG mice demonstrated improvements in memory, which made them indistinguishable from WT mice on all tasks. In addition, animals provided with an immobile wheel exhibited improvement in some, but not all cognitive measures. Our findings demonstrate that exercise can improve cognitive performance in a mouse model of AD even if applied after the development of pathology.
Keywords: exercise, Alzheimer's, Tg2576, aging, RAWM, intervention
Introduction
Cognitive decline has been consistently reported in human aging. [29, 33] This age-related decline of cognitive ability also occurs in mice. [32] Cognitive decline, however, can be modified by environmental factors. For example, several researchers show that changing from a sedentary lifestyle to an active lifestyle results in cognitive benefits in both humans and mice. [9, 16, 25] Further, exercise improves cognition in aging humans and can delay the onset of dementia. [25] Exercise is beneficial through multiple pathways such as increased growth factors, increased metabolism, improved cerebrovascular function, increased neurogenesis and plasticity, and increased immune system efficacy. [6, 10, 11, 25] One important question that remains is whether exercise improves cognitive ability in advanced states of dementia. Transgenic mouse models, developed to mimic the pathological progression of Alzheimer's disease, the most common of the dementias, provide opportunities to explore the effects of exercise on AD. Recently published data by Adlard et al. using the TgCRND8 model of AD demonstrates that 5 months of voluntary wheel running begun at 1 month of age improves performance on the Morris water maze (MWM) compared to sedentary animals of the same age. [2] Adlard's findings support an exercise-induced improvement in cognition if exercise is begun at a young age, prior to the development of AD pathology.
Though previous studies demonstrate that exercise begun in youth can contribute to a healthy cognitive profile throughout old age, we do not yet know if exercise initiated late in life has cognitive benefits. Until a recent study published by van Praag et al. (2005), it was unclear whether exercise could enhance learning in aged mice, even those not engineered to develop AD pathology. [32] In 19 month C57Bl/6 males (the background strain for the Tg2576 mouse model of AD used in the current study), 6 weeks of voluntary wheel running increased the amount of time animals spent in the target quadrant of the platform in the MWM, though escape latency improved only in young (3 month) runners. [32] This evidence suggests that aged wild-type mice may benefit from exercise begun late in life. It is not known if AD models, such as the Tg2576, will respond similarly to exercise begun in old age.
A possible role of exercise in improving cognition in aging animals is suggested by experiments with environmental enrichment. Typically an enriched rodent environment includes access to a running wheel and a larger cage size. Previous studies have demonstrated a beneficial effect of such environmental enrichment on cognition in mouse AD models. [4, 20] It is unclear what the mechanism is for this cognitive improvement. Lazarov et al. (2005) exposed transgenic AD mice at 1 month of age to 5 months of environmental enrichment and discovered decreases in Aβ pathology. [24] Jankowsky et al. (2005) found cognitive improvement in the same transgenic model as Lazarov, using a similar enrichment paradigm, but found increases in Aβ pathology. [19] Both studies included exercise wheels in the enriched environment, but exercise alone was not evaluated. In Arendash et al. (2004), enrichment included a large living space, with toys, tunnels, and an exercise wheel present in the environment. [4] This environmental enrichment improved cognitive performance in old (16-22 mos) Tg2576 mice, confirming enrichment can enhance cognitive ability in aged mice. [4] Kempermann et al. (2002) demonstrated that enrichment results in neurogenesis in aged C57Bl/6 if begun at middle age (10months). [20] In an important study by van Praag et al (1999), exercise alone increased neuronal proliferation as much as the enriched environment, demonstrating that running alone could be responsible for the effects of enrichment on improved cognitive performance. [31] Conversely, a recent paper by Pietropaolo et al. (2006) that compared enriched housing with a running wheel, enriched housing with a locked wheel, standard housing with a running wheel, and standard housing with a locked wheel suggests enrichment alone is most beneficial on cognitive performance. [28] Pietropaolo et al. found the combination of enrichment and running had similar effects to enrichment with a locked wheel present. Both improved the acquisition of the platform location in Morris water maze. However, the animals that ran in a standard housing environment were unique from the other groups in their lengthened time needed to extinguish a learned response.
Our primary goal was to determine the effects of three weeks of voluntary wheel running in aged Tg2576 (TG) and C57Bl/6 (WT) mice. The Tg2576 mouse strain over-expresses the 695-amino acid form of the human amyloid precursor protein containing a Lys670 ➝ Asn, Met671 ➝ Leu mutation. [17] Tg2576 mice express 5.5 times the amount of APP endogenous to the wild type. The pathological features of this model have been well characterized, and suggest that the Tg2576 is a useful model of Alzheimer's disease. [7, 17, 21, 30] Elevated levels of Aβ1-40 and Aβ1-42/43, pathological hallmarks of AD whose causal role is still under debate, are present at 11 months as determined by ELISA, as are dense core classic senile plaques. [17]
Though the Tg2576 has been tested on several behavioral tasks, the results of tests for learning and memory impairments have been varied and difficult to interpret. (Table 1) We therefore felt it was important to employ a task that engages memory at both long-term and short-term intervals in a spatial task where the animal must remember the platform location in order to escape, as well as search through multiple arms to find locate the platform. The radial-arm water maze (RAWM) is a recent addition to the behavioral tasks available to investigators of learning and memory. [26] The RAWM combines the spatial working memory task of the radial arm maze with the spatial reference memory task of the traditional Morris water maze. [27] Though experiments have not been done to correlate performance on RAWM to Aβ levels, the RAWM has proven more sensitive than Morris water maze in detecting cognitive deficit in several transgenic AD models. [5, 8, 26] The present study compares TG and WT at a time-point (17-19 months) when significant AD-like pathology is established in the Tg2576. [17, 18, 22, 30] Three weeks of wheel running was chosen because multiple neuroprotective mechanisms are engaged at this duration of exercise, including increased neurogenesis, increased expression of brain-derived growth factor, improved cerebrovascular function, resistance to ischemic damage, and improved immune function. [1, 12, 13, 15, 22] Thus, we felt that initial improvements in cognitive function would be detectable at this same time-point.
