Abstract
Gut immune components are severely compromised among persons with AIDS, which allows increased translocation of bacterial lipopolysaccharides (LPS) into the systemic circulation. These microbial LPS are reportedly increased in chronically HIV-infected individuals and findings have correlated convincingly with measures of immune activation. Immune reconstitution inflammatory syndrome (IRIS) is an adverse consequence of the restoration of pathogen-specific immune responses in a subset of HIV-infected subjects with underlying latent infections during the initial months of highly active antiretroviral treatment (HAART). Whether IRIS is the result of a response to a high antigen burden, an excessive response by the recovering immune system, exacerbated production of pro-inflammatory cytokines or a lack of immune regulation due to inability to produce regulatory cytokines remains to be determined. We theorize that those who develop IRIS have a high burden of proinflammatory cytokines produced also in response to systemic bacterial LPS that nonspecifically act on latent mycobacterial antigens. We also hypothesize that subjects that do not develop IRIS could have developed either tolerance (anergy) to persistent LPS/tubercle antigens or could have normal FOXP3+ gene and that those with defective FOXP3+ gene or those with enormous plasma LPS could be vulnerable to IRIS. The measure of microbial LPS, anti-LPS antibodies and nonspecific plasma cytokines in subjects on HAART shall predict the role of these components in IRIS.
Background
Immune reconstitution inflammatory syndrome (IRIS): An existing lacuna in HIV immunology?
IRIS is an adverse consequence of the restoration of pathogen-specific immune responses in HIV-infected patients during the initial months of highly active antiretroviral treatment (HAART) [1]. Even though IRIS is also closely associated with certain other infectious (mycobacteria, varicella zoster, herpesviruses, and cytomegalovirus) and non-infectious (autoimmune) conditions [2-10], the morbidity associated with HIV/tuberculosis (TB) is more important [1,11] as the crisis seem to be alarming in third-world nations, where the proportion of HIV/TB IRIS is reportedly high, ranging from 11% to 43% [12-15]. This could be due to differences in cohort characteristics, case definitions and differences in the mean time interval between TB diagnosis and antiretroviral therapy (ART) initiation. Data from resource-limited countries on TB-IRIS is scarce; a rate of 8% was reported from India [1]. Immunology of IRIS in HIV/TB is deficient and HIV-specific T lymphocyte responses have repeatedly shown to be defective [16]. To understand the immunopathogenesis of IRIS it will be crucial to elucidate the intrinsic dynamics of immune cells after initiation of HAART [17]. Preliminary investigations have shown that an acute exacerbation of mycobacteria-specific Th1 response after HIV infection control by HAART causes IRIS in HIV/TB [17,18].
Does CD4+ T-cell depletion lead to a breach in gut immune cell integrity to initiate the proinflammatory cytokine saga?
In the context of an HIV infected subject with latent pulmonary TB, progressing to AIDS stage of HIV disease, the acute stage of the infection is characterized by eventual depletion in the number of CD4+ T-cells, the key orchestrator of all immune mechanisms in the body. Recent research has re-examined the rate of immunopathologic events in HIV disease, where the first few weeks is characterized by massive viremia and depletion of ~50% memory CD4+ T-cell (CCR5+) population especially in the gut [19-26]. Since the gut associated lymphoid tissue (GALT) comprises ~60% of entire lymphoid organ system, rich in memory cells, its depletion has a strong consequence on the entire CD4+ T-cell population. Memory CD4+ T-cells in the lamina propria is depleted principally by Fas-FasL-mediated cell death [26]. In addition, productive HIV infection is favored by an inflammatory environment, because Th1 cytokines (IL-2, IL-12, TNF-α) increase NFkB activation in T-cells, which drives HIV transcription. Early breach in the gut mucosal integrity and epithelial microenvironment [19-21,27-30] leads to increased translocation of luminal microbial products [20] because the gut is thought to be the principal source of microbial products (especially LPS) and because it has a massive bacterial load compared to other anatomical sites [31-33]. Translocation results in chronic inflammation via Toll-like receptor (TLR-4) stimulation, resulting in cytokine and chemokine release driving persistent T-cell activation and (tat mediated) apoptosis via activation-induced cell death (AICD) [21]. However, due to lack of sufficient CD4+ T-cells, complex inflammatory mechanisms might not be expected due to anergy.
