Abstract
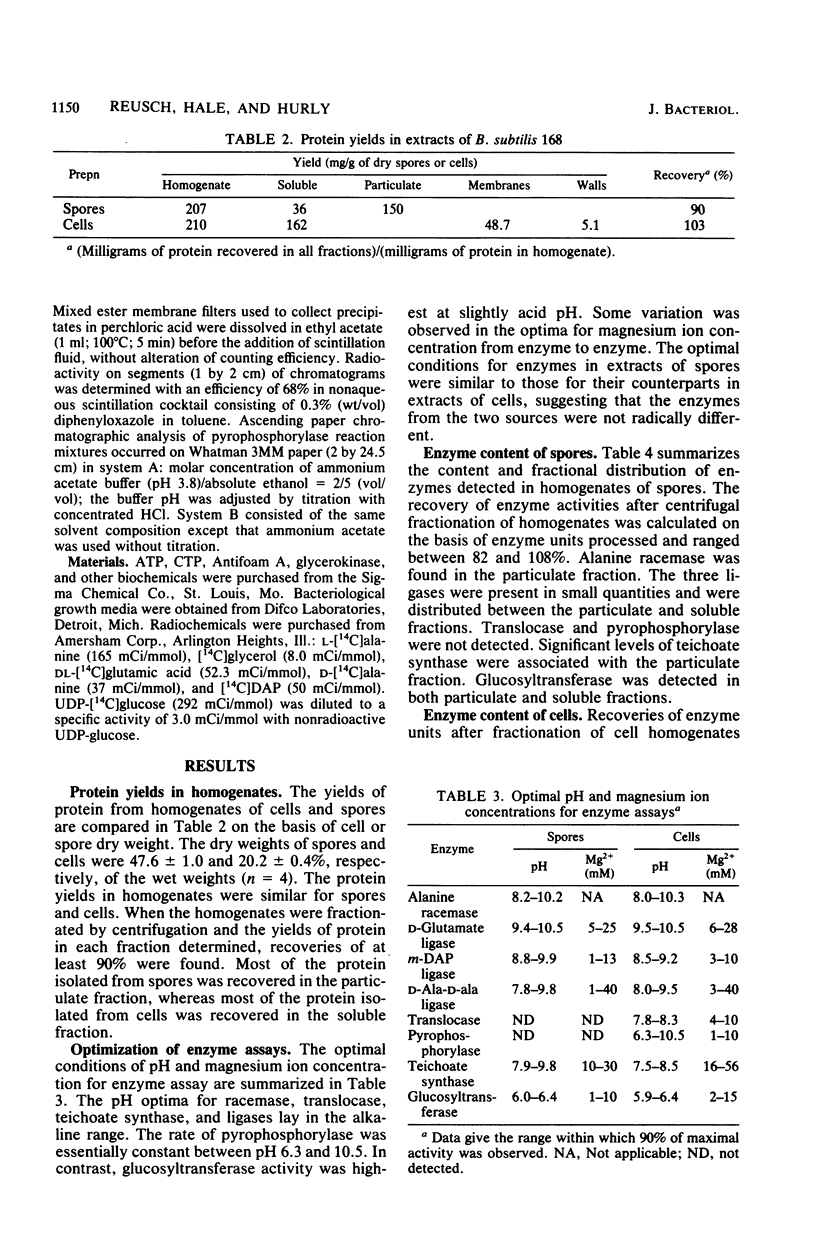
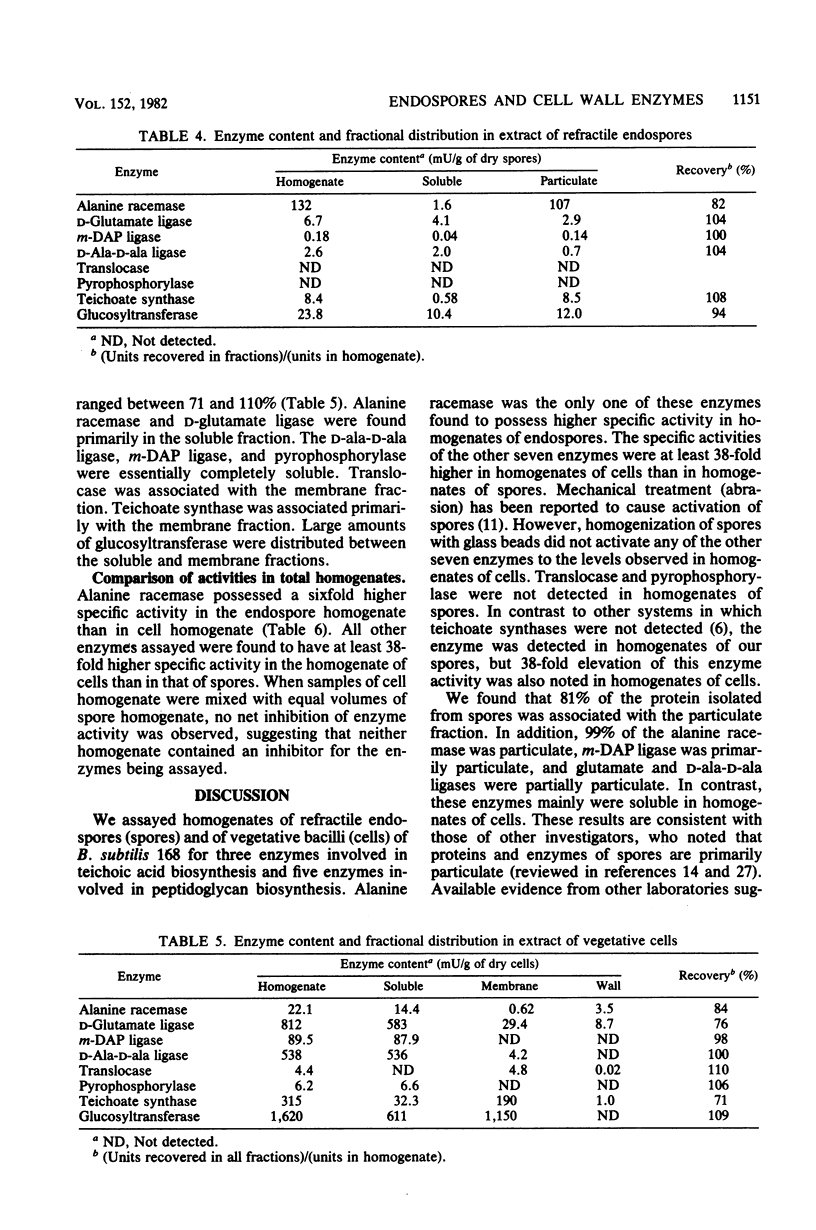
Vegetative bacilli and refractile endospores of Bacillus subtilis 168 were disrupted by homogenization with glass beads and fractionated by differential centrifugation. Most of the protein of endospores was particulate, whereas for bacilli most was soluble. Alanine racemase activity was sixfold higher in extract of endospores than in extract of bacilli and was particulate, whereas the enzyme from bacilli was soluble. The specific activities of seven other enzymes involved in peptidoglycan and teichoic acid biosynthesis were higher in extracts of bacilli than in those of endospores. The results suggest that restoration of activities of these seven enzymes to vegetative levels occurs during germination and outgrowth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Anderson J. S., Meadow P. M., Haskin M. A., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. I. Utilization of uridine diphosphate acetylmuramyl pentapeptide and uridine diphosphate acetylglucosamine for peptidoglycan synthesis by particulate enzymes from Staphylococcus aureus and Micrococcus lysodeikticus. Arch Biochem Biophys. 1966 Sep 26;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- BURGER M. M., GLASER L. THE SYNTHESIS OF TEICHOIC ACIDS. I. POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3168–3177. [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W., Ensign J. C. Ratio of teichoic acid and peptidoglycan in cell walls of Bacillus subtilis following spire germination and during vegetative growth. J Bacteriol. 1968 Aug;96(2):421–427. doi: 10.1128/jb.96.2.421-427.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin T., Younger J., Glaser L. Synthesis of teichoic acids. VII. Synthesis of teichoic acids during spore germination. J Bacteriol. 