TABLE 1. Cognitive measures observed in Tg2576 compared to the WT.
| Y-maze | T-maze | MWM I | MWM II | RAM | RAWM I | RAWM II | Visual platform | Circular holeboard | |
|---|---|---|---|---|---|---|---|---|---|
| Hsiao, 1996 | ↔3 mos
↓10 mos |
↔2, 6 mos*
↓ 9-10 mos ↓ 12-15 mos |
↓ 10 mos | ↔ 9-10mos** | |||||
| Pompl, 1999 | ↔7 mos (learning),
↓ reversal learning |
||||||||
| Chapman, 1999 | ↔2 mos
↓10 mos ↓16 mos |
||||||||
| Arendash, 2001 (Pre-vaccination) | ↔5-7mos | ↔5-7 mos | ↓15mos (IgG vaccinated) | ↔5-7mos | ↔5-7 mos | ||||
| King & Arendash, 2002 | ↓3 mos
↔9 mos ↔ 14 mos ↓19 mos |
↔3 mos
↔9 mos ↔14 mos ↔19 mos |
↔3 mos
↔9 mos ↔14mos ↔19 mos |
↓3 mos
↓9 mos ↓14 mos ↓19 mos |
↔3 mos
↔9 mos ↔14 mos ↔19 mos |
||||
| Sigurdsson, 2004 (IgG vaccinated) | ↓19 mos | ||||||||
| Ognibene, 2005 | ↓7-12 mos | ↑7-12 mos*** |
↓=performed worse than WT
↑=performed better than WT
↔=no different than WT
Y-maze: tendency to alternate arm choice, T-maze: choice of novel arm, MWM I: escape latency, MWM II: time spent in target quadrant after platform removal, RAM: errors, RAWM I: latency, RAWM II: errors
6month Tg animals differed from WT on last day of testing only
10 mos Tg animals differed from WT on days 2 and 4, but no differences were observed on day 1
elevated plus maze measure of exploration of arms
To investigate the exercise effects versus the potentially enriching effects of simply having a wheel in the cage, we included a wheel locked control group. While the main focus was on the aged AD model, we study also examined exercise-induced improvements on performance in aged WT mice. To our knowledge, no studies have previously examined the effects of exercise in the aged Tg2576 model. We hypothesized that cognitive deficits would be present in the TG mice compared to the WT mice. We further hypothesized that three weeks of voluntary wheel running would improve the cognitive performance of the TG mice. We do not hypothesize as great an improvement in cognition in the wheel locked group.
Materials and Methods
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. We used C57Bl6 (WT) and Tg2576 (TG) (Hsiao, 1997) from an established colony (ca. 2000) at University of California, Irvine. [17] We assigned WT (n=47) and TG (n=37) mice to exercise, sedentary or wheel locked groups at 16-18 months of age. Distribution of groups and genders is shown in Table 1. Running was voluntary and monitored by computer software (Vital-view©, Mini-Mitter) to record distance run by each animal over the three week period. Animals were cognitively tested at approximately 17-19 months of age.
Radial-arm water maze
The RAWM protocol used in this study was provided by Dr. David Morgan (Health Science Center, University of South Florida) (personal communication) and consisted of three days of testing. It has been previously used in studies of Tg2576 animals with success. [3, 34] We performed RAWM testing in a room with visual cues posted on the four walls. The maze consisted of five-arms, placed in a water bath such that the tops of the dividers were above water. We placed the platform in one arm for each mouse (arm placement randomized between animals), kept consistent throughout the first two days for that mouse. We placed individual mice in a different arm location sequentially on all trials. We tested the animals in cohorts of four animals, with a minimum of 30 minutes and a maximum of 60 minutes consolidation between blocks of 5 trials. Each animal received a total of 15 trials per day. At the end of each trial, the mouse was allowed to sit on the platform for 20 seconds. The experimenter guided the animal to the platform if it failed to find the platform on its own after 60 seconds. If the animal chose an incorrect arm (arm not containing platform), it was gently pulled back to the start arm. We scored animals on latency (i.e. time to find platform), errors (entries of entire body into arm not containing platform), and failures (failure to find the platform after 60 seconds) on days 1 and 2. We adapted the method for separating reference and working memory scores from Hyde et al as follows. [18] For all cognitive measurements, the experimenter scoring the animals was blind to group assignment and genotype.
