Abstract
Background
Antibody-dependent cellular cytotoxicity (ADCC) is greatly enhanced by the absence of the core fucose of oligosaccharides attached to the Fc, and is closely related to the clinical efficacy of anticancer activity in humans in vivo. Unfortunately, all licensed therapeutic antibodies and almost all currently-developed therapeutic antibodies are heavily fucosylated and fail to optimize ADCC, which leads to a large dose requirement at a very high cost for the administration of antibody therapy to cancer patients. In this study, we explored the possibility of converting already-established antibody-producing cells to cells that produce antibodies fully lacking core fucosylation in order to facilitate the rapid development of next-generation therapeutic antibodies.
Results
Firstly, loss-of-function analyses using small interfering RNAs (siRNAs) against the three key genes involved in oligosaccharide fucose modification, i.e. α1,6-fucosyltransferase (FUT8), GDP-mannose 4,6-dehydratase (GMD), and GDP-fucose transporter (GFT), revealed that single-gene knockdown of each target was insufficient to completely defucosylate the products in antibody-producing cells, even though the most effective siRNA (>90% depression of the target mRNA) was employed. Interestingly, beyond our expectations, synergistic effects of FUT8 and GMD siRNAs on the reduction in fucosylation were observed, but not when these were used in combination with GFT siRNA. Secondly, we successfully developed an effective short hairpin siRNA tandem expression vector that facilitated the double knockdown of FUT8 and GMD, and we converted antibody-producing Chinese hamster ovary (CHO) cells to fully non-fucosylated antibody producers within two months, and with high converting frequency. Finally, the stable manufacture of fully non-fucosylated antibodies with enhanced ADCC was confirmed using the converted cells in serum-free fed-batch culture.
Conclusion
Our results suggest that FUT8 and GMD collaborate synergistically in the process of intracellular oligosaccharide fucosylation. We also demonstrated that double knockdown of FUT8 and GMD in antibody-producing cells could serve as a new strategy for producing next-generation therapeutic antibodies fully lacking core fucosylation and with enhanced ADCC. This approach offers tremendous cost- and time-sparing advantages for the development of next-generation therapeutic antibodies.
Background
Antibodies of the human IgG1 isotype containing two biantennary complex-type N-linked oligosaccharides in the constant region (Fc) [1] are commonly used therapeutically. As regards cancer treatment in particular, the antibody effector function of antibody-dependent cellular cytotoxicity (ADCC) is known to be important and is closely related to clinical efficacy in humans in vivo [2-4]. Through the Fc, therapeutic antibodies can mediate effector functions, and ADCC is greatly influenced by Fc oligosaccharide structure [5,6]. Removal of the core fucose from Fc oligosaccharides is widely recognized as being important for the effector function of ADCC [7,8]. Antibodies in which the Fc oligosaccharide structure lacks the core fucose exhibit more potent efficacy than do fucosylated antibodies, both in vitro and in vivo [9-13]. Therapeutic antibodies fully lacking core fucosylation are able to escape the inhibitory effects of both human serum IgG and other contaminating fucosylated antibody ingredients to achieve optimal ADCC [6,14-17]. Unfortunately, almost all licensed therapeutic antibodies developed to date are heavily fucosylated, i.e., the majority of antibody molecules possess Fc oligosaccharides with the core fucose [18,19], which results in a failure to optimize ADCC. The presence of this core fucose is largely due to the fact that the antibodies are produced by rodent mammalian cell lines with intrinsic fucosyltransferase activity (e.g., Chinese hamster ovary (CHO), mouse myeloma NS0 and SP2/0, and mouse hybridoma cell lines).
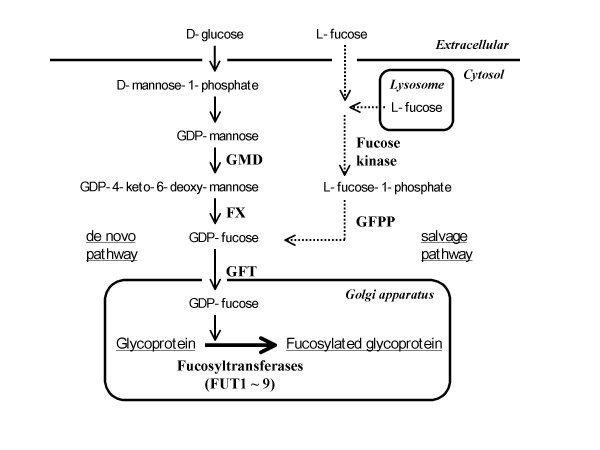
In mammalian cells, core fucosylation of the Fc oligosaccharides is mediated by the only gene, α1,6-fucosyltransferase (FUT8), that catalyzes the transfer of fucose from GDP-fucose to the innermost N-acetylglucosamine (GlcNAc) of Fc oligosaccharides via an α1,6-linkage [20]. The intracellular GDP-fucose, an essential substrate of oligosaccharide fucosylation, is synthesized in the cytoplasm via both a de novo pathway and the salvage pathway shown in Fig. 1. The de novo pathway transforms GDP-mannose, which originates from D-glucose taken into the cytoplasm from the extracellular environment, to GDP-fucose, via three enzymatic reactions carried out by two proteins: GDP-mannose 4,6-dehydratase (GMD) and GDP-keo-6-deoxymannose 3,5-epimerase, 4-reductase (FX) [21,22]. The salvage pathway synthesizes GDP-fucose from free L-fucose derived from extracellular or lysosomal sources. Most of the intracellular GDP-fucose is generated via the de novo pathway, and the metabolite-free L-fucose is also reutilized through the salvage pathway [22]. The GDP-fucose, which accumulates in the cytoplasm, is transported into the lumen of the Golgi apparatus by a GDP-fucose transporter (GFT) anchored at the Golgi membrane [23], and then serves as a substrate in the synthesis of fucosylated glycoconjugates by fucosyltransferases [22,24,25].
Figure 1.
Oligosaccharide fucosylation and GDP-fucose synthesis in mammalian cells. In mammalian cells, GDP-fucose is synthesized via two distinct pathways, the de novo and salvage pathways. The transport of GDP-fucose into the Golgi apparatus, where the fucosyltransferases are located, is accomplished by a GDP-fucose transporter.