1968 Jun;95(6):2044–2050. doi: 10.1128/jb.95.6.2044-2050.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., McDannel M. L., Helman J. R., Streips U. N. Distribution of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):152–158. doi: 10.1128/jb.122.1.152-158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L., BURGER M. M. THE SYNTHESIS OF TEICHOIC ACIDS. 3. GLUCOSYLATION OF POLYGLYCEROPHOSPHATE. J Biol Chem. 1964 Oct;239:3187–3191. [PubMed] [Google Scholar]
- Good C. M., Tipper D. J. Conditional mutants of Staphylococcus aureus defective in cell wall precursor synthesis. J Bacteriol. 1972 Jul;111(1):231–241. doi: 10.1128/jb.111.1.231-241.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Gauther J. J., Tipper D. J. Ultrastructural studies of sporulation in Bacillus sphaericus. J Bacteriol. 1975 Jun;122(3):1322–1338. doi: 10.1128/jb.122.3.1322-1338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert M. P., Neuhaus F. C. Mechanism of D-cycloserine action: alanine racemase from Escherichia coli W. J Bacteriol. 1972 Jun;110(3):978–987. doi: 10.1128/jb.110.3.978-987.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Tipper D. J. Cell wall polymers of Bacillus sphaericus: activities of enzymes involved in peptidoglycan precursor synthesis during sporulation. J Bacteriol. 1974 Oct;120(1):342–354. doi: 10.1128/jb.120.1.342-354.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Tipper D. J. Transcriptional control of peptidoglycan precursor synthesis during sporulation in Bacillus sphaericus. J Bacteriol. 1976 Feb;125(2):565–574. doi: 10.1128/jb.125.2.565-574.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUHAUS F. C., STRUVE W. G. ENZYMATIC SYNTHESIS OF ANALOGS OF THE CELL-WALL PRECURSOR. I. KINETICS AND SPECIFICITY OF URIDINE DIPHOSPHO-N-ACETYLMURAMYL-L-ALANYL-D-GLUTAMYL-L-LYSINE:D-ALANYL-D-ALANINE LIGASE (ADENOSINE DIPHOSPHATE) FROM STREPTOCOCCUS FAECALIS R. Biochemistry. 