Reference/ “Long-term” memory
For each block of five trials, the first trial after a 30+ minute consolidation period is considered a reference or “long-term” memory trial. Thus, on day 1, animals were scored for reference memory latency and errors on trials 6 and 11. On day 2, animals were scored on trials 1, 6, and 11. The first trial of day 2 occurs more than 12 hours after the last trial of day 1.
Working/ “Short term” memory
The remaining trials are working or “short-term” memory trials. Less than 4 minutes elapses between each working memory trial for each mouse. On day 1, we scored animals on working memory latency and errors on trials 1-5, 7-10, and 12-15. On day 2, animals were scored for latency and errors on trials 2-5, 7-10, and 12-15.
Failures
We recorded failures to locate the platform across both days 1 and 2. A failure consisted of an inability to find the platform after the maximum allowable time of 60 seconds.
Extinction
On day 3, we placed the platform in a new location for each animal. On the first five trials, we analyzed the number of times the animals returned to the old platform location as memory for the original platform location.
Control measures
We performed a final test for swim speed and a test with a visible platform one day after completion of testing. The swim speed task involved timing the animal over a fixed distance. For the visual task, we drained roughly 1 inch of water from the tank so that the platform surface was visible above the water line. We recorded latency to find and crawl onto the visible platform. If the animal failed climb onto the platform after 120 seconds in the visual task, it was given the maximum latency score of 120 seconds.
Statistics
A 2×3 repeated measures ANOVA was performed for reference memory latency and working memory errors. Posthoc analysis was by Fisher's LSD. Due to unequal variance, non-parametric tests (Friedman's rank test) were performed for the reference memory errors and working memory latency, and results analyzed posthoc using Dunnett's T3 post hoc comparison for groups of unequal variance. One-way ANOVAs were performed for genotype effects and gender effects on swim speed, visual task latency, and for failures. One way ANOVAs were also performed for genotype effects and gender effects on swim speed and visual task latency. Gender was run as a covariate in all other tests. It was found not to contribute significantly to measures other than swim speed and was therefore removed as a predictor. For all tests, p≤0.05 was considered significant.
Results
Prior to statistical analyses, we normalized animals by their total running distances over the three week intervention. To normalize running behavior, we excluded animals with running distances greater than or less than two standard deviations from the mean from all cognitive tests (total excluded animals: WT n=6, TG n=4). A separate analysis of the excluded animals showed effects on reference and working memory in the same direction as those discussed below. The average distance run per day of the included animals did not differ significantly between WT and TG (mean rotations per day were 4794 ± 996 and 4390 ± 820, respectively). Gender had no effect on distance run in either genotype.
We did not observe any significant differences in working or reference memory in WT mice with exercise or locked wheels compared to sedentary. Because only TG groups showed an effect of exercise and/or wheel presence, we will focus on their results in further analysis.
Failures
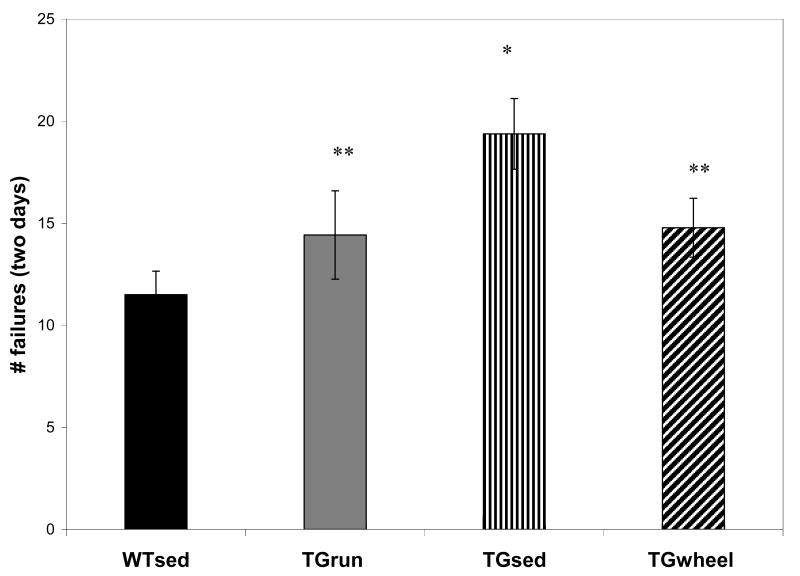
We analyzed the number of times animals achieved the maximum time allotted (60 seconds) without locating the platform as “failures.” We found significant effects of treatment condition (WHEEL, SED, RUN) and genotype (TG, WT) on number of failures across both days of testing using a one-way ANOVA for group assignment (F5, 69 =4.218, p=0.002). In post-hoc analysis, TGSED failed to find the platform significantly more often than WTSED (p=0.002) and TGRUN (p=0.02). Conversely, TGRUN did not statistically differ from WTSED. (Figure 1)
FIGURE 1.