To date, only a few studies have addressed the regulation of Fc oligosaccharide fucosylation in mammalian cells using the following approaches: 1) the application of a mutant CHO cell line, Lec13, partially deficient in GMD [7] or that of a rat hybridoma cell line, YB2/0 [8], as host cells; 2) the introduction of a small interfering RNA (siRNA) against FUT8 [26]; and 3) the co-expression of β-1,4-N-acetylglucosaminyltransferase III (GnT-III) and Golgi α-mannosidase II (ManII) [27]. Of these, the gene knockout of FUT8 and GMD is the only strategy for manufacturing fully non-fucosylated recombinant therapeutics in mammalian cells [28-30]. However, gene targeting in mammalian somatic cells is difficult to achieve and is a laborious and time-consuming process, because in somatic cells, non-homologous recombination events occur several orders of magnitude more frequently than homologous recombination [28,31]. Thus, gene targeting in mammalian somatic cells remains very difficult to apply to each antibody-producing clone as a simple means of controlling fucosylation.
In this study, we explored the possibility of the high-frequency conversion of already-established antibody-producing cells (1st-generation) to cells that produce antibodies fully lacking core fucosylation (2nd-generation) within a few months. Moreover, it was considered industrially useful to generate 2nd-generation cells that would yield the equivalent antibody productivity of the original cells, without inducing any changes in cell character beyond the lack of fucosylation. To this end, we applied an RNA interference (RNAi) technique in combination with a cellular phenotypic selection strategy using Lens culinaris agglutinin (LCA) lectin, which recognizes the α1,6 fucosylated trimannose-core structure of N-linked oligosaccharides and commits cells expressing this structure to a cell-death pathway. In our previous study, single-gene knockdown of FUT8 resulted in a substantial, but not full, reduction of antibody fucosylation in the products [26]. Here, we identified synergistic effects of the double knockdown of FUT8 and GMD on oligosaccharide fucosylation in mammalian cells. This finding enabled us to design a new conversion strategy for the manufacture of next-generation therapeutic antibodies fully lacking core fucosylation and with enhanced ADCC.
Results
Single gene knockdown of FUT8, GMD, and GFT in antibody-producing cells
Three constitutive short hairpin siRNA expression vectors against Chinese hamster FUT8, GMD, and GFT were generated and introduced into an IgG1 antibody-producing CHO/DG44 clone, 32-05-12 [26], to evaluate the effects of target gene knockdown on the levels of fucosylation of the products. Puromycin-resistant clones transformed by the vectors appeared after transfection with a transformation efficiency of approximately 1,300 per 1.6 × 106 electroporated cells, irrespective of the vector differences. However, subsequent selection of LCA-resistant clones showed clear differences in the appearance of surviving colonies. The ratios of LCA-resistant clones to puromycin-resistant clones transformed by the three siRNA expression vectors against FUT8, GMD, and GFT were 11.5%, 14.8%, and 0.8%, respectively (Table 1). The resultant LCA-resistant clones were expanded in medium without LCA, and the mRNA expression of FUT8, GMD, and GFT, as well as the oligosaccharide structure of the antibodies produced in these clones, were quantitatively analyzed. The target gene mRNA was reduced to a level exceeding 90% in clones appearing in all three siRNA-introduced transformants; however, in none of the clones was Fc oligosaccharide fucosylation completely abolished. The most effective rates of reduction in fucosylation among the clones transformed by the FUT8, GMD, and GFT siRNA expression vectors were approximately 72%, 79%, and 42%, respectively.
Table 1.
Ratio of LCA-resistant clones to drug-resistant clones transformed by the introduction of siRNA expression vectors
| Vector | Target mRNA | Drugr clonesa | LCAr clonesb | % LCAr/Drugr |
| FUT8shRNA/lib3/pPUR | FUT8 | 1344 | 155 | 11.5 |
| pPUR/GMDshB | GMD | 1440 | 213 | 14.8 |
| GFT_3G10/pPUR | GFT | 1296 | 11 | 0.8 |
| FT8lib3_GMDB/pAGE | GMD·FUT8 | 1500 | 730 | 48.7 |
aDrugr, puromycin resistance or hygromycin resistance.
bLCAr, LCA resistance
Synergistic effects of double knockdown of FUT8 and GMD on fucosylation
To explore the effects of double knockdown of the target genes FUT8 and GMD, two GMD siRNA-introduced clones exhibiting a maximum reduction in product fucosylation, designated as GMDb2 and GMDb5, were selected and re-electroporated with the FUT8 siRNA expression vector. LCA-resistant clones were selected in the presence of 100 μM L-fucose after drug-resistant selection had been carried out, and two clones expressing highly non-fucosylated antibodies were selected independently from each GMD siRNA-introduced clone in the medium. In the four established clones, the FUT8 mRNA expression levels decreased to roughly one-tenth of the parental expression levels, while the original 90% decrease in GMD mRNA was retained. Monosaccharide composition analysis showed that the ratio of non-fucosylated oligosaccharides of the antibodies produced by the four clones had increased significantly in each case (i.e., up to 91%, 95%, 97%, and 98%), compared to those of parental GMD siRNA-introduced clones (Table 2). The increase in non-fucosylated product levels was cancelled when the clones were cultured in the presence of L-fucose; this cancellation was ascribed to the absence of a GMD knockdown effect due to activation of the salvage pathway of GDP-fucose synthesis. No significant effect on fucosylation levels in antibody-producing cells was observed with a combination of either FUT8 and GFT or GMD and GFT double knockdown.
Table 2.
Monosaccharide composition of the N-linked oligosaccharide core structure of IgG1 from clones transformed with siRNA expression vectors
| Clone Name | siRNAa | LFb | Relative composition of monosaccharide | Fucose(-)%d | ||
| Fucosec | GlcNAc | Mannose | ||||
| 32-05-12 | - | - | 0.97 | 4.00 | 2.67 | 3 |
| GMDb2 | GMD | - | 0.22 | 4.00 | 2.52 | 78 |
| GMDb5 | GMD | - | 0.21 | 4.00 | 2.53 | 79 |
| GMDb2-F3 | GMD·FUT8 | - | 0.02 | 4.00 | 2.70 | 98 |
| GMDb2-F5 | GMD·FUT8 | - | 0.03 | 4.00 | 2.71 | 97 |
| GMDb5-F2 | GMD·FUT8 | - | 0.05 | 4.00 | 2.77 | 95 |
| GMDb5-F4 | GMD·FUT8 | - | 0.09 | 4.00 | 2.70 | 91 |
| 32-05-12 | - | + | 0.94 | 4.00 | 2.56 | 6 |
| GMDb2 | GMD | + | 0.94 | 4.00 | 2.56 | 6 |
| GMDb5 | GMD | + | 0.92 | 4.00 | 2.60 | 8 |
| GMDb2-F3 | GMD·FUT8 | + | 0.19 | 4.00 | 2.52 | 81 |
| GMDb2-F5 | GMD·FUT8 | + | 0.21 | 4.00 | 2.54 | 79 |
| GMDb5-F2 | GMD·FUT8 | + | 0.19 | 4.00 | 2.55 | 81 |
| GMDb5-F4 | GMD·FUT8 | + | 0.33 | 4.00 | 2.68 | 67 |
aGMD, introduction of GMD siRNA expression vector; GMD·FUT8, introduction of FUT8 siRNA expression vector to GMD siRNA-introduced cells.
bLF, addition of 100 μM L-fucose into the culture medium.
cMolar ratios calculated vs. 4 GlcNAc.
dTotal percentage of non-fucosylated Fc oligosaccharides calculated by the formula (1-c) × 100.