1965 Jan;4:120–131. doi: 10.1021/bi00877a020. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- Reusch V. M., Jr, Burger M. M. Distribution of marker enzymes between mesosomal and protoplast membranes. J Biol Chem. 1974 Aug 25;249(16):5337–5345. [PubMed] [Google Scholar]
- Reusch V. M., Panos C. Defective synthesis of lipid intermediates for peptidoglycan formation in a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1976 Apr;126(1):300–311. doi: 10.1128/jb.126.1.300-311.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW D. R. Pyrophosphorolysis and enzymic synthesis of cytidine diphosphate glycerol and cytidine diphosphate ribitol. Biochem J. 1962 Feb;82:297–312. doi: 10.1042/bj0820297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Linnett P. E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976 Apr;126(1):213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Molecular structure of the bacterial spore. Adv Microb Physiol. 1978;17:1–45. doi: 10.1016/s0065-2911(08)60056-9. [DOI] [PubMed] [Google Scholar]
- Wickus G. G., Rubenstein P. A., Warth A. D., Strominger J. L. Partial purification and some properties of the uridine diphospho-N-acetylglucosamine-enolpyruvate reductase from Staphylococcus epidermidis. J Bacteriol. 1973 Jan;113(1):291–294. doi: 10.1128/jb.113.1.291-294.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickus G. G., Strominger J. L. Partial purification and properties of the pyruvate-uridine diphospho-N-acetylglucosamine transferase from Staphylococcus epidermidis. J Bacteriol. 1973 Jan;113(1):287–290. doi: 10.1128/jb.113.1.287-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