Number of failures is defined as the number of trials in which animals reached the maximum allowable time (60 seconds) without locating the platform. TGSED had significantly more failures than WTSED, though TGRUN and TGWHEEL were not significantly different from WTSED. TGRUN and TGWHEEL had significantly fewer failures than TGSED. Error bars represent ± standard error. *sig. different from WTSED **sig. different from TGSED
Reference/ “Long term” memory
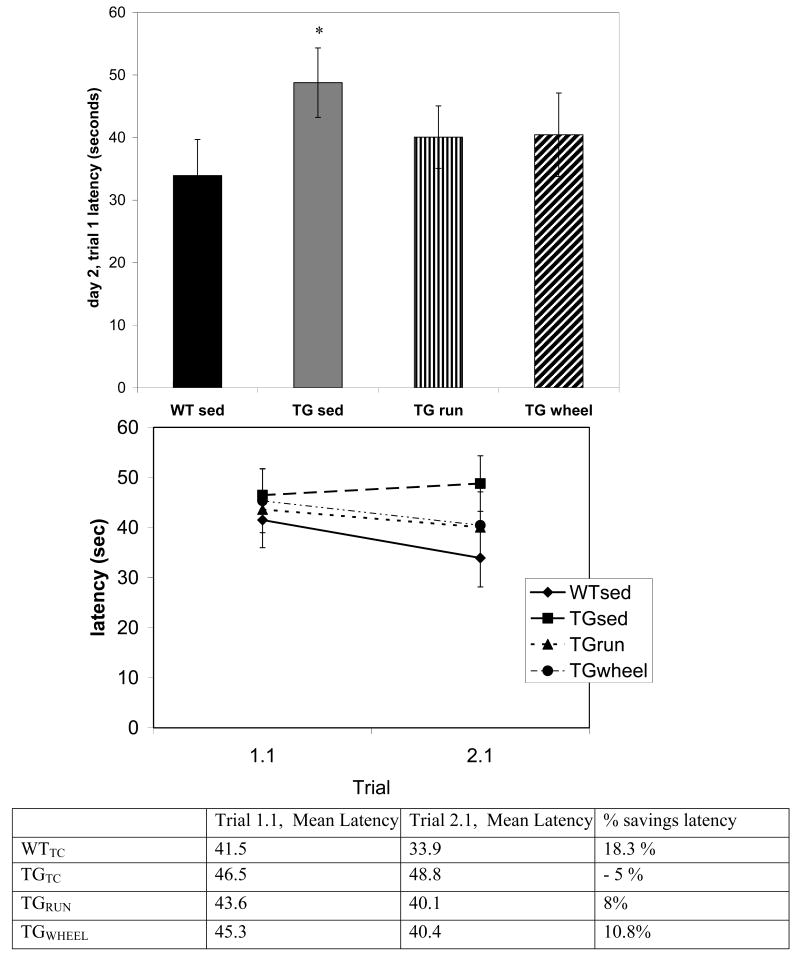
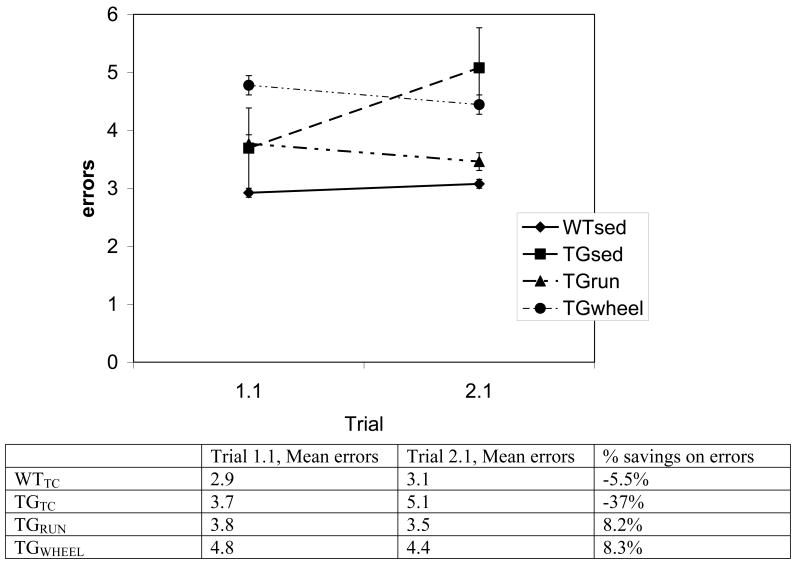
We found a significant effect of genotype (TG, WT) (F1, 69 =6.57, p=0.01) and also condition (RUN, WHEEL, SED) (F2, 69 =2.98, p=0.05) on reference memory latency during day 2 of testing. Posthoc analysis of individual trials by Fisher's LSD revealed that on the first day of testing, no significant differences existed for escape latency on trials 6 and 11. However, on the first trial of the second day, when more than 12h had passed since the previous trial, TGSED took significantly longer to find the platform than the WTSED (p=0.05) (Figure 2, top). Interestingly, at this timepoint the TGRUN does not differ from WTSED. The TGSED was the only group that did not improve (evaluated as percent savings) between day 1, trial 1, and day 2, trial 1. (Figure 2, bottom) We found no significant differences in reference memory errors (data not shown). However, it is of note than in reference memory errors, TGSED again failed to improve (% savings) from day 1, trial 1 to day 2, trial 1. (Figure 3).
FIGURE 2.