Conversion of fucosylated antibody-producing cells to non-fucosylated antibody producers
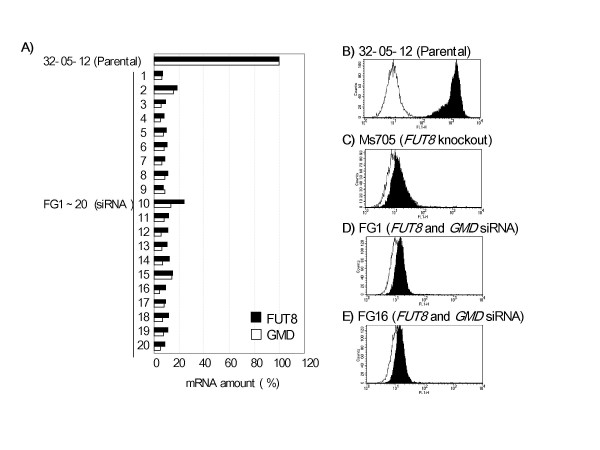
To facilitate the double knockdown of FUT8 and GMD, the siRNA tandem expression vector for targeting these genes was generated and introduced into IgG1 antibody-producing CHO/DG44 32-05-12 cells. Hygromycin-resistant clones transformed by the vectors appeared with a common transformation efficiency of approximately 1,500 per 1.6 × 106 electroporated cells. However, subsequent selection of LCA-resistant clones revealed clear differences in the appearance of surviving colonies, as compared to that of single siRNA-introduced clones (Table 1). The ratio of LCA-resistant clones to hygromycin-resistant clones transformed by the siRNA tandem expression vector was much higher (more than triple) than that of clones transformed by the vector bearing each single siRNA expression cassette; approximately half of the hygromycin-resistant clones showed resistance to LCA, even in the culture condition containing L-fucose. Among the resultant LCA-resistant clones, twenty were randomly selected and expanded in medium lacking both LCA and L-fucose. The mRNA expression of both target genes, FUT8 and GMD, in each clone decreased to approximately less than 20% of that of the parental clone (Fig. 2A). Out of twenty clones, eight exhibited relatively low levels of both target genes, and these were selected for further analysis of the oligosaccharide structure of the antibody products. The Fc oligosaccharide structures of the antibodies produced by these eight clones showed almost complete non-fucosylation; there were no detectable L-fucose residues among the products from six clones (Table 3). These six clones exhibited very low reactivity to LCA, even after adaptation to serum-free medium, as was also observed in the case of the FUT8-knockout CHO cell line Ms705. The results obtained from two representative clones, designated as FG1 and FG16, are shown in Fig. 2B–2E.
Figure 2.
Analyses of clones transformed by the FUT8 and GMD siRNA tandem expression vector. The relative amounts of FUT8 (filled columns) and GMD (open columns) mRNA were quantified in the tandem siRNA expression vector-introduced LCA-resistant cells and parental cells (A). Each cell type was harvested after 3-day culture and the samples were analyzed by real-time PCR. Each mRNA amount was normalized to the amount of β-actin mRNA; the results are shown as the relative percent with respect to the parental cells (100%). The LCA reactivity of cells (32-05-12 (B), Ms705 (C), FG1 (D), and FG16 (E)) was analyzed after adaptation of the cells to serum-free medium. Each cell type was harvested after 6-day culture and stained with FITC-labeled LCA (filled peak) or FITC-labeled streptavidin (open peak) as a negative control, and the results were analyzed by FACS.
Table 3.
Monosaccharide composition of the N-linked oligosaccharide core structure of IgG1 from clones transformed with the FUT8 and GMD siRNA tandem expression vector
| Clone Name | siRNAa | Relative composition of monosaccharide | Fucose(-)%c | ||
| Fucoseb | GlcNAc | Mannose | |||
| 32-05-12 | - | 0.96 | 4.00 | 2.60 | 4 |
| FG1 | + | n.d. | 4.00 | 2.58 | 100 |
| FG3 | + | n.d. | 4.00 | 2.65 | 100 |
| FG4 | + | n.d. | 4.00 | 2.63 | 100 |
| FG7 | + | 0.02 | 4.00 | 2.57 | 98 |
| FG9 | + | n.d. | 4.00 | 2.61 | 100 |
| FG13 | + | 0.02 | 4.00 | 2.55 | 98 |
| FG16 | + | n.d. | 4.00 | 2.57 | 100 |
| FG20 | + | n.d. | 4.00 | 2.62 | 100 |
aIntroduction of FUT8 and GMD siRNA tandem expression vector.
bMolar ratios calculated vs. 4 GlcNAc; n.d., not detectable.
cTotal percentage of non-fucosylated Fc oligosaccharides calculated by the formula (1-b) × 100.
