Abstract
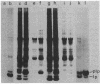
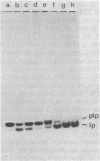
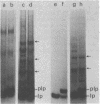
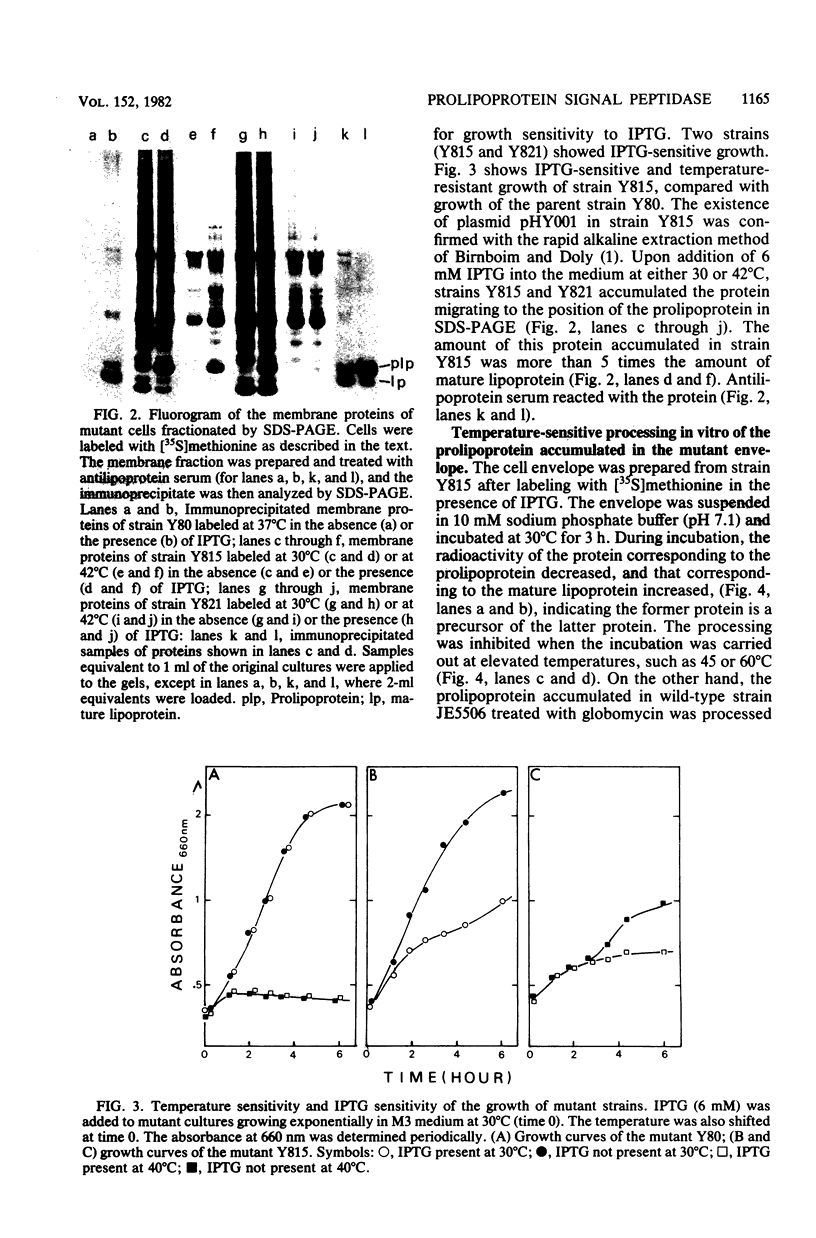
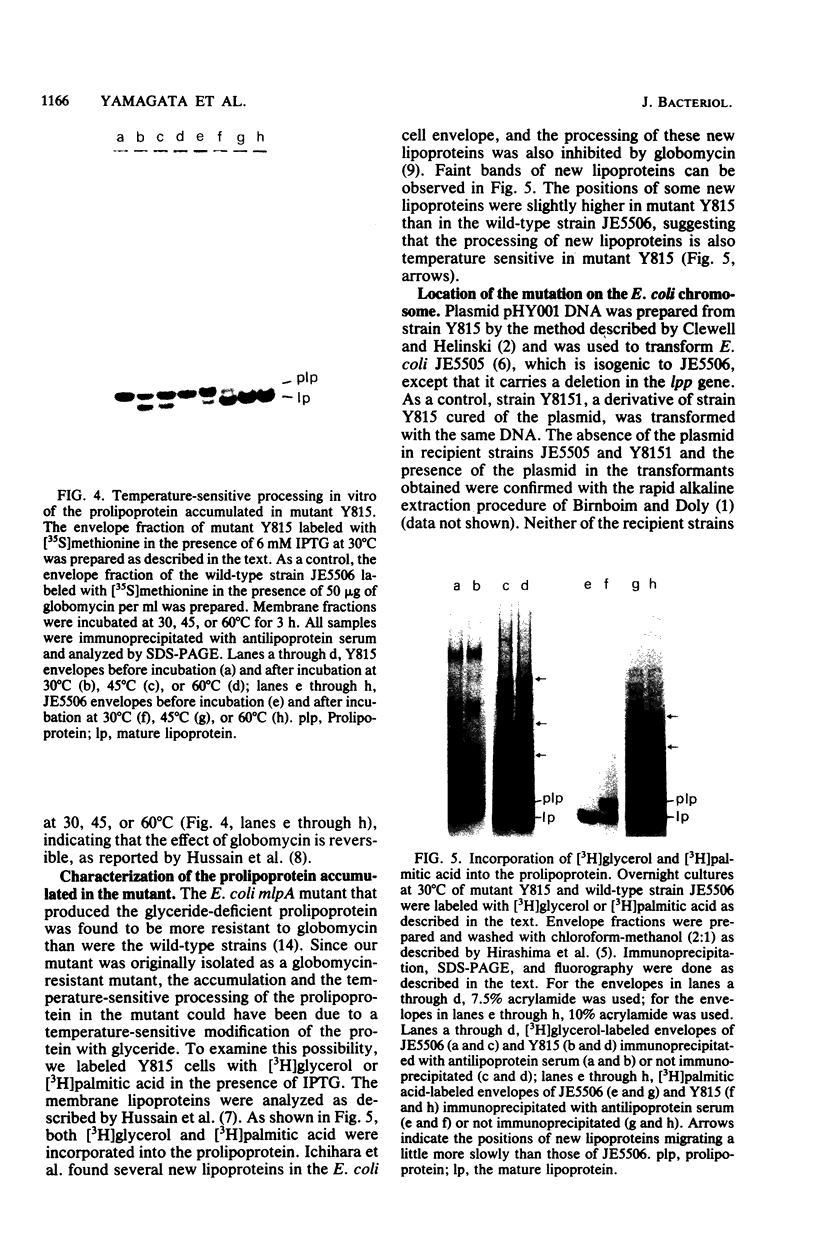
A mutant of Escherichia coli that accumulated prolipoprotein, a secretory precursor of the outer membrane lipoprotein, was isolated. The prolipoprotein accumulated in this mutant was modified by glyceride, but the in vitro cleavage of the signal peptide of the accumulated prolipoprotein was found to be temperature sensitive. The mutation appears to be located outside the gene for the lipoprotein, thus suggesting that the gene for the signal peptidase for the prolipoprotein was mutated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Hirashima A., Inouye M. Existence of a free form of a specific membrane lipoprotein in gram-negative bacteria. J Bacteriol. 1974 Dec;120(3):1204–1208. doi: 10.1128/jb.120.3.1204-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J Biol Chem. 1980 Apr 25;255(8):3707–3712. [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1982 May 10;257(9):5177–5182. [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Inouye M. Lipoprotein of the outer membrane of Escherichia coli. Biomembranes. 