HAART, immunological restoration and the inflammatory milieu: Who are the possible mediators?
Most of the subjects with HIV disease attend HIV testing centers in India only after advanced clinical HIV disease (AIDS) sets in and when their CD4+ T-cell counts are low [1,11]. In spite of initiation of HAART, some experience a 'discordant response', whereby the HIV-1 RNA plasma level is below the limit of detection but the CD4+ cell count response is blunted. We propose that these individuals with HIV/TB coinfection might not progress to clinical IRIS owing to poor immune reconstitution despite considerable virological recovery. As a consequence, a substantial proportion of treated individuals show poor CD4+ T-cell recovery [40]. This has also been correlated with a lower nadir pretreatment CD4+ T-cell count, suggestive of more extensive depletion of CD4+ T-cells in the GALT during acute HIV infection, which may be refractory to reconstitution with ART [19,41]. Initiation of HAART allows 'partial' immune restoration [42], which however, can result in the substantial proliferation and differentiation of most of the immune components [43,44]. Due to immune restoration, an inflammatory response against infectious and non-infectious antigens (LPS) is mounted leading to noticeable 'paradoxical worsening' [43-45], with a shift toward a Th1 receptor profile, which increases the levels of IFN-γ and IL-2 [46-51]. Therefore, persons with latent TB or other systemic commensal antigens (LPS) could lead to exaggerated inflammatory responses. Studies also show that an inflammatory response is required for the elimination of any gram-negative infection (i.e. LPS) [52]. HAART treatment (that enable 'partial' immune reconstitution) considerably reduces circulating LPS although total clearance may not be feasible for considerable periods of time.
Bacterial LPS, the microbe-associated molecular patterns (MAMP) of gram-negative bacteria are known potent activators of cells of inflammatory system. Plasma LPS levels have been directly associated with the degree of intestinal permeability following invasive gastrointestinal surgery [34], inflammatory bowel disease (IBD) [35] and graft-versus host disease (GVHD) [31,35-39]. Experimental SIV infection of macaques resulted in raised circulating LPS levels [21]. Recent studies have found significantly elevated levels of plasma LPS in chronically HIV-infected humans with progressive disease [21] and has correlated convincingly with measures of innate and adaptive immune activation. Besides, the study also has shown the association between LPS and chronic in vivo stimulation of monocytes, an association between raised plasma LPS; and an association between reduction in plasma LPS and CD4+ T-cell reconstitution with HAART [21]. Due to abrupt increase in the numbers of CD4+ T-cells, the pattern recognition receptors (PRR) induce signal transduction pathway molecules like NFkB, IL-1 receptor, TNF receptor, MAP kinase receptor etc. [53]. Cytokines such as IL-1 can also stimulate the NFkB binding molecule to activate NFkB [54-56], which induces the expression of cyclooxygenase-2 (COX-2), which consequently leads to tissue inflammation at the site where latent TB antigens are located. Interestingly, the expression of the COX-2-encoding gene, believed to be responsible for the massive production of prostaglandins at inflammatory sites, is transcriptionally regulated by NFkB [54]. NFkB resides in the cytoplasm and is bound to its inhibitor. Furthermore, injurious and inflammatory stimuli, such as free radicals present in the plasma of the immune deteriorated host leads to NFkB release that subsequently moves into the nucleus to activate the genes responsible for COX-2 expression.