Serum-free fed-batch culture of siRNA-introduced cells producing non-fucosylated antibodies
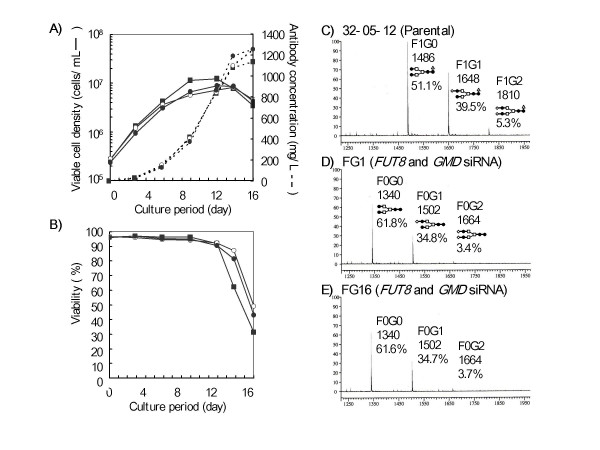
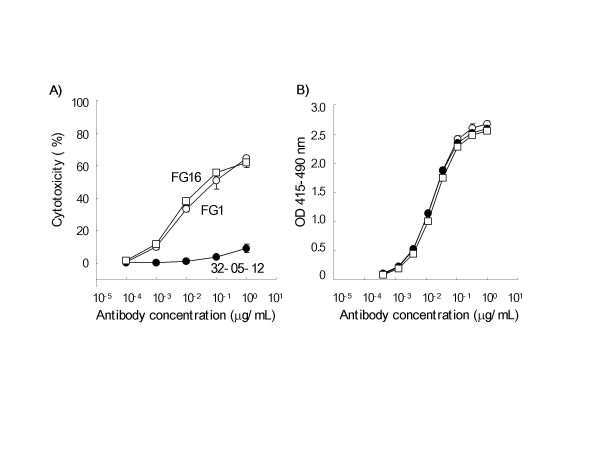
Serum-free fed-batch culture of the clones transformed by the FUT8 and GMD siRNA tandem expression vector was carried out using 1L-scale spinner bioreactors with pH and DO controls. Two FUT8 and GMD siRNA-introduced clones, FG1 and FG16, and their parental IgG1-producing clone, CHO/DG44 32-05-12, were adapted to serum-free medium, and the performance of the fed-batch cultures was compared in a head-to-head analysis (Fig. 3A, 3B). The fed-batch cultures were maintained until the cell viability decreased to less than 50%, which occurred at day 16 post-inoculation. Culture aliquots were taken at days 3, 6, 9, 12, 14, and 16 to analyze the cells and antibody products. Two siRNA-introduced cell lines grew logarithmically, with a slight difference in the specific production rate, maximum viable cell density, and the day upon which the viable cell density reached the maximum level; two siRNA-introduced hygromycin-resistant descendants showed slightly slower growth and death rates than those of the parent cells. However, the productivity of two clones transformed by FUT8 and GMD siRNAs reached a production level of approximately 1200 mg/L, which was basically equivalent to that of the parental cells at the end of the fed-batch culture period. During the culture period, the levels of cellular FUT8 and GMD mRNAs in the two siRNA-introduced clones remained at the original low level, i.e., less than 20% of the parental level. Monosaccharide composition analysis revealed that the two siRNA-introduced clones stably produced non-fucosylated antibodies throughout the culture period (Table 4). The oligosaccharide profile analysis of products purified from the final culture medium confirmed that the N-linked Fc oligosaccharides of the products were of the biantennary complex type, and the products from the two siRNA-introduced clones fully lacked core fucosylation (Fig. 3D, 3E). It was also confirmed that there was no significant change in the oligosaccharide profile during the culture period, and the non-fucosylated products showed two orders of magnitude higher ADCC than the antibodies from the parental CHO/DG44 cells, without any changes in antigen binding (Fig. 4).
Figure 3.
Serum-free fed-batch culture of clones transformed by the FUT8 and GMD tandem siRNA expression vector. Serum-free fed-batch culture using a 1L spinner bioreactor was carried out using cells transformed by the FUT8 and GMD tandem siRNA expression vector (FG1 (open circles) and FG16 (filled circles)). The parental cell line 32-05-12 (filled squares) was cultured as a control. Viable cell density (A, solid lines), antibody concentration in the culture supernatant (A, dotted lines), and cell viability (B) were analyzed in the fed-batch culture. The oligosaccharide structures of the final products from 32-05-12 (C), FG1 (D), and FG16 (E) were analyzed using MALDI-TOF MS. The relative composition of each peak is shown as the relative amount to the total amount of oligosaccharide detected.
Table 4.
Monosaccharide composition of the N-linked oligosaccharide core structure of IgG1 from clones transformed with the FUT8 and GMD siRNA tandem expression vector during fed-batch culture
| Clone Name | Culture period (day) | Relative composition of monosaccharide | Fucose(-)%b | ||
| Fucosea | GlcNAc | Mannose | |||
| 32-05-12 | 6 | 0.92 | 4.00 | 2.86 | 8 |
| 12 | 0.94 | 4.00 | 2.99 | 6 | |
| 16 | 0.97 | 4.00 | 3.01 | 3 | |
| FG1 | 6 | n.d. | 4.00 | 2.85 | 100 |
| 12 | n.d. | 4.00 | 2.97 | 100 | |
| 16 | n.d. | 4.00 | 2.92 | 100 | |
| FG16 | 6 | n.d. | 4.00 | 2.82 | 100 |
| 12 | n.d. | 4.00 | 2.96 | 100 | |
| 16 | n.d. | 4.00 | 2.96 | 100 | |
aMolar ratios calculated vs. 4 GlcNAc; n.d., not detectable.
bTotal percentage of non-fucosylated Fc oligosaccharides calculated by the formula (1-a) × 100.
Figure 4.
Biological activity of antibodies from clones transformed with the FUT8 and GMD siRNA tandem expression vector. Lysis of antigen-expressing cells targeted by human PBMCs at a target:effector ratio of 1:20 in the presence of different antibody concentrations was quantified by detecting lactate dehydrogenase activity (A). The antigen-binding activity of the antibody was measured by ELISA (B). Antibody purified from the serum-free fed-batch cultures of cells transformed by the FUT8 and GMD tandem siRNA expression vector, FG1 (open circles), FG16 (open squares), and the parental cell line 32-05-12 (filled circles) are shown. Cytotoxicity (%) and absorbance are indicated as the mean values ± SD of triplicates.
Discussion
The conversion of fucosylated therapeutic antibodies to antibodies lacking the core fucose in the Fc is recognized as an attractive approach for generating next-generation therapeutics. In this study, we explored the possibility of the high-frequency conversion of already-established antibody-producing cells (1st-generation) to cells that produce antibodies fully lacking core fucosylation (2nd-generation) within a few months, while almost all original features of the 1st-generation antibody-producing cells, including cell growth and recombinant protein productivity, were retained. The present approach is expected to be industrially useful for the establishment of a master cell bank of antibody-producing cells for the rapid development of 2nd-generation therapeutic antibodies.
