Abstract
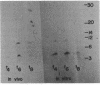
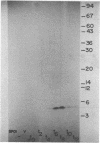
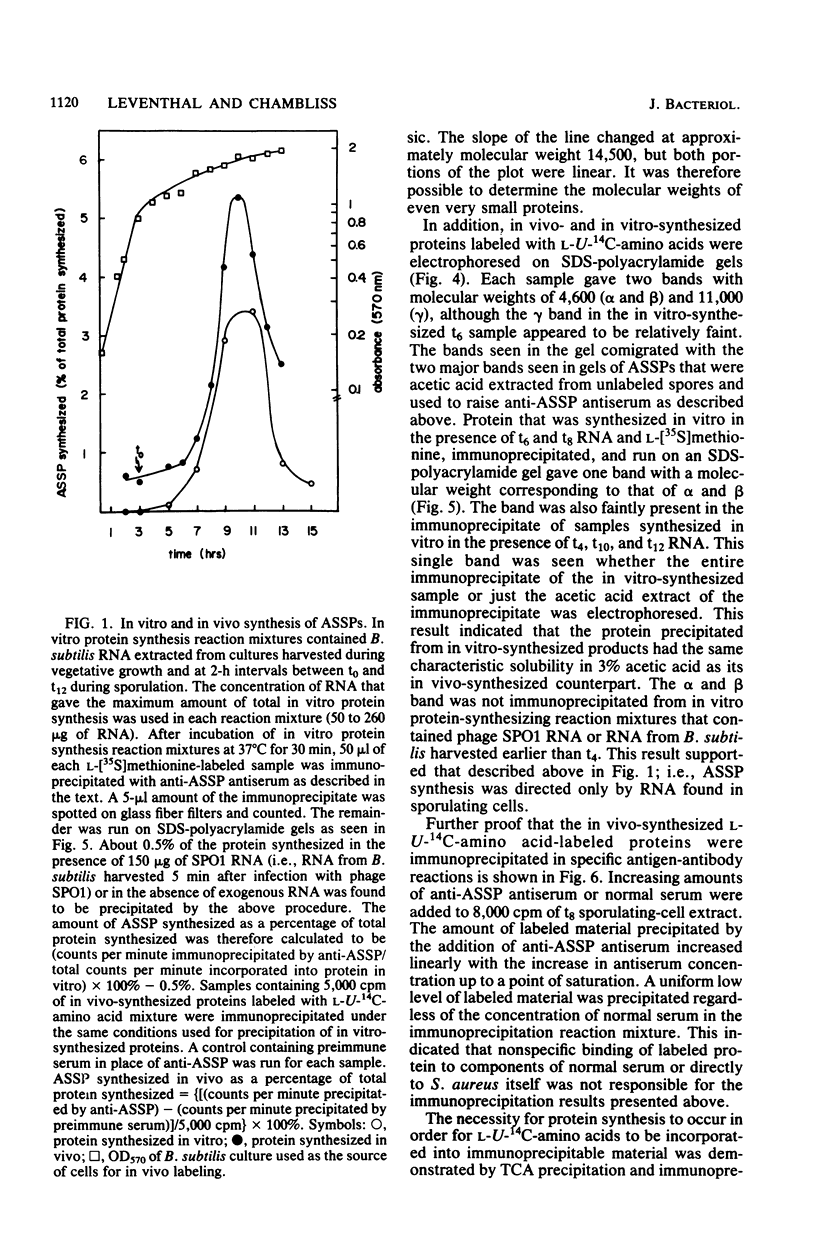
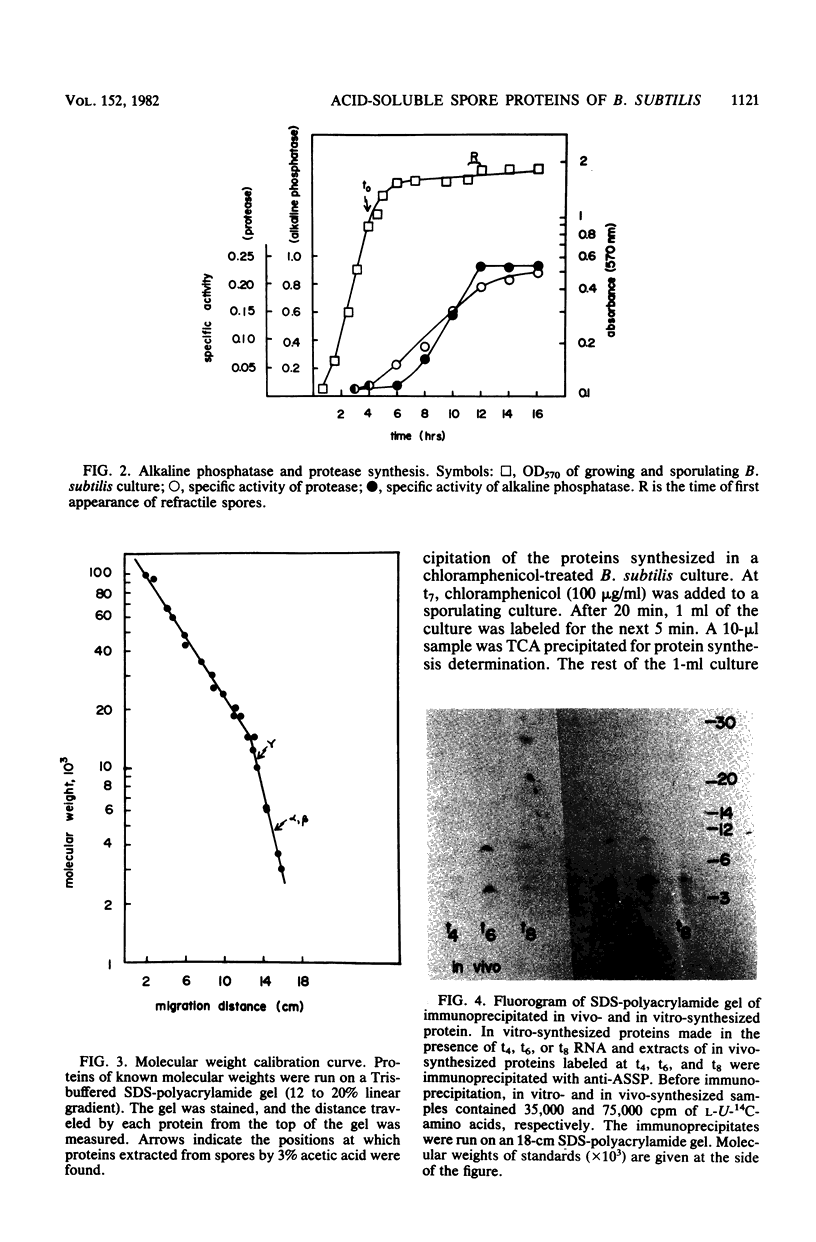
The major acid-soluble spore proteins (ASSPs) of Bacillus subtilis were detected by immunoprecipitation of radioactively labeled in vitro- and in vivo-synthesized proteins. ASSP synthesis in vivo began 2 h after the initiation of sporulation (t2) and reached its maximum rate at t7. This corresponded to the time of synthesis of mRNA that stimulated the maximum rate of ASSP synthesis in vitro. Under the set of conditions used in these experiments, protease synthesis began near t0, alkaline phosphatase synthesis began at about t2, and refractile spores were first observed between t7 and t8. In vivo- and in vitro-synthesized ASSPs comigrated in sodium dodecyl sulfate-polyacrylamide gels. Their molecular weights were 4,600 (alpha and beta) and 11,000 (gamma). The average half-life of the ASSP messages was 11 min when either rifampin (10 micrograms/ml) or actinomycin D (1 microgram/ml) was used to inhibit RNA synthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte-Panvier A., Davies J. E., Gritz L. R., Littlewood B. S. Studies of ribosomal proteins of yeast species and their hybrids: gel electrophoresis and immunochemical cross-reactions. Mol Gen Genet. 1980;179(2):273–282. doi: 10.1007/BF00425454. [DOI] [PubMed] [Google Scholar]
- Dignam S. S., Setlow P. In vivo and in vitro synthesis of the spore-specific proteins A and C of bacillus megaterium. J Biol Chem. 1980 Sep 25;255(18):8417–8423. [PubMed] [Google Scholar]
- Johnson W. C., Tipper D. J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legault-Demare L., Chambliss G. H. Natural messenger ribonucleic acid-directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal J. M., Chambliss G. H. DNA-directed cell-free protein-synthesizing system of Bacillus subtilis. Biochim Biophys Acta. 1979 Aug 29;564(1):162–171. doi: 10.1016/0005-2787(79)90197-7. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Johnson W. C., Tipper D. J., Setlow P. Comparison of various properties of low-molecular-weight proteins from dormant spores of several Bacillus species. J Bacteriol. 1981 Jun;146(3):965–971. doi: 10.1128/jb.146.3.965-971.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]