Abstract
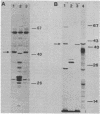
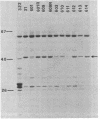
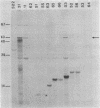
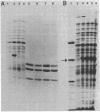
A collection of hybrid plasmids carrying either the wild-type or mutated glpT gene was generated in vitro and used to characterize the glpT-dependent active transport system for sn-glycerol-3-phosphate in Escherichia coli K-12. Restriction endonuclease analysis and recloning of DNA fragments localized glpT to a 3-kilobase pair PstI-HpaI segment of DNA. Comparison of DNA carrying glpT-lacZ fusions with DNA carrying intact glpT allowed determination of the direction of transcription. Through characterization of the proteins synthesized by strains harboring hybrid plasmids carrying amber, missense, or deletion mutations in glpT, it was shown that glpT is a promoter-proximal gene in an operon consisting of at least two genes. The gene product of glpT, the sn-glycerol-3-phosphate permease, was found associated with the inner membrane. It could be solubilized by treatment with sodium dodecyl sulfate at 50°C. Its molecular weight, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was dependent upon sample treatment before electrophoresis. The apparent molecular weight was 44,000 when membrane fractions were heated to 50°C; subsequent treatment at 95°C modified the protein such that it migrated faster (apparent molecular weight = 33,000). Several missense mutations in glpT were negatively dominant over wild-type glpT, indicating that the active form of the permease is multimeric. A gene (named glpQ) promoter distal to glpT codes for a periplasmic protein. This protein had previously been named GLPT protein to indicate its relationship to the glpT gene. The present report demonstrates that it is not the gene product of glpT and is not required for active transport of sn-glycerol-3-phosphate.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Argast M., Boos W. Co-regulation in Escherichia coli of a novel transport system for sn-glycerol-3-phosphate and outer membrane protein Ic (e, E) with alkaline phosphatase and phosphate-binding protein. J Bacteriol. 1980 Jul;143(1):142–150. doi: 10.1128/jb.143.1.142-150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast M., Schumacher G., Boos W. Characterization of a periplasmic protein related to sn-glycerol-3-phosphate transport in escherichia coli. J Supramol Struct. 1977;6(1):135–153. doi: 10.1002/jss.400060111. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boos W., Hartig-Beecken I., Altendorf K. Purification and properties of a periplasmic protein related to sn-glycerol-3-phosphate transport in Escherichia coli. Eur J Biochem. 1977 Feb;72(3):571–581. doi: 10.1111/j.1432-1033.1977.tb11280.x. [DOI] [PubMed] [Google Scholar]
- Bremer E., Beck E., Hindennach I., Sonntag I., Henning U. Cloned structural gene (ompA) for an integral outer membrane protein of Escherichia coli K-12: localization on hybrid plasmid pTU100 and expression of a fragment of the gene. Mol Gen Genet. 1980;179(1):13–20. doi: 10.1007/BF00268440. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Fried V. A. Membrane biogenesis. Evidence that a soluble chimeric polypeptide can serve as a precursor of a mutant lac permease in Escherichia coli. J Biol Chem. 1981 Jan 10;256(1):244–252. [PubMed] [Google Scholar]
- Giphart-Gassler M., Reeve J., van de Putte P. Polypeptides encoded by the early region of bacteriophage Mu synthesized in minicells of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):165–191. doi: 10.1016/0022-2836(81)90339-9. [DOI] [PubMed] [Google Scholar]
- Guth A., Engel R., Tropp B. E. Uptake of glycerol 3-phosphate and some of its analogs by the hexose phosphate transport system of Escherichia coli. J Bacteriol. 1980 Jul;143(1):538–539. doi: 10.1128/jb.143.1.538-539.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. D., Selsing E., Wells R. D. A rapid microscale technique for isolation of recombinant plasmid DNA suitable for restriction enzyme analysis. Plasmid. 1980 Jan;3(1):88–91. doi: 10.1016/s0147-619x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Lightner V. A., Green P. R., Modrich P., Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Identification of the sn-glycerol-3-phosphate acyltransferase polypeptide as the plsB gene product. J Biol Chem. 1980 Oct 10;255(19):9421–9426. [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Ludtke D., Larson T. J., Beck C., Boos W. Only one gene is required for the glpT-dependent transport of sn-glycerol-3-phosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):540–547. doi: 10.1007/BF00337962. [DOI] [PubMed] [Google Scholar]
- Mieschendahl M., Büchel D., Bocklage H., Müller-Hill B. Mutations in the lacY gene of Escherichia coli define functional organization of lactose permease. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7652–7656. doi: 10.1073/pnas.78.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Silhavy T. J., Andrews K. J. Resolution of glpA and glpT loci into separate operons in Escherichia coli K-12 strains. J Bacteriol. 1979 Apr;138(1):268–269. doi: 10.1128/jb.138.1.268-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Hofer M., Schmeissner U., Sommer H., Schmitz A., Lu P. Genetic studies of the lac repressor. IX. Generation of altered proteins by the suppression of nonsence mutations. J Mol Biol. 1979 Jun 25;131(2):191–222. doi: 10.1016/0022-2836(79)90073-1. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Oeschger M. P., Oeschger N. S., Wiprud G. T., Woods S. L. High efficiency temperature-sensitive amber suppressor strains of Escherichia coli K12: isolation of strains with suppressor-enhancing mutations. Mol Gen Genet. 1980;177(4):545–552. doi: 10.1007/BF00272662. [DOI] [PubMed] [Google Scholar]
- Rood J. I., Sneddon M. K., Morrison J. F. Instability in tyrR strains of plasmids carrying the tyrosine operon: isolation and characterization of plasmid derivatives with insertions or deletions. J Bacteriol. 1980 Nov;144(2):552–559. doi: 10.1128/jb.144.2.552-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A., Weiner J. H. The anaerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli. Purification and characterization. J Biol Chem. 1981 Oct 10;256(19):9959–9965. [PubMed] [Google Scholar]
- Silhavy T. J., Hartig-Beecken I., Boos W. Periplasmic protein related to the sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1976 May;126(2):951–958. doi: 10.1128/jb.126.2.951-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Enquist L., Silhavy T. J. Identification of OmpR: a positive regulatory protein controlling expression of the major outer membrane matrix porin proteins of Escherichia coli K-12. J Bacteriol. 1981 Jul;147(1):255–258. doi: 10.1128/jb.147.1.255-258.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Overduin P., Lugtenberg B., Bergmans H. Cloning of phoE, the structural gene for the Escherichia coli phosphate limitation-inducible outer membrane pore protein. J Bacteriol. 1982 Feb;149(2):668–672. doi: 10.1128/jb.149.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Lohmeier E., Schryvers A. Cloning and expression of the glycerol-3-phosphate transport genes of Escherichia coli. Can J Biochem. 1978 Jun;56(6):611–617. doi: 10.1139/o78-092. [DOI] [PubMed] [Google Scholar]
- Zipser D., Bhavsar P. Missense mutations in the lacZ gene that result in degradation of beta-galactosidase structural protein. J Bacteriol. 1976 Sep;127(3):1538–1542. doi: 10.1128/jb.127.3.1538-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]