Here, we focused on three key genes involved in the oligosaccharide fucosylation pathway in mammalian cells (FUT8, GMD, and GFT), and we identified an effective siRNA of each gene among more than ten siRNA candidates using a transient expression system with a target gene-GFP fusion reporter construct, as previously reported (data not shown) [32]. Every identified siRNA showed high efficacy in terms of depressing the target mRNA to levels of over 90%, when it was expressed in the form of short hairpin siRNA under the control of U6 or the tRNAval promoter in antibody-producing cells in which the siRNA expression unit was integrated in the genome. The present results thus demonstrated that our established siRNA expression system was able to sufficiently decrease level of target mRNA to the current maximum level achievable with RNAi technology. The levels of reduction in Fc fucosylation among the products were found to vary, depending on both target gene and clone, even when specific target genes were reduced to comparable levels in the siRNA-introduced cells. Cellular phenotypic selection of resistance to LCA was conducted to enrich transformants with cellular features equivalent to those of FUT8-knockout cells. However, among clones transformed by either siRNA alone, none produced fully lacking the core fucose of the Fc. These results suggest that there is a fucosylation-reducing limit associated with single-gene knockdown of FUT8, GMD, and GFT using RNAi technology.
On the other hand, we clearly observed a noteworthy synergistic effect of double knockdown of FUT8 and GMD on the fucosylation of products in antibody-producing cells (Table 2), although neither the combination of FUT8 and GFT, nor GMD and GFT double knockdown caused a significant change. This synergistic effect was not accounted for by the levels of mRNA regulation of target genes, because no additive effect on the level of depression of each target mRNA was observed with the FUT8 and GMD double knockdown. Some previous studies have shown that there is no functional redundancy in FUT8 and GMD, although there is a fuctional redundancy in GFT [28,30,33,34]. The loss of GFT seemed to be compensated for by other genes possessing transporter activity. In ordinary cell cultures, FUT8 is considered to be the only rate-limiting step enzyme of fucosylation, since mammalian cells retain excess endogenous intracellular GDP-fucose [30,35]. In theory, the level of product fucosylation should be controlled, either in a manner dependent on endogenous FUT8 activity under cellular conditions of abundant intracellular GDP-fucose, or in a manner dependent on endogenous GMD activity under condition of intracellular GDP-fucose starvation; these genes are not currently believed to act in an interdependent manner. However, the present results clearly demonstrated that residual FUT8 activity in single FUT8-gene knockdown in mammalian CHO cells with a reduced substrate supply due to a second GMD-gene knockdown yields an even lower rate of fucose transfer to the oligosaccharides of the products than that achieved with single FUT8-gene knockdown cells. The intracellular GDP-fucose concentration in the Golgi apparatus might have an effect on the function of FUT8, although the precise reasons for the observed synergy of FUT8 and GMD double knockdown in terms of reducing intracellular oligosaccharide fucosylation remain unclear.
Single-step conversion of already-established antibody-producing cells to non-fucosylated antibody producers by double knockdown of FUT8 and GMD was attempted in order to verify the industrial applicability of such a system. Our transformation strategy was found to function quite well; in brief, we introduced an siRNA tandem expression vector for introducing the double knockdown of FUT8 and GMD into antibody-producing cells, and we carried out the cellular phenotypic selection of resistance to LCA in the presence of L-fucose. We successfully established antibody-producing clones in which no fucosylated oligosaccharides were detected by either monosaccharide composition or MALDI-TOF MS analyses (Table 3, 4, Fig. 3D, 3E). Due to the very high conversion frequency achieved with this approach, only two months were required to complete all steps of the conversion; the ratio of LCA-resistant clones to drug-resistant clones transformed by the siRNA tandem expression vector was higher than that of clones transformed by each single siRNA expression unit (Table 1), and six clones out of the twenty LCA-resistant transformants randomly selected were found to be converted precisely to the desired clones (Table 3). The established converted clones proved capable of consistently producing fully non-fucosylated antibodies throughout the duration of a serum-free fed-batch culture period; furthermore, a constant ratio of F0G0, F0G1, and F0G2 oligosaccharides was maintained until the end of the culture period (Fig. 3, Table 4). The antibody productivity of the clones was also found to be equivalent to that of the parental cells at the end of the culture period. The purified antibodies obtained from the cultures exhibited approximately 100-fold higher ADCC compared to that of the original antibodies obtained from the parental cells (Fig. 4). The stability of the converted clones was confirmed for a long culture period of over 30 passages with stable integration of the siRNA expression unit into the genome (data not shown). The production and purification processes established for the 1st-generation therapeutic antibodies could be applied, with only marginal modifications, to establish the 2nd-generation processes. Thus, the present approach could provide substantial time and cost benefits for the development of next-generation therapeutic antibodies. Our preliminary experiments have also indicated that the tandem siRNA expression vector designed for the double knockdown of mouse FUT8 and GMD also worked in mouse myeloma cell lines NS0 and SP2/0.
Conclusion
The double knockdown of FUT8 and GMD in antibody-producing cells is worth considering as a novel strategy for generating therapeutic antibodies fully lacking core fucosylation with enhanced ADCC. The tremendous cost- and time-sparing advantages of this approach are expected to facilitate the development of next-generation therapeutic antibodies. This approach is based on our observation of the synergistic effects of double knockdown of FUT8 and GMD on intracellular oligosaccharide fucose modification in mammalian cells.
Methods
Cell lines
The dihydrofolate reductase-deficient CHO cell line, CHO/DG44 [36], was obtained from Dr. Lawrence Chasin of Columbia University (New York). A recombinant mouse/human chimeric IgG1-producing CHO/DG44 cell line, 32-05-12, generated as described previously [26], was cultured in IMDM medium (Invitrogen, Carlsbad, CA) containing 10% (v/v) dialyzed fetal bovine serum (dFBS; Invitrogen) and 500 nM methotrexate (MTX; Sigma-Aldrich, St. Louis, MO).
Construction of siRNA expression plasmids
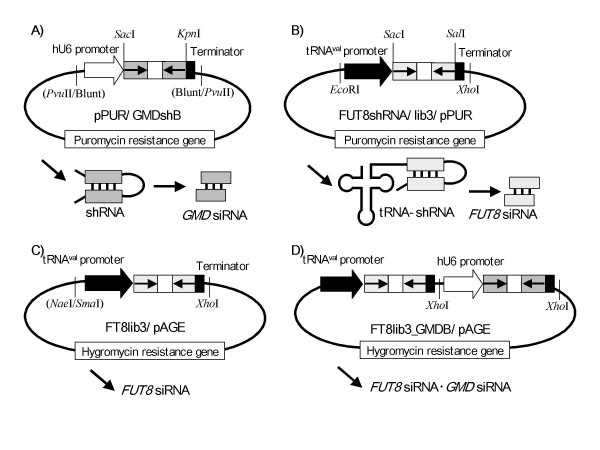
An siRNA expression plasmid for targeting Chinese hamster GMD (GenBank: AF525364), pPUR/GMDshB, was generated as described below (Fig. 5A). The plasmid contained a puromycin resistance gene as a selection marker and a GMD short hairpin siRNA expression cassette controlled by the human U6 promoter. The fragment of human U6 promoter was prepared by PCR with KOD polymerase (TOYOBO, Tokyo, Japan) from the plasmid U6_FUT8_B_puro [26] using the primers 5'-CCCAAGCTTG ATATCAAGGT CGGGCAGGAA GAGGGCCTAT-3' and 5'-GCTCTAGAGA TATCAAAAAA GGTACCGAGC TCGGTGTTTC GTCCTTTCCA CA-3'. The amplified human U6 promoter was inserted into pPUR (Clontech, Mountain View, CA) at the PvuII site, and the synthetic dsDNA coding short hairpin siRNA against GMD (5'-GAGCTCTATA AGAATCCACA GGCTCATATT GAAGGCTTCC TGTCACCTTC AATATGAGCC TGTGGATTCT TATAGGTACC-3') was inserted immediately downstream of the human U6 promoter at the SacI and KpnI sites.