Reference memory latencies from day 2. On the first trial of day 2 of testing (2.1), TGSED mice took significantly longer to escape the RAWM. This trial occurs more than 12 hours after the previous day of testing. The increased latency indicates an impairment in reference memory for the platform location. TGRUN and TGWHEEL did not differ from the WTSED. The overall difference in latency between the first trial of day 1(1.1) and the first trial of day 2 (2.1) is shown in the lower panel as % savings: (mean trial 1.1 -mean trial 2.1) / mean trial 1.1. A positive value represents improvement and a negative value represents a decrement in performance between days. Error bars represent ± standard error. *sig. different from WTSED **sig. different from TGSED
FIGURE 3.
Reference memory errors from days 1 and 2. Errors did not differ significantly between groups. Overall differences from the first trial of day 1 (1.1) and the first trial of day 2 (2.1) are shown as % savings: (mean trial 1.1 - mean trial 2.1) / mean trial 1.1. A positive value represents improvement and a negative value represents a decrement in performance between days. TGSED demonstrated an increase in number of errors between day 1 and day 2. Error bars represent ± standard error.
Working/ “Short term” Memory
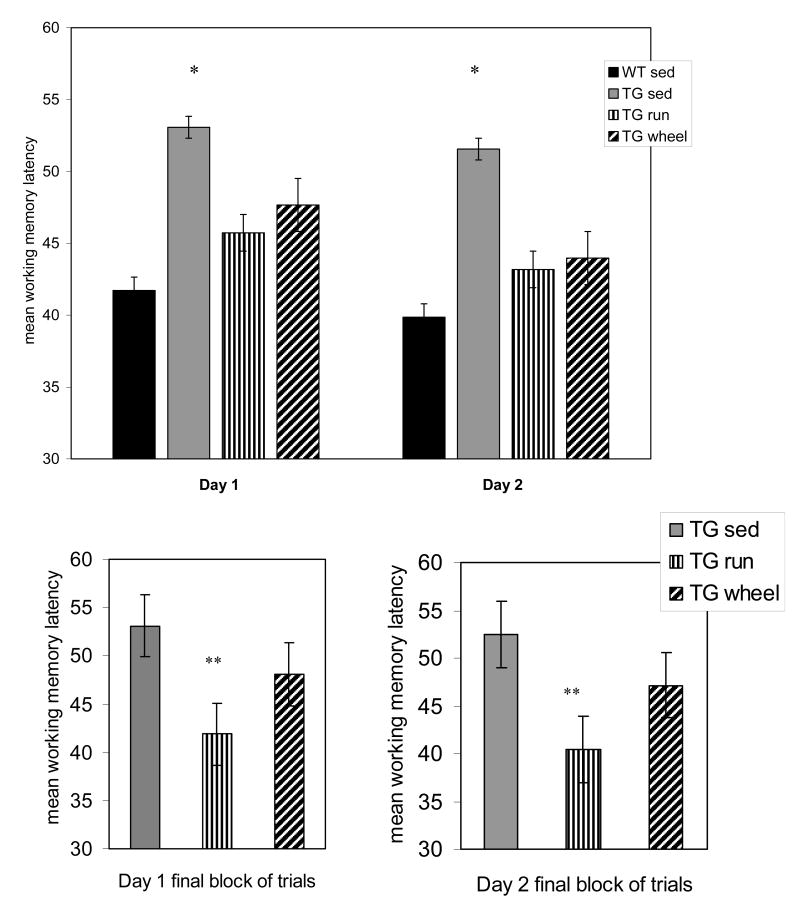
Group × Condition significantly affected working memory latency across trials (χ2 = 21.04, p=0.001). TGSED took significantly longer to find the platform on than WTSED across all working memory trials (p≤0.05). (Figure 4, top) TGRUN did not significantly differ from the WTSED on any trial. On the final group of trials (trials 11-15) on both days, TGRUN time to find the platform was significantly less than that of TGSED (day 1 p=0.005, day 2, p=0.02). (Figure 4, bottom) No differences were observed in number of errors during working memory trials.
FIGURE 4.
