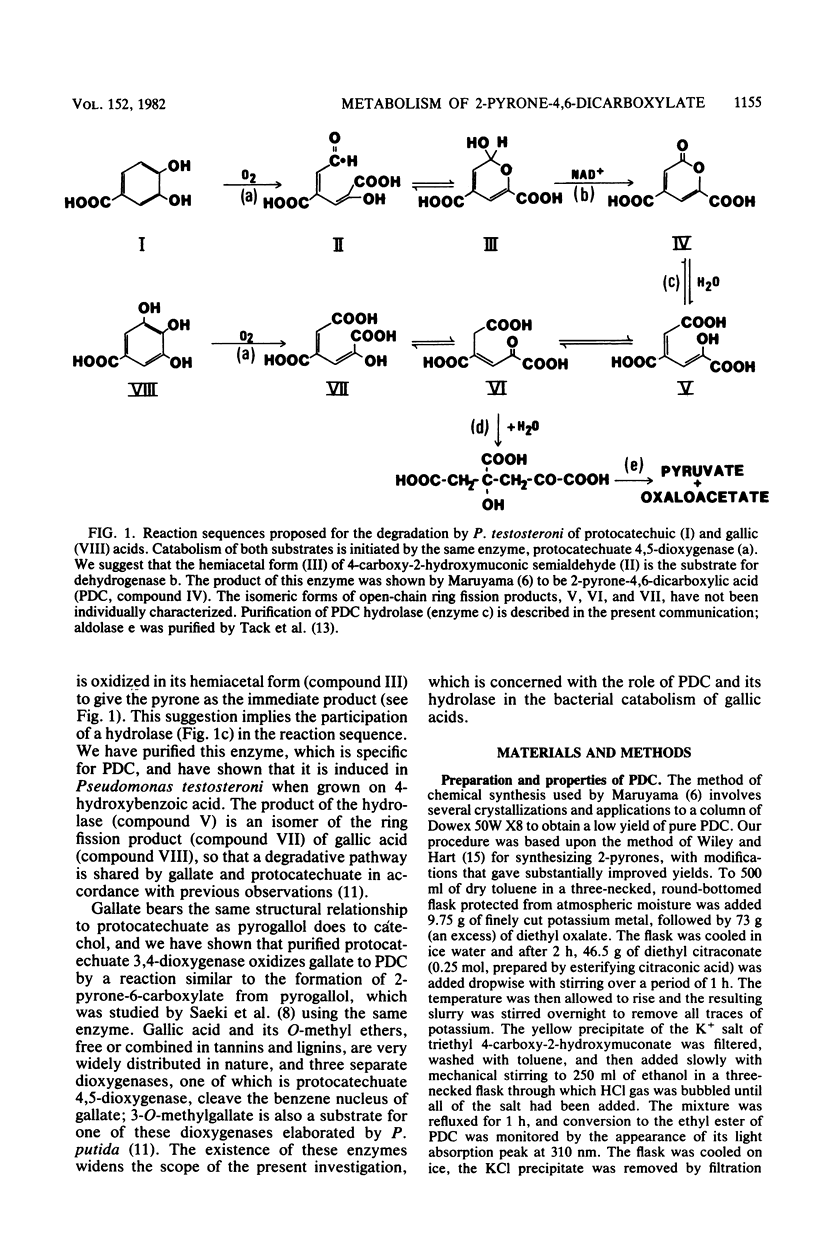
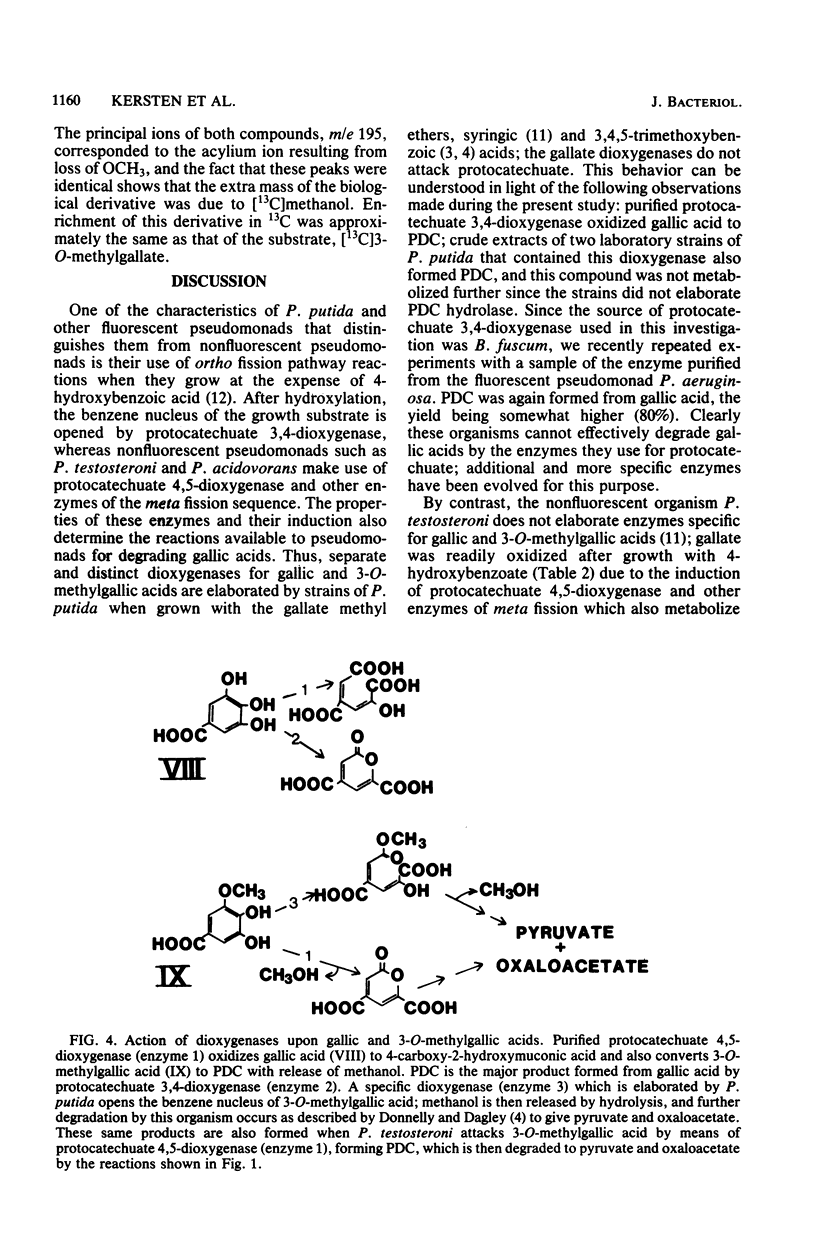
Abstract
2-Pyrone-4,6-dicarboxylate hydrolase was purified from 4-hydroxybenzoate-grown Pseudomonas testosteroni. Gel filtration and electrophoretic measurements indicated that the preparation was homogeneous and gave a molecular weight of 37,200 for the single subunit of the enzyme. Hydrolytic activity was dependent upon a functioning sulfhydryl group(s) and was freely reversible; the equilibrium position was dependent upon pH, with equimolar amounts of pyrone and open-chain form present at pH 7.9. Since the hydrolase was strongly induced when the nonfluorescent organisms P. testosteroni and P. acidovorans grew with 4-hydroxybenzoate, it is suggested that 2-pyrone-4,6-dicarboxylate is a normal intermediate in the meta fission degradative pathway of protocatechuate. Laboratory strains of fluorescent pseudomonads did not metabolize 2-pyrone-4,6-dicarboxylate, but a strain of P. putida was isolated from soil that utilized this compound for growth; the hydrolase was then induced, but it was absent from extracts of 4-hydroxybenzoate-grown cells that readily catabolized protocatechuate by ortho fission reactions. 2-Pyrone-4,6-dicarboxylic acid was the major product formed when gallic acid was oxidized by purified protocatechuate 3,4-dioxygenase. Protocatechuate 4,5-dioxygenase gave only the open-chain ring fission product when gallic acid was oxidized, but the enzyme attacked 3-O-methylgallic acid, giving 2-pyrone-4,6-dicarboxylic acid as the major product. Cell suspensions of 4-hydroxybenzoate-grown P. testosteroni readily oxidized 3-O-methylgallate with accumulation of methanol.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Dagley S. Catabolism of tryptophan, anthranilate, and 2,3-dihydroxybenzoate in Trichosporon cutaneum. J Bacteriol. 