Figure 5.
Structure of siRNA expression plasmids. The GMD siRNA expression plasmid consisted of a puromycin resistance gene and a short hairpin siRNA expression cassette controlled by the human U6 promoter (A). FUT8 siRNA expression plasmids consisted of a puromycin or hygromycin resistance gene and a short hairpin siRNA expression cassette controlled by the human tRNAval promoter (B or C). The siRNA tandem expression plasmid consisted of a hygromycin resistance gene and two short hairpin siRNA expression cassettes targeting FUT8 and GMD (D). The transcribed shRNAs and tRNA-shRNA fusion product were processed into siRNAs by Dicer.
An siRNA expression plasmid for targeting Chinese hamster FUT8 [28], FUT8shRNA/lib3/pPUR, contained a puromycin resistance gene as a selection marker and a FUT8 short hairpin siRNA expression cassette controlled by the human tRNAval promoter (Fig. 5B). The fragment of human tRNAval promoter was prepared by PCR from the plasmid ptRNA-SS [37] using the primers 5'-TTCCCAGTCA CGACGTT-3' and 5'-CAGGAAACAG CTATGAC-3'. The amplified human tRNAval promoter was inserted into pPUR using the PvuII site, and the synthetic dsDNA coding short hairpin siRNA against FUT8 (5'-GAGCTCAAAT CCAAAAGAAT TTCATCTGCA TGTCTTTGGG GATCCCCAAA GACATGCAGA TGAAATTCTT TTGGATTTGT CGAC-3') was inserted immediately downstream of the human tRNAval promoter at the SacI and SalI sites.
Another FUT8 siRNA expression plasmid, FT8lib3/pAGE (Fig. 5C), was constructed by insertion of the FUT8 short hairpin siRNA expression cassette from FUT8shRNA/lib3/pPUR into pAGE249 [8] as follows. The FUT8 short hairpin siRNA expression cassette was excised from FUT8shRNA/lib3/pPUR using the EcoRI and XhoI sites, and its EcoRI terminus was converted to SmaI by subcloning into pBlusecriptII KS(+) (Stratagene, La Jolla, CA). The SmaI-XhoI fragment containing the FUT8 short hairpin siRNA expression cassette was inserted into pAGE249 at the NaeI and XhoI sites to construct FT8lib3/pAGE.
Two siRNA expression plasmids for targeting Chinese hamster GFT (GenBank: AB222037), GFT_3G10/pPUR and GFT_3G10/pAGE, were constructed by replacement of the FUT8 dsDNA of FUT8shRNA/lib3/pPUR and FT8lib3/pAGE, respectively, with the dsDNA coding short hairpin siRNA against GFT (5'-GAGCTCCCAA AGAGGGTGAG AAGAGTGCTA TTGGGATCCCAATAGCACTC TTCTCACCCT CTTTGGGTCG AC-3').
Finally, an siRNA tandem expression plasmid for the double knockdown of FUT8 and GMD, FT8lib3_GMDB/pAGE, was constructed by insertion of the GMD short hairpin siRNA expression cassette (amplified by PCR from pPUR/GMDshB using the primers 5'-CCGCTCGAGA GCGCCTGATG CGGTATT-3' and 5'-CCGCTCGAGG GACTTTCCAC ACCTGGT-3') into FT8lib3/pAGE at the XhoI site (Fig. 5D). In this siRNA tandem expression vector, different PolIII promoters, tRNAval and U6, were designed to prevent unnecessary interference between the two promoter systems.
Real-Time PCR analyses of GMD, FUT8, and GFT
In order to quantify the amounts of GMD, FUT8, and GFT mRNA in the cells, real-time PCR analysis was carried out using TaKaRa Ex Taq™ R-PCR Version (TaKaRa, Shiga, Japan) and the following four sets of primers: 5'-ATCCTCGTCC TCCTTACTTA CC-3' and 5'-TCCAGCTGAC CAAGAAATAG AG-3' for FUT8, 5'-AAGCCCAGGA AGGTGGCGCT CATCAC-3'and 5'-CACTAGTTGA GGCCTGGTAG AACTTCAC-3' for GMD, 5'-ATCATCATTG GTGGTTTCTG G-3' and 5'-TCTCTTCATA GTAGAGCACG GC-3' for GFT, and 5'-GATATCGCTG CGCTCGTCGT CGAC-3' and 5'-CAGGAAGGAA GGCTGGAAGA GAGC-3' for β-actin as an internal standard gene. Total RNA was isolated from 5 × 106 cells using an RNeasy minikit (Qiagen, Hilden, Germany). Single-strand cDNA was synthesized from 3 μg total RNA using the Superscript™ III first-strand synthesis system for RT-PCR (Invitrogen). A 50-fold diluted reaction mixture was used as a template. PCR was carried out by heating the mixture at 94°C for 5 min followed by 40 cycles of 94°C for 20 s, 65°C for 1 min, and 72°C for 30 s in 20 μL of reaction mixture containing 1 unit of TaKaRa Ex Taq™ R-PCR Version, 1 μL of 2500-fold diluted SYBR Green I (TaKaRa), 5 μL of the diluted single-strand cDNA, and 6 pmol of primers using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. After PCR amplification, data acquisition and analyses were performed using the GeneAmp 7700 sequence detection system version 1.7 (Applied Biosystems).
LCA-staining analysis
Cells (2 × 105) were suspended in phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA), and either 2 μg/mL fluorescein isothiocyanate (FITC)-labeled LCA (Vector Laboratories, Burlingame, CA) or 2 μg/mL FITC-labeled streptavidin (KPL, Gaithersburg, MD) were added to the suspension. After incubation at 4°C for 30 min, 1 × 104 stained cells were analyzed by FACScalibur (BD Biosciences, San Jose, CA) to evaluate the reactivity to LCA on the cell surface.
