Abstract