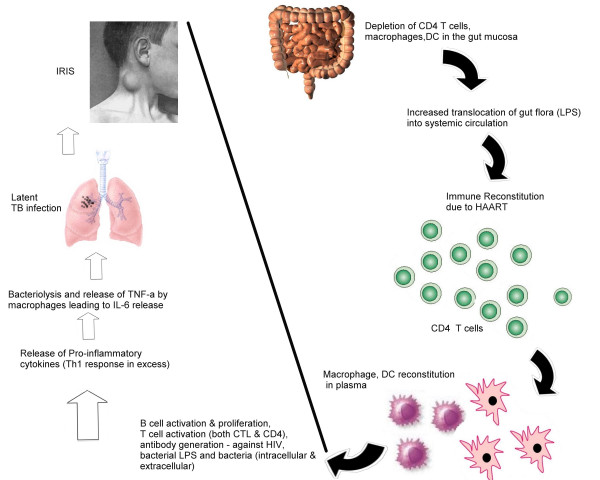
Alternatively, effector T-cells of the Th1 subset activates macrophages by CD154 – CD4+0 interactions and by secreting IFN-γ. Th1 subsets produce the proinflammatory cytokines, IL-2, IFN-γ, and TNF-α, and Th2 cells, the anti-inflammatory cytokines, IL-4, 5, 6, 10, and 13. In addition, macrophages that have phagocytosed TB bacilli produce IL-12 that stimulates the differentiation of naïve CD4+ T-cells to the Th1 subset, which again produces IFN-γ on encountering macrophage-associated microbial antigens; IL-12 also increases the amount of IFN-γ produced by these T-cells. In different T-cell mediated diseases, tissue injury is caused by a delayed-type hypersensitivity response mediated by CD4+ T-cells or by lysis of host cells by CD8+ CTLs. Some studies suggest that circulating IL-6 levels prior to HAART may be associated with IRIS [53]. CD4+ T-cells may react against cell or tissue antigens and secrete cytokines that induce local inflammation and activate macrophages. The actual tissue injury is caused by the macrophages and other inflammatory cells. CD8+ T-cells specific for antigens on autologous cells may directly kill these cells. Increased LPS-binding protein (LBP) may also increase the host response and potentiate injury. We hypothesize that the excessive presence of LPS in HIV/TB coinfected subjects accounts for the progression of IRIS and those that have LPS in limited concentrations may not. In studies in which normal human subjects were treated with LPS intravenously, there was a shift toward a Th2 response with increased expression of IL-10, [57-59] and the pretreatment of healthy human volunteers with IL-10 reduced the LPS-induced increases in chemokines [60,61]. Data from studies in normal human volunteers suggest that LPS increase the production of circulating IL-10, which would then blunt the proinflammatory response to a second bacterial challenge [60,61]. The Th2 shift in sepsis suggests that an excess of anti-inflammatory cytokines may result in impaired lung host response. We therefore hypothesize that this situation could also lead to extensive multiplication of TB bacilli. A brief overview of the concept is illustrated in figure 1.
Figure 1.
One possible mechanism that illustrates the immunology of IRIS in a subject with HIV/TB coinfection. Compromised gut immunity leads to increased translocation of luminal gram negative bacterial LPS into the systemic circulation. Initiation of HAART in the subject leads to abrupt restoration of CD4+ T-cells and almost any pathogen-specific immune response. IRIS developers have a high burden of LPS and proinflammatory cytokines produced against LPS could result in an exaggerated, nonspecific attack on latent mycobacterial antigens that are presented in the local lymph nodes leading to localized inflammation. We also hypothesize that subjects that do not develop IRIS could have developed either tolerance (anergy) to persistent LPS and tubercle antigens or could have normal FOXP3+ gene (not shown) and that those with defective FOXP3+ gene or enormous plasma LPS could be vulnerable to IRIS (as demonstrated by researchers that defective FOXP3+ gene is associated with increased risk for inflammatory conditions). (Bold lines indicate the availability of clinical/experimental evidence and dashed lines indicate the possible mechanism).