Analyses of antibody-derived N-linked Fc oligosaccharides
Recombinant antibodies were purified from the serum-free culture supernatant by Protein A-affinity chromatography using MabSelect™ (Amersham Biosciences, Piscataway, NJ) and the samples were stored in 10 mM KH2PO4. The concentration of purified antibodies was measured by absorbance at 280 nm. The monosaccharide composition of each purified IgG1 was characterized by modified high-performance anion exchange chromatography (HPAEC) as previously described [8]. The oligosaccharide profile of each purified antibody was characterized by modified matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) in positive-ion mode, as described previously [29].
Biological activity analysis of antibodies
An ADCC assay for each purified IgG1 was performed by lactate dehydrogenase (LDH) release assay using human peripheral blood mononuclear cells (PBMCs) from healthy donors as effector cells at an E:T ratio of 20:1, as described previously [26]. The antigen-binding activities of the antibodies were measured by antigen-binding ELISA, as previously described [26].
Isolation of FUT8, GMD, or GFT knockdown clones
An IgG1 antibody-producing clone, CHO/DG44 32-05-12 (1.6 × 106 cells), was transformed by electroporation with 10 μg of each siRNA expression vector linearized at the FspI site (pPUR/GMDshB, FUT8shRNA/lib3/pPUR, or GFT_3G10/pPUR). Transfectants were selected in 12 μg/mL puromycin (Sigma-Aldrich) for 7 days, and then the drug-resistant clones were subjected to 7-day selection with 500 μg/mL LCA. The resultant LCA-resistant clones were isolated and expanded in IMDM medium containing 10% (v/v) dFBS, 500 nM MTX, and 12 μg/mL puromycin. Cells were collected for real-time PCR analysis after they had grown to confluence in a tissue-culture flask (Greiner, Frickenhausen, Germany). The serum-free culture supernatants were recovered for antibody analysis after a 7-day culture of the confluent cells in serum-free EX-CELL™ 301 medium (JRH Biosciences, Lenexa, KS).
Isolation of clones transformed by two siRNA expression vectors
The isolated GMD siRNA-introduced clones (1.6 × 106 cells) were electroporated again with 10 μg of FUT8 or GFT siRNA expression vector linearized at the FspI site (FT8lib3/pAGE or GFT_3G10/pAGE). Transfectants were selected in 3 μg/mL puromycin and 400 μg/mL hygromycin (Wako Pure Chemical Industries, Osaka, Japan) for 8 days, and then the drug-resistant clones were subjected to 7-day selection with 500 μg/mL LCA in the presence of 100 μM L-fucose. The resultant LCA-resistant clones were isolated and expanded in IMDM medium containing 10% (v/v) dFBS, 500 nM MTX, 3 μg/mL puromycin, and 400 μg/mL hygromycin for further real-time PCR and antibody analyses, as described above.
Conversion of antibody-producing cells to cells producing non-fucosylated antibodies
The CHO/DG44 32-05-12 (1.6 × 106) cells were transformed by electroporation with 10 μg of the FUT8 and GMD siRNA tandem expression vector linearized at the FspI site (FT8lib3_GMDB/pAGE). Transfectants were selected in 500 μg/mL hygromycin for 8 days, and then the drug-resistant clones were subjected to 7-day selection with 500 μg/mL LCA and 1 mM L-fucose. The resultant LCA-resistant clones were isolated and expanded in IMDM medium containing 10% (v/v) dFBS, 500 nM MTX, and 500 μg/mL hygromycin for further real-time PCR and antibody analyses as described above. The established clones were adapted to serum-free medium EX-CELL™ 302 (JRH Biosciences) supplemented with 6 mM L-glutamine, 500 nM MTX, and 500 μg/mL hygromycin for LCA-reactivity analysis and serum-free fed-batch culture.
Serum-free fed-batch culture
A 1L-scale spinner bioreactor (ABLE, Tokyo, Japan) was employed to maintain controlled, serum-free, fed-batch culture conditions of 35°C, pH 7.1, 50% DO. Each clone was inoculated at 3.0 × 105 cells/mL in EX-CELL™302 medium supplemented with 6 mM L-glutamine and 500 nM MTX, and was fed with serum-free IMDM-based feeding medium containing 5.3 μM human recombinant insulin (Invitrogen) at days 3, 5, 7, 9, and 11 post-inoculation to maintain a glucose content of approximately 5.0 g/L. Culture aliquots were drawn on days 3, 6, 9, 12, 14, and 16 prior to addition of the feeding medium. Viable cell density was measured with an automatic cell counter, Vi-CELL™ XR (Beckman Coulter, Fullerton, CA), using trypan blue exclusion. The antibody concentration in the culture supernatant was measured by an enzyme-linked immunosorbent assay (ELISA) specific for human IgG1, as previously described [38].
Authors' contributions
HI designed the study; participated in the construction of plasmids and the screening of siRNA; carried out cell cultures, transfection, real-time PCR, purification of antibodies; and drafted the manuscript.
KM initiated and worked on the study, and participated in plasmid construction and the screening of siRNA.
MI participated in carrying out the serum-free fed-batch culture.
MW participated in the analyses of antibody-derived N-linked Fc oligosaccharides.
SI participated in carrying out the serum-free fed-batch culture, the biological activity analysis, and helped to draft the manuscript.
KS was the head of the laboratories that carried out the present study and also discussed the study from both scientific and industrial points of view.
MS is the head of the laboratories that carried out the present study, initially conceived of the study, and helped to design the study and draft the manuscript.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors would like to thank Dr. Lawrence Chasin and Dr. Gail Urlaub Chasin (Columbia University) for their generous gift of the CHO/DG44 cell lines. We are also very grateful for the technical support provided by Ms. Ai Sato-Kubota.
Contributor Information
Harue Imai-Nishiya, Email: harue.imai@kyowa.co.jp.
Katsuhiro Mori, Email: mkatsu@kyowa.co.jp.
Miho Inoue, Email: miho.inoue@kyowa.co.jp.
Masako Wakitani, Email: masako.wakitani@kyowa.co.jp.
Shigeru Iida, Email: s.iida@kyowa.co.jp.
Kenya Shitara, Email: kshitara@kyowa.co.jp.
Mitsuo Satoh, Email: msatoh@kyowa.co.jp.