1979;10:141–208. doi: 10.1007/978-1-4615-6564-2_4. [DOI] [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Philbrick W. M., Hayashi S., Inukai M., Arai M., Hirota Y., Wu H. C. Globomycin sensitivity of Escherichia coli and Salmonella typhimurium: effects of mutations affecting structures of murein lipoprotein. J Bacteriol. 1981 Jan;145(1):657–660. doi: 10.1128/jb.145.1.657-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Dombou M., Sato T., Mizushima S., Uchida H. Deletion mapping and heterogenote analysis of a mutation responsible for osmosis-sensitive growth, spectinomycin resistance, and alteration of cytoplasmic membrane in Escherichia coli. J Bacteriol. 1980 Aug;143(2):661–667. doi: 10.1128/jb.143.2.661-667.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Nakamura K., Inouye M. Comparison of the lipoprotein gene among the Enterobacteriaceae. DNA sequence of Erwinia amylovora lipoprotein gene. J Biol Chem. 1981 Mar 10;256(5):2194–2198. [PubMed] [Google Scholar]
- Zwiebel L. J., Inukai M., Nakamura K., Inouye M. Preferential selection of deletion mutations of the outer membrane lipoprotein gene of Escherichia coli by globomycin. J Bacteriol. 1981 Jan;145(1):654–656. doi: 10.1128/jb.145.1.654-656.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]