Average working memory latencies from days 1 and 2. TGSED demonstrated longer average escape latencies than WTSED on working memory trials across both days of testing. TGRUN and TGWHEEL did not differ from the WTSED. (top) TGRUN performed significantly better than TGSED on the third block of working memory trials on both days. (bottom) Error bars represent ± standard error. *sig. different from WTSED **sig. different from TGSED
Extinction
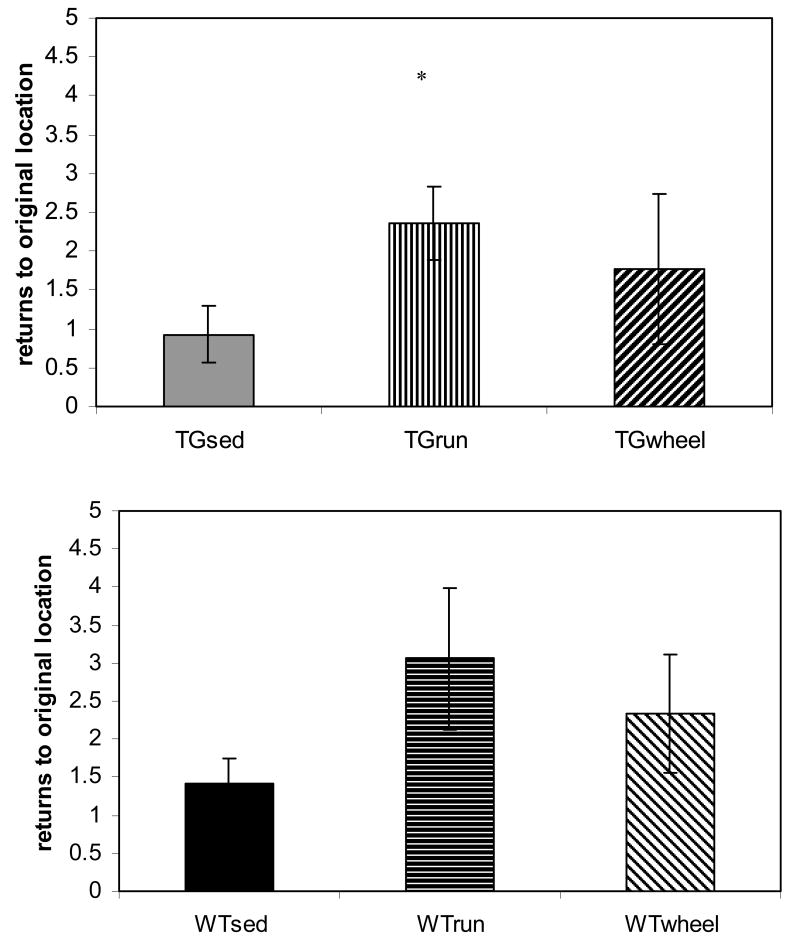
On the third day of testing, we moved the platform to a new location. Animals who learn the task on days 1 and 2 return more frequently to the old arm during this testing. As expected, the TGRUN animals returned to their old platform location significantly more often than the TGSED animals on the first two trials of day 3 (p=0.02). (Figure 5, top) WTRUN also exhibited a similar trend to return to the old arm more than WTSED over the first five trials, though it did not achieve statistical significance (p=0.1). (Figure 5, bottom)
FIGURE 5.
In reversal training, returns to the original arm indicate a higher amount of retention for the platform's previous location, similar to a probe trial in standard Morris water maze. TGRUN animals returned to their old arm significantly more than TGSED (p=0.02). WTRUN had a trend to return to the old arm more than WTSED over the first five trials (p=0.1). Error bars represent ± standard error.*sig. different from WTSED **sig. different from TGSED
Wheel locked animals
In the post-hoc analysis of failures, TGWHEEL showed fewer failures to find the platform than TGSED (p=0.04) and like TGRUN, no longer differed from WTSED. (Figure 1) On the long-term memory trial, comparing trial 1 of day 2 to trial 1 of day 1, TGWHEEL showed a decrease in escape latency such that it no longer differed from WTSED. (Figure 2, top) Interestingly, when we look at percent savings from day 1 to day 2, TGWHEEL improved similar to TGRUN. (Figure 2, bottom) Like TGRUN, TGWHEEL did not significantly differ from the WTSED on any working memory/short-term memory trial. However, on the final group of working memory trials (trials 11-15) on both days, TGWHEEL improved to point midway between the TGSED and TGRUN. (Figure 4, bottom) Unlike TGRUN, the TGWHEEL group did not differ from TGSED group on day 3 of testing. TGWHEEL did not show an increased tendency to return to the old platform location. (Figure 5, top)
Control measures
Mean swim speeds and latencies to find the visible platform are shown in Table 2. We did not observe any differences in swim speed between TG and WT groups. Regardless of genotype, males showed significantly slower swim speeds than females (p=0.03). It is important to note that we did not observe any significant differences in swim speed between treatments in either genotype (RUN, WHEEL, SED). In testing for latency to find the visible platform, we found no differences in visual task times due to gender. The mean time to find and mount the platform was lower in pooled WT animals (i.e. all 3 conditions) than the mean of all TG animals (p<0.01). This was not perceived as a vision failure, as TG mice approached the platform and then swam away from it (instead of crawling onto it), but rather a failure in ability to shift strategy to locate the platform with dividers absent (see discussion). Further, we observed no significant differences between conditions in either genotype in finding the visible platform.
TABLE 2.