1981 Apr;146(1):291–297. doi: 10.1128/jb.146.1.291-297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Dagley S. Bacterial degradation of 3,4,5-trimethoxycinnamic acid with production of methanol. J Bacteriol. 1981 Aug;147(2):471–476. doi: 10.1128/jb.147.2.471-476.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. I., Dagley S. Production of methanol from aromatic acids by Pseudomonas putida. J Bacteriol. 1980 Jun;142(3):916–924. doi: 10.1128/jb.142.3.916-924.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K. Isolation and identification of the reaction product of alpha-hydroxy-gamma-carboxymuconic epsilon-semialdehyde dehydrogenase. J Biochem. 1979 Dec;86(6):1671–1677. doi: 10.1093/oxfordjournals.jbchem.a132687. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Nozaki M., Senoh S. Cleavage of pyrogallol by non-heme iron-containing dioxygenases. J Biol Chem. 1980 Sep 25;255(18):8465–8471. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Murray K., Williams P. A. The metabolic divergence in the meta cleavage of catechols by Pseudomonas putida NCIB 10015. Physiological significance and evolutionary implications. Eur J Biochem. 1972 Jul 24;28(3):347–356. doi: 10.1111/j.1432-1033.1972.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Dagley S. Alternative routes of aromatic catabolism in Pseudomonas acidovorans and Pseudomonas putida: gallic acid as a substrate and inhibitor of dioxygenases. J Bacteriol. 1975 Dec;124(3):1374–1381. doi: 10.1128/jb.124.3.1374-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-4-methyl-2-oxoglutarate aldolase. J Biol Chem. 1972 Oct 25;247(20):6444–6449. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zabinski R., Münck E., Champion P. M., Wood J. M. Kinetic and Mössbauer studies on the mechanism of protocatechuic acid 4,5-oxygenase. Biochemistry. 1972 Aug 15;11(17):3212–3219. doi: 10.1021/bi00767a012. [DOI] [PubMed] [Google Scholar]