The likelihood of 'normal' FOXP3+ gene and endotoxin-tolerance among IRIS non-developers – Why?
This 'paradoxical worsening' could also be attributable to additional presence of defective FOXP3+ gene among IRIS developers. Presence of defective FOXP3+ gene in T-cells has been reported to confer increased risk of inflammatory conditions in human beings in contrast to a normal FOXP3+ gene. After an initial exposure to LPS, monocytes and macrophages become refractory to subsequent LPS challenge (endotoxin-tolerance) (57 – 61). This initially was believed to be protective against septic shock. However, recent evidence suggests that endotoxin-tolerance impairs the host response to a second bacterial challenge [62,63]. The prolonged presence of TB antigens (and a normal FOXP3+ gene) could also lead to anergy and poor immune responses to TB antigens despite HAART. Monocytes obtained from septic patients have functional defects that include profound defects in IL-1, 6, and TNF-α production; loss of HLA class II antigen expression; and impaired antigen presentation [64-69]. In patients with sepsis, monocytes from survivors showed normal cytokine response following LPS stimulation [64]. A potential mechanism whereby endotoxin-tolerance develops is a down-regulation of LPS receptors such as membrane CD14 on macrophages [70]. The exposure of monocytes and macrophages to the anti-inflammatory cytokines, IL-10 and TGF-β, is a second mechanism that may be responsible for the monocyte deactivation that resembles endotoxin-tolerance [71]. Studies performed with human alveolar macrophages exposed to IL-10 in vitro show increased intracellular bacterial replication of Legionella pneumophila, [72] and decreased production of proinflammatory cytokines [73]. These suggest that macrophages and monocytes in septic patients may develop a phenotype similar to that observed in endotoxin-tolerance, which could result in an impaired response to lung pathogens. The development of tolerance was hypothesized to be beneficial by diminishing the proinflammatory response in patients with sepsis. However, some data suggest that the development of tolerance may worsen clinical outcomes because monocytes and macrophages may not respond adequately to a bacterial challenge [62,65,74]. The CD14/TLR complex and associated signaling pathways are essential for the recognition of LPS by macrophages, and several studies suggest that down-regulation of CD14/TLR complexes on macrophages is responsible for the development of tolerance [63,70,75,76]. However, the development of tolerance does not correlate with down-regulation of LPS-binding sites [77], suggesting the possible role of other mechanisms including the disruption of CD14/TLR signaling pathways [78] and the macrophage exposure to anti-inflammatory IL-10 [79]. Therefore, it is hypothesized that subjects that do not progress to develop IRIS (IRIS tolerant) despite HAART initiation could develop tolerance (anergy) to persistent LPS/tubercle antigens.
Conclusion
It is hypothesized that proinflammatory cytokines produced excessively in response to systemic bacterial LPS nonspecifically act on latent mycobacterial antigens leading to clinical deterioration and 'paradoxical worsening' of inflammatory responses against both infectious (HIV/TB) and non-infectious (LPS) microbial antigens. This 'paradoxical worsening' could also be attributable to additional presence of defective FOXP3+ gene among IRIS developers. Subjects that do not progress to develop IRIS (IRIS tolerant) despite HAART initiation could develop either tolerance (anergy) to persistently existing LPS and tubercle antigens. Thus far, no single treatment option exists against IRIS and depends on the underlying infectious agent and its clinical presentation. However, since the pathogenesis is an inflammatory one, systemic corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDS) may assuage the symptoms. Therefore, studies must be attempted to assess the role of immunological correlates and possible markers of IRIS needs to be evaluated to better understand the mechanisms behind IRIS in HIV/TB or other opportunistic coinfections, which would largely facilitate the timely management of IRIS in HIV/AIDS.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
EMS, RV and NK conceived and proposed the hypothesis. RV, KGM, PB, CAL, RS, SS, and NK provided additional inputs to further develop the scientific concept; EMS, RV and PB drafted the manuscript; SS and NK shared their clinical expertise and critically revised the manuscript. All authors read and approved the final manuscript. EMS, RV and NK are the guarantors of the paper.