| WTSED m=3, f=8 |
TGSED m=6, f=5 |
WTRUN m=8, f=9 |
TGRUN m=8, f=4 |
WTWHEEL m=3, f=7 |
TGWHEEL m=4, f=3 |
ALL WT
m=14, f=24 |
ALL TG
m=18, f=12 |
|
|---|---|---|---|---|---|---|---|---|
| Swim speed (meters/sec) | 0.15±0.02
m=0.18±0.06 f=0.14±0.02 |
0.11±0.03
m=0.05±0.01 f=0.23±0.02 |
0.16±0.03
m=0.15+0.03 f=0.17+0.04 |
0.18±0.03
m=0.16±0.03 f=0.23±0.04 |
0.23±0.02
m=0.29±0.00 f=0.22±0.02 |
0.19±0.03
m=0.17±0.03 f=0.11±0.00 |
0.15±0.01
m=0.17±0.02 f=0.17±0.02 |
0.17±0.01
m=0.11±0.02 f=0.22±0.02 |
| Visual Task (sec) | 25.6±5.7
m=21±6.7 f=26.7±14 |
54.4±15*
m=59.5+20.5 f=55.2+26.5 |
31.8±5.7
m=18.5+3.1 f=32.9±8.9 |
46.6±10
m=48.6±16.5 f=25.5±11.7 |
30.0±11
m=18.3±2.2 f=35±15 |
57.4±16
m=82±21.9 f=24.7±0.88 |
27.0±3.9
m=18.8±2.5 f=31.45±5.7 |
50.9±8*
m=59.7±10.9 f=37.7±11.8 |
WTSED= sedentary wild-type, WTRUN= exercised wildtype, WTWHEEL=locked wheel exposed wildtype
TGSED=sedentary Tg2576, TGRUN= exercised Tg2576, TGWHEEL=locked wheel exposed Tg2576
ALL= grouped conditions for given genotype
significantly different from WT
Discussion
Using the RAWM, our study reveals clear reference/long-term memory deficits in TG at 17-19 months of age, compared to their WT counterparts, as well as impaired working or short-term memory. Both impairments are reduced in the Tg2576 mouse exposed to three weeks of exercise. In addition, three weeks exposure to a locked wheel also reduces the TG impairment on long term memory, as revealed on trial 1 of day 2. The TGRUN mice were indistinguishable from the WTSED on all memory measures. The TGRUN animals showed significant improvement on working memory measures compared to the TGSED. This is not true of the TGWHEEL animals, which showed moderate, but not significant, improvement compared to TGSED. TGRUN returned to the original platform location significantly more often than TGSED during the extinction trials on day 3 (when the platform was moved), confirming improved long term memory, but perhaps also suggesting a decreased extinction of previously learned platform location. These data are the first to indicate that voluntary exercise improves memory impairment in aged animals transgenic for Alzheimer's pathology.
TG animals exposed to an immobile running wheel (TGWHEEL) were also indistinguishable from WTSED animals in most working and reference memory measures and in number of failures. However, the TGWHEEL group did not improve as much as TGRUN on the final block of working memory trials, nor did the TGWHEEL show the preference for the old platform location on day 3 extinction trials as TGRUN. The TGWHEEL group showed performance at levels intermediate to the TGSED and TGRUN groups on the final block of working or short term memory trials. These data indicate that the presence of a locked wheel in an animal's home cage partially improves performance on the RAWM, but not as extensively as exercising on the wheel. The locked wheel itself may have served as a mild form of enrichment, consistent with a report by Arendash et al. (2004) in which similar decreases in escape latency in the RAWM following environmental enrichment were observed. [4] Our data are also interesting in light of the recent Pietropaolo study in which enrichment effects were studied with and without exercise by having either free or immobile wheels in the enriched environment. [28] In this enriched environment, there was equivalent improvement of the immobile wheel and free wheel groups. It is possible the larger housing of the enriched environment itself provided greater physical activity. In the standard (non-enriched) environment, differences in extinction tasks were obvious between mice with an immobile wheel and mice with a free wheel. Similar to our day 3 extinction trials the standard housed animals with a free wheel showed difficulty extinguishing prior learning compared to the standard cages with immobile wheels. It is unfortunate that the study did not further assess comparisons between the standard housed free wheel and immobile wheel groups. Our data suggest that both groups show improvement, but that the effect of the free wheel is greater than that of the immobile wheel's presence alone. It is impossible to say with any certainty if this was due to enrichment or exercise on the immobile wheel, though Koteja et al. (1999) have shown wheel climbing activity in mice provided a locked wheel nearly equal to running in mice with a free wheel. [23] It is possible that the mice in our locked wheel group climbed on the immobile wheel resulting in an unanticipated exercise effect.
The TGSED mice showed a greater number of failures than WTSED animals. It is not possible from our data to discern if these failures represent a failure to learn or simply reflect a lack of motivation in the TGSED animals. Importantly, the TGWHEEL and the TGRUN groups did not differ from WTSED group on number of failures, indicating that both conditions improve performance either by improving learning or increasing motivation.
Voluntary exercise did not alter reference or working memory performance in the WT animals. This conflicts with the recent findings of van Praag et al., who showed 45 days of running improved MWM performance in 19 month old C57Bl6 (WT) mice. [32] It is possible that the extra 24 days of running employed by van Praag (2005) represented the threshold for improvement in the WT mouse. Differences in protocol for the two tests may also contribute, as van Praag et al. did not see improvements in escape latency in their WTRUN mice, but rather increased amount of time in the target quadrant. Our protocol did not include any quadrant time analysis, only latency and error. Finally, MWM involves repeated testing over a longer period of time (5 days) compared to the 3 day testing protocol used in the RAWM procedure. It is possible that the extended testing period allowed for greater memory consolidation and thus allowed for greater differences to be observed in the WT mice. In support of this idea, WTRUN mice do show a trend to return to the original platform more often than WTSED on the extinction trials of day 3.
For the TG animals, latency to find the visual platform was significantly higher than that of the WT animals. We do not believe this is due to any visual dysfunction, as we observed animals swimming up to and around the visual platform, rather than approaching it and climbing onto it like WT animals. TGRUN animals showed improvement in finding the platform across the testing days, which relies on the ability to use the visual cues in the room. It has been suggested by King & Arendash (2002) that the Tg2576 mouse displays fear of the platform, but as the animals in our study do approach the platform, it is hard to interpret the behavior. [21] We suggest that future studies measure time to locate the platform and make contact, rather than climbing onto the platform. The visual task requires a partial strategy shift in that the arms of the maze are no longer present (though spatial cues are still present). It is possible aged Tg2576 mice are impaired in making this shift in strategies. Previous studies have observed deficiencies in strategy/task shifting in the Tg2576 mouse. [2, 19] This inability to shift strategies may be analogous to executive functions of planning and problem solving in the human. [33] Our extinction data could also be interpreted as further demonstrating this impairment. While the return to the old platform location represents a better memory for the previous location, it also represents a failure to shift strategies and begin searching for a new location.
