Abstract
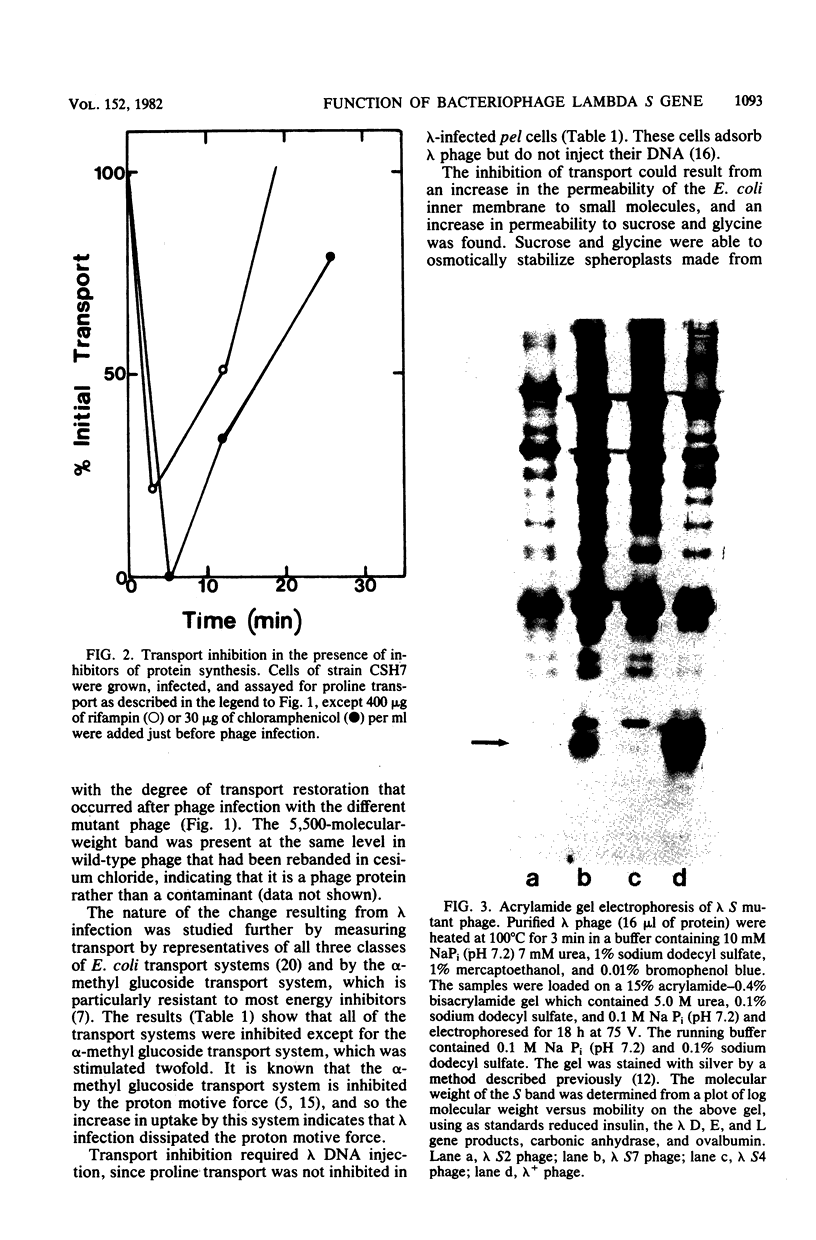
Infection of Escherichia coli by bacteriophage lambda caused an immediate inhibition of uptake by members of all three classes of E. coli active transport systems and made the inner membrane permeable to sucrose and glycine; however, infection stimulated alpha-methyl glucoside uptake. Phage infection caused a dramatic drop in the ATP pool of the cell, but the membrane did not become permeable to nucleotides. Infection by only one phage per cell was sufficient to cause transport inhibition. However, adsorption of phage to the lambda receptor did not cause transport inhibition; DNA injection was required. The inhibition of transport caused by lambda phage infection was transient, and by 20 min after infection, transport had returned to its initial level. The recovery of transport activity appeared to require a lambda structural protein with a molecular weight of 5,500. This protein was present in wild-type phage and at a reduced level in S7 mutant phage but was missing in S2 and S4 mutant phage. Cells infected with S7 phage had a partial recovery of active transport, whereas cells infected with S2 or S4 phage did not recover active transport. Neither the inhibition of transport caused by phage infection nor its recovery were affected by the protein synthesis inhibitors chloramphenicol and rifampin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. Biological activity of bacteriophage ghosts and "take-over" of host functions by bacteriophage. Bacteriol Rev. 1970 Sep;34(3):344–363. doi: 10.1128/br.34.3.344-363.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Hernandez-Asensio M., Ramirez J. M., Del Campo F. F. The control by respiration of the uptake of alpha-methyl glucoside in Escherichia coli K12. Arch Microbiol. 1975 Apr 7;103(2):155–162. doi: 10.1007/BF00436343. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Signer E. R. A site essential for expression of all late genes in bacteriophage lambda. J Mol Biol. 1970 Feb 14;47(3):545–556. doi: 10.1016/0022-2836(70)90321-9. [DOI] [PubMed] [Google Scholar]
- Israeli M., Artman M. Leakage of beta-galactosidase from phage-infected Escherichia coli: a re-evaluation. J Gen Virol. 1970;7(2):137–142. doi: 10.1099/0022-1317-7-2-137. [DOI] [PubMed] [Google Scholar]
- Labedan B., Letellier L. Membrane potential changes during the first steps of coliphage infection. Proc Natl Acad Sci U S A. 1981 Jan;78(1):215–219. doi: 10.1073/pnas.78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. II. Demonstration of cyclic permeability change accompanying virus infection of Escherichia coli B cells. J Exp Med. 1955 Feb 1;101(2):151–175. doi: 10.1084/jem.101.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. Physical mechanism for regulation of phosphoenolpyruvate-dependent glucose transport activity in Escherichia coli. Biochemistry. 1981 Aug 18;20(17):5025–5032. doi: 10.1021/bi00520a032. [DOI] [PubMed] [Google Scholar]
- Scandella D., Arber W. An Escherichia coli mutant which inhibits the injection of phage lambda DNA. Virology. 1974 Apr;58(2):504–513. doi: 10.1016/0042-6822(74)90084-1. [DOI] [PubMed] [Google Scholar]
- Shapira A., Giberman E., Kohn A. Recoverable potassium fluxes variations following adsorption of T4 phage and their ghosts on Escherichia coli B. J Gen Virol. 1974 May;23(2):159–171. doi: 10.1099/0022-1317-23-2-159. [DOI] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Effect of the lambda S gene product on properties of the Escherichia coli inner membrane. J Bacteriol. 1982 Sep;151(3):1403–1410. doi: 10.1128/jb.151.3.1403-1410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