References
- Rademacher TW, Homans SW, Parekh RB, Dwek RA. Immunoglobulin G as a glycoprotein. Biochem Soc Symp. 1986;51:131–148. [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, Kuni-Kamochi R, Nakano R, Yano K, Kakita S, Shitara K, Satoh M. Comparison of biological activity among non-fucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2006;17:104–118. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcgammaRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, Matsushima K, Ueda R, Hanai N, Shitara K. Defucosylated anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T cell leukemia and lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.CAN-03-2068. [DOI] [PubMed] [Google Scholar]
- Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, Yokoi H, Nakamura K, Shitara K. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, Nakamura K, Shitara K. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11:2327–2336. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Niwa R, Saji S, Muta M, Hirose M, Iida S, Shiotsu Y, Satoh M, Shitara K, Kondo M, Toi M. A non-fucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin Cancer Res. 2007;13:1875–1882. doi: 10.1158/1078-0432.CCR-06-1335. [DOI] [PubMed] [Google Scholar]
- Iida S, Misaka H, Inoue M, Shibata M, Nakano R, Yamane-Ohnuki N, Wakitani M, Yano K, Shitara K, Saoh M. Non-fucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcγIIIa. Clin Cancer Res. 2006;12:2879–2887. doi: 10.1158/1078-0432.CCR-05-2619. [DOI] [PubMed] [Google Scholar]
- Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- Preithner S, Elm S, Lippold S, Locher M, Wolf A, da Silva AJ, Baeuerle PA, Prang NS. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol. 2006;43:1183–1193. doi: 10.1016/j.molimm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Nechansky A, Schuster M, Jost W, Siegl P, Wiederkum S, Gorr G, Kircheis R. Compensation of endogenous IgG mediated inhibition of antibody-dependent cellular cytotoxicity by glyco-engineering of therapeutic antibodies. Mol Immunol. 2007;44:1815–1817. doi: 10.1016/j.molimm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Schenerman MA, Hope JN, Kletke C, Singh JK, Kimura R, Tsao EI, Folena-Wasserman G. Comparability testing of a humanized monoclonal antibody (SynagisR) to support cell line stability, process validation, and scale-up for manufacturing. Biologicals. 1999;27:203–215. doi: 10.1006/biol.1999.0179. [DOI] [PubMed] [Google Scholar]
- Kamoda S, Nomura C, Kinoshita M, Nishiura S, Ishikawa R, Kakehi K, Kawasaki N, Hayakawa T. Profiling analysis of oligosaccharides in antibody pharmaceuticals by capillary electrophoresis. J Chromatogr A. 2004;1050:211–216. doi: 10.1016/j.chroma.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Noda K, Yamaguchi Y, Inoue S, Ikeda Y, Wang W, Ko JH, Uozumi N, Li W, Taniguchi N. The alpha1-6-fucosyltransferase gene and its biological significance. Biochim Biophys Acta. 1999;1473:9–20. doi: 10.1016/s0304-4165(99)00166-x. [DOI] [PubMed] [Google Scholar]
- Tonetti M, Sturla L, Bisso A, Benatti U, De FA. Synthesis of GDP-L-fucose by the human FX protein. J Biol Chem. 1996;271:27274–27279. doi: 10.1074/jbc.271.44.27274. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Hirschberg CB. Reconstitution, identification, and purification of the rat liver Golgi membrane GDP-fucose transporter. J Biol Chem. 1999;274:35596–35600. doi: 10.1074/jbc.274.50.35596. [DOI] [PubMed] [Google Scholar]
- de Vries T, Knegtel RM, Holmes EH, Macher BA. Fucosyltransferases: structure/function studies. Glycobiology. 2001;11:119R–128R. doi: 10.1093/glycob/11.10.119R. [DOI] [PubMed] [Google Scholar]
- Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- Mori K, Kuni-Kamochi R, Yamane-Ohnuki N, Wakitani M, Yamano K, Imai H, Kanda Y, Niwa R, Iida S, Uchida K, Shitara K, Satoh M. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng. 2004;88:901–908. doi: 10.1002/bit.20326. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Brunker P, Suter T, Moser S, Puntener U, Umana P. Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng. 2006;93:851–861. doi: 10.1002/bit.20777. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M. Establishment of FUT8 knockout Chinese hamster ovary cells; an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, Yano K, Shitara K, Hosoi S, Satoh M. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng. 2006;94:680–688. doi: 10.1002/bit.20880. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Imai-Nishiya H, Kuni-Kamochi R, Mori K, Inoue M, Kitajima-Miyama K, Okazaki A, Iida S, Shitara K, Satoh M. Establishment of a GDP-mannose 4,6-dehydratase (GMD) knockout host cell line: A new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol. 2007;130:300–310. doi: 10.1016/j.jbiotec.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sedivy JM, Sharp PA. Positive genetic selection for gene disruption in mammalian cells by homologous recombination. Proc Natl Acad Sci USA. 1989;86:227–231. doi: 10.1073/pnas.86.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P, Arndt-Jovin DJ, Jovin TM. Small interfering RNAs suppress the expression of endogenous and GFP-fused epidermal growth factor receptor (erbB1) and induce apoptosis in erbB1-overexpressing cells. Exp Cell Res. 2003;285:39–49. doi: 10.1016/S0014-4827(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, Taniguchi N. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci USA. 2005;102:15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellbusch CC, Sperandio M, Frommhold D, Yakubenia S, Wild MK, Popovici D, Vestweber D, Grone HJ, von Figura K, Lubke T, Korner C. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J Biol Chem. 2007;282:10762–10772. doi: 10.1074/jbc.M700314200. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Atkinson PH. Fucosyl-glycoprotein and precursor polls in HeLa cells. Biochemistry. 1975;14:3107–3114. doi: 10.1021/bi00685a011. [DOI] [PubMed] [Google Scholar]
- Urlaub G, Mitchell PJ, Kas E, Chasin LA, Funanage VL, Myoda TT, Hamlin J. Effect of gamma rays at the dihydrofolate reductase locus: Deletions and inversions. Somatic Cell Mol Genet. 1986;12:555–556. doi: 10.1007/BF01671941. [DOI] [PubMed] [Google Scholar]
- Fukano H, Hayatsu N, Goto R, Suzuki Y. A technique to enzymatically construct libraries which express short hairpin RNA of arbitrary stem length. Biochem Biophys Res Commun. 2006;347:543–550. doi: 10.1016/j.bbrc.2006.05.124. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Tanaka Y, Fujino I, Hirayama N, Shitara K, Hanai N. Dissection and optimization of immune effector functions of humanized anti-ganglioside GM2 monoclonal antibody. Mol Immunol. 2000;37:1035–1046. doi: 10.1016/S0161-5890(01)00021-9. [DOI] [PubMed] [Google Scholar]