Conclusion
While three weeks of exercise or immobile wheel exposure did not eliminate spatial learning and memory deficits in TG mice, both interventions did improve RAWM performance and decrease failure rates to those observed in WT mice. The effects were greatest in the TGRUN animals. The lack of extensive improvement in the WTRUN and WTWHEEL, coupled with previous studies showing longer exercise exposures do result in improvements in WT mice, suggests that the threshold for exercise-induced learning improvement may be lower in the TG animals. [32] Perhaps because of their greater impairment, aged TG mice exhibit the positive cognitive effects of an exercise regime sooner than WT mice. This is consistent with the concept of cognitive reserve translated to human study. The cognitive reserve hypothesis states that the more cognitive reserve (education, intellect, social interaction, etc.) an individual has, the longer a cognitive deficit will take to appear. It follows then that perhaps the more cognitively impaired or at risk an individual is, the easier it may be to effect a noticeable improvement. Recent findings by Etnier et al. (2007) indicate a greater cognitive effect of exercise in women who carry a genetic risk factor for AD, while little effect is observed in non-carriers. [14]
Perhaps more exciting is that our data supports the meta-analysis of exercise studies in the elderly cognitively impaired by Heyn et al. (2004), indicating that those in late stages of Alzheimer's pathology could show cognitive improvement with exercise even if that exercise is not begun until after the pathology is present. [16] While a total reversal of cognitive deficits was not observed, our findings lend hope to those patients looking to improve their cognitive function who have not exercised in life prior to diagnosis with Alzheimer's. Our data also suggest that the threshold for exercise induced improvement may be lower in cases of Alzheimer pathology than in normal age related decline.
Acknowledgments
Support contributed by NIAMSD AR047752 and NIA AG000538
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124(4):985–92. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of alzheimer's disease. J Neurosci. 2005;25(17):4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1(4):1671–9. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 4.Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged alzheimer's transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15(11):1751–4. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- 5.Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D. Behavioral assessment of alzheimer's transgenic mice following long-term abeta vaccination: Task specificity and correlations between abeta deposition and spatial memory. DNA Cell Biol. 2001;20(11):737–44. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- 6.Ari Z, Kutlu N, Uyanik BS, Taneli F, Buyukyazi G, Tavli T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int J Neurosci. 2004;114(5):623–37. doi: 10.1080/00207450490430499. [DOI] [PubMed] [Google Scholar]
- 7.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2(3):271–6. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 8.Chen GH, Wang YJ, Wang XM, Zhou JN. Accelerated senescence prone mouse-8 shows early onset of deficits in spatial learning and memory in the radial six-arm water maze. Physiol Behav. 2004;82(5):883–90. doi: 10.1016/j.physbeh.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23(5):941–55. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 10.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 12.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, Nickenig G, Kuschinsky W, Dirnagl U, Laufs U. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54(5):582–90. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 13.Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22(1):157–71. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 14.Etnier JL, Berry M. Fluid intelligence in an older copd sample after short- or long-term exercise. Med Sci Sports Exerc. 2001;33(10):1620–8. doi: 10.1097/00005768-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Filteau SM, Menzies RA, Kaido TJ, O'Grady MP, Gelderd JB, Hall NR. Effects of exercise on immune functions of undernourished mice. Life Sci. 1992;51(8):565–74. doi: 10.1016/0024-3205(92)90225-e. [DOI] [PubMed] [Google Scholar]
- 16.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice [see comments] Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 18.Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: Learning in three inbred strains of mice. Brain Res. 1998;785(2):236–44. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- 19.Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of alzheimer's disease. J Neurosci. 2005;25(21):5217–24. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52(2):135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 21.King DL, Arendash GW. Behavioral characterization of the tg2576 transgenic model of alzheimer's disease through 19 months. Physiol Behav. 2002;75(5):627–42. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking nmda receptor epsilon 1 subunit. Neurosci Res. 2003;47(1):55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- 23.Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim Behav. 1999;58(6):1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- 24.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–13. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 25.McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: A brief review. Brain Behav Immun. 2004;18(3):214–20. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of alzheimer's disease. Nature. 2000;408(6815):982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 27.Morris RGM. Spatial localization does not require the presence of local cues. Learning and Motivation. 1981;12(2):239–260. [Google Scholar]
- 28.Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: A behavioral analysis. Behav Neurosci. 2006;120(4):787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- 29.Schafer D. No old man ever forgot where he buried his treasure: Concepts of cognitive impairment in old age circa 1700. J Am Geriatr Soc. 2005;53(11):2023–7. doi: 10.1111/j.1532-5415.2005.53558.x. [DOI] [PubMed] [Google Scholar]
- 30.Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ. Repetitive mild brain trauma accelerates abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of alzheimer amyloidosis. J Neurosci. 2002;22(2):446–54. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 32.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 34.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26(20):5340–6. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]