Acknowledgments
Acknowledgements
The authors are grateful to all the staff and patients of YRG CARE without whose support and facilitation, this manuscript could not have been conceived and drafted.
Contributor Information
Esaki Muthu Shankar, Email: shankarem@yrgcare.org.
Ramachandran Vignesh, Email: vignesh@yrgcare.org.
Kailapuri G Murugavel, Email: murugavel@yrgcare.org.
Pachamuthu Balakrishnan, Email: bala@yrgcare.org.
Ramalingam Sekar, Email: sekarlingam@gmail.com.
Charmaine AC Lloyd, Email: charmaine@yrgcare.org.
Suniti Solomon, Email: suniti@yrgcare.org.
Nagalingeswaran Kumarasamy, Email: kumarasamy@yrgcare.org.
References
- Kumarasamy N, Chaguturu S, Mayer KH, Solomon S, Yepthomi HT, Balakrishnan P, Flanigan TP. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–1576. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- Keane NM, Price P, Lee S, Almeida CA, Stone SF, James I, French MA. Restoration of CD4 T-cell responses to cytomegalovirus is short-lived in severely immunodeficient HIV-infected patients responding to highly active antiretroviral therapy. HIV Med. 2004;5:407–414. doi: 10.1111/j.1468-1293.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpes virus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- Koval CE, Gigliotti F, Nevins D, Demeter LM. Immune reconstitution syndrome after successful treatment of Pneumocystis carinii pneumonia in a man with human immunodeficiency virus type 1 infection. Clin Infect Dis. 2002;35:491–493. doi: 10.1086/341974. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Bicanic TA, Macallan DC. Pyomyositis and cutaneous abscesses due to Mycobacterium avium : an immune reconstitution manifestation in a patient with AIDS. Clin Infect Dis. 2004;38:461–463. doi: 10.1086/381033. [DOI] [PubMed] [Google Scholar]
- Sereti I, Sarlis NJ, Arioglu E, Turner ML, Mican JM. Alopecia universalis and Graves' disease in the setting of immune restoration after highly active antiretroviral therapy. AIDS. 2001;15:138–140. doi: 10.1097/00002030-200101050-00026. [DOI] [PubMed] [Google Scholar]
- Mirmirani P, Maurer TA, Herndier B, McGrath M, Weinstein MD, Berger TG. Sarcoidosis in a patient with AIDS: a manifestation of immune restoration syndrome. J Am Acad Dermatol. 1999;41:285–286. doi: 10.1016/S0190-9622(99)70364-6. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Checkley A, Wansbrough-Jones MH. Acute bilateral parotitis caused by Mycobacterium scrofulaceum : immune reconstitution disease in a patient with AIDS. Sex Transm Infect. 2005;81:517–518. doi: 10.1136/sti.2005.014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CL, Subbarao V, Gayed S, Ustianowski AP. Immune reconstitution syndrome to Strongyloides stercoralis infection. AIDS. 2007;21:649–650. doi: 10.1097/QAD.0b013e3280117f94. [DOI] [PubMed] [Google Scholar]
- Kumarasamy N, Vallabhaneni S, Flanigan TP, Mayer KH, Solomon S. Clinical profile of HIV in India. Indian J Med Res. 2005;121:377–394. [PubMed] [Google Scholar]
- Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo MD, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV infected persons. Chest. 2001;120:193–197. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, Hamill RJ. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- Shao H, Crump J, Ramadhani H, Uiso L, Sendui-Nguyaine , Kiwera R, Ndosi1 E, Shao J, Bartlett J, Thielman N. A randomised trial of early versus delayed fixed dose combination zidovudine/lamivudine/abacavir in patients coinfected with HIV and tuberculosis: early findings of the tuberculosis and immune reconstitution syndrome trial. Thirteenth Conference on Retroviruses and Opportunistic Infections Denver, CO, February 2006. [abstract 796]
- Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57:167–170. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- Race EM, Adelson-Mitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, Japour AJ. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet. 1998;351:252–255. doi: 10.1016/S0140-6736(97)04352-3. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- Sharpstone D, Neild P, Crane R, Taylor C, Hodgson C, Sherwood R, Gazzard B, Bjarnason I. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45:70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–448. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg. 2006;243:359–363. doi: 10.1097/01.sla.0000201455.89037.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- Hill GR, Teshima T, Gerbitz A, Pan L, Cooke KR, Brinson YS, Crawford JM, Ferrara JL. Differential roles of IL-1 and TNF-α on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietroma M, Cappelli S, Carlei F, Di Giuro G, Agnifili A, Recchia CL, Antonellis M, Amicucci G. Intestinal and systemic endotoxaemia after laparotomic or laparoscopic cholecystectomy. Chir Ital. 2006;58:171–177. [PubMed] [Google Scholar]
- Wellmann W, Fink PC, Benner F, Schmidt FW. Endotoxaemia in active Crohn's disease. Treatment with whole gut irrigation and 5-aminosalicylic acid. Gut. 1986;27:814–820. doi: 10.1136/gut.27.7.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-H. [DOI] [PubMed] [Google Scholar]
- Nicastri E, Chiesi A, Angeletti C, Sarmati L, Palmisano L, Geraci A, Andreoni M, Vella S, Italian Antiretroviral Treatment Group (IATG) Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. J Med Virol. 2005;76:153–160. doi: 10.1002/jmv.20352. [DOI] [PubMed] [Google Scholar]
- Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active antiretroviral therapy (HAART) prolongs time to treatment failure in Kaposi's sarcoma. AIDS. 1999;13:2105–2111. doi: 10.1097/00002030-199910220-00014. [DOI] [PubMed] [Google Scholar]
- Chien J, Johnson H. Paradoxical reactions in HIV and pulmonary TB. Chest. 1998;114:933–936. doi: 10.1378/chest.114.3.933. [DOI] [PubMed] [Google Scholar]
- Kunimoto DY, Chui L, Nobert E, Houston S. Immune mediated "HAART" attack during treatment for tuberculosis. Int J Tuberc Lung Dis. 1999;3:944–947. [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, Gathe JC, Jr, Visnegarwala F, Trautner BW. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu R. HIV protease inhibitors: immunological insights. AIDS. 1999;13:S19–S27. [PubMed] [Google Scholar]
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- John M, French MAH. Exacerbation of the inflammatory response to Mycobacterium tuberculosis after antiretroviral therapy. Med J Aust. 1998;169:473–474. doi: 10.5694/j.1326-5377.1998.tb123372.x. [DOI] [PubMed] [Google Scholar]
- Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43–49. doi: 10.2214/ajr.174.1.1740043. [DOI] [PubMed] [Google Scholar]
- Furrer H, Malinverni R. Systemic inflammatory reaction after starting highly active antiretroviral therapy in AIDS patients treated for extrapulmonary tuberculosis. Am J Med. 1999;106:371–372. doi: 10.1016/S0002-9343(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- Shahin RD, Engberg I, Hagberg L, Eden CS. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G. Cooperation of multiple signaling pathways in CD4+0-regulated gene expression in B lymphocytes. Proc Natl Acad Sci USA. 2002;5:1497–1502. doi: 10.1073/pnas.032665099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acquisto F, May MJ, Ghosh S. Inhibition of nuclear factor kappa B (NFkB): an emerging theme in anti-inflammatory therapies. Molec Interv. 2002;2:22–35. doi: 10.1124/mi.2.1.22. [DOI] [PubMed] [Google Scholar]
- Seaman DR. Joint complex dysfunction, a novel term to replace subluxation/subluxation complex: etiological and treatment considerations. J Manipulative Physiol Ther. 1997;20:634–644. [PubMed] [Google Scholar]
- Seaman DR. The diet-induced proinflammatory state: a cause of chronic pain and other degenerative diseases? J Manipulative Physiol Ther. 2002;25:168–179. doi: 10.1067/mmt.2002.122324. [DOI] [PubMed] [Google Scholar]
- van der Poll T, de Wall Malefyt R, Coyle SM, Lowry SF. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10, and IL-13. J Infect Dis. 1997;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- Zimmer S, Pollard V, Marshall GD. Effects of endotoxin on the Th1/Th2 response in humans. J Burn Care Rehabil. 1996;17:491–496. doi: 10.1097/00004630-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Lauw FN, Lauw FN, ten Hove T, Dekkers PE, de Jonge E, van Deventer SJ, van Der Poll T. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect Immun. 2000;68:1014–1018. doi: 10.1128/IAI.68.3.1014-1018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszyna DP, Pajkrt D, van Deventer SJ, van der Poll T. Effect of interleukin 10 on the release of the CXC chemokines growth related oncogene GRO-alpha and epithelial cell-derived neutrophil activating peptide (ENA)-78 during human endotoxemia. Immunol Lett. 2001;78:41–44. doi: 10.1016/S0165-2478(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Olszyna DP, Pajkrt D, Lauw FN, van Deventer SJ, van Der Poll T. Interleukin 10 inhibits the release of CC chemokines during human endotoxemia. J Infect Dis. 2000;181:613–620. doi: 10.1086/315275. [DOI] [PubMed] [Google Scholar]
- West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30:S64–S73. doi: 10.1097/00003246-200201001-00009. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/S1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- Munoz C, Carlet J, Fitting C, Misset B, Blériot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HD, Thieme M, Heym S, Döcke WD, Ruppe U, Tausch W, Manger D, Zuckermann S, Golosubow A, Nieter B, et al. Alterations in function and phenotype of monocytes from patients with septic disease: predictive value and new therapeutic strategies. Behring Inst Mitt. 1991;88:208–215. [PubMed] [Google Scholar]
- Wilson CS, Seatter SC, Rodriguez JL, Bellingham J, Clair L, West MA. In vivo endotoxin tolerance: impaired LPS-stimulated TNF release of monocytes from patients with sepsis, but not SIRS. J Surg Res. 1997;69:101–106. doi: 10.1006/jsre.1997.5040. [DOI] [PubMed] [Google Scholar]
- Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-g treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–223. [PubMed] [Google Scholar]
- Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162:1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol. 2000;165:411–418. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]
- Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DR, Skerrett SJ. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-gamma: differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- Raychaudhuri B, Fisher CJ, Farver CF, Malur A, Drazba J, Kavuru MS, et al. Interleukin 10 (IL-10)-mediated inhibition of inflammatory cytokine production by human alveolar macrophages. Cytokine. 2000;12:1348–1355. doi: 10.1006/cyto.2000.0721. [DOI] [PubMed] [Google Scholar]
- Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, Thomassen MJ. Impaired ex vivo lipopolysaccharide stimulated whole blood tumor necrosis factor production may identify "septic' intensive care unit patients. Shock. 2000;14:271–276. doi: 10.1097/00024382-200014030-00005. [DOI] [PubMed] [Google Scholar]
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Sato S, Nomura F, Kawai T, Takeuchi O, Mühlradt PF, Takeda K, Akira S. Synergy and crosstolerance between toll-like receptor (TLR) 2- and TLR-4- mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- Fahmi H, Chaby R. Desensitization of macrophages to endotoxin effects is not correlated with a down-regulation of lipopolysaccharide-binding sites. Cell Immunol. 1993;150:219–229. doi: 10.1006/cimm.1993.1191. [DOI] [PubMed] [Google Scholar]
- Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharideand lipoteichoic acid induced tolerance and cross-tolerance: distinct alterations in il-1 receptor-associated kinase. J Immunol. 2002;168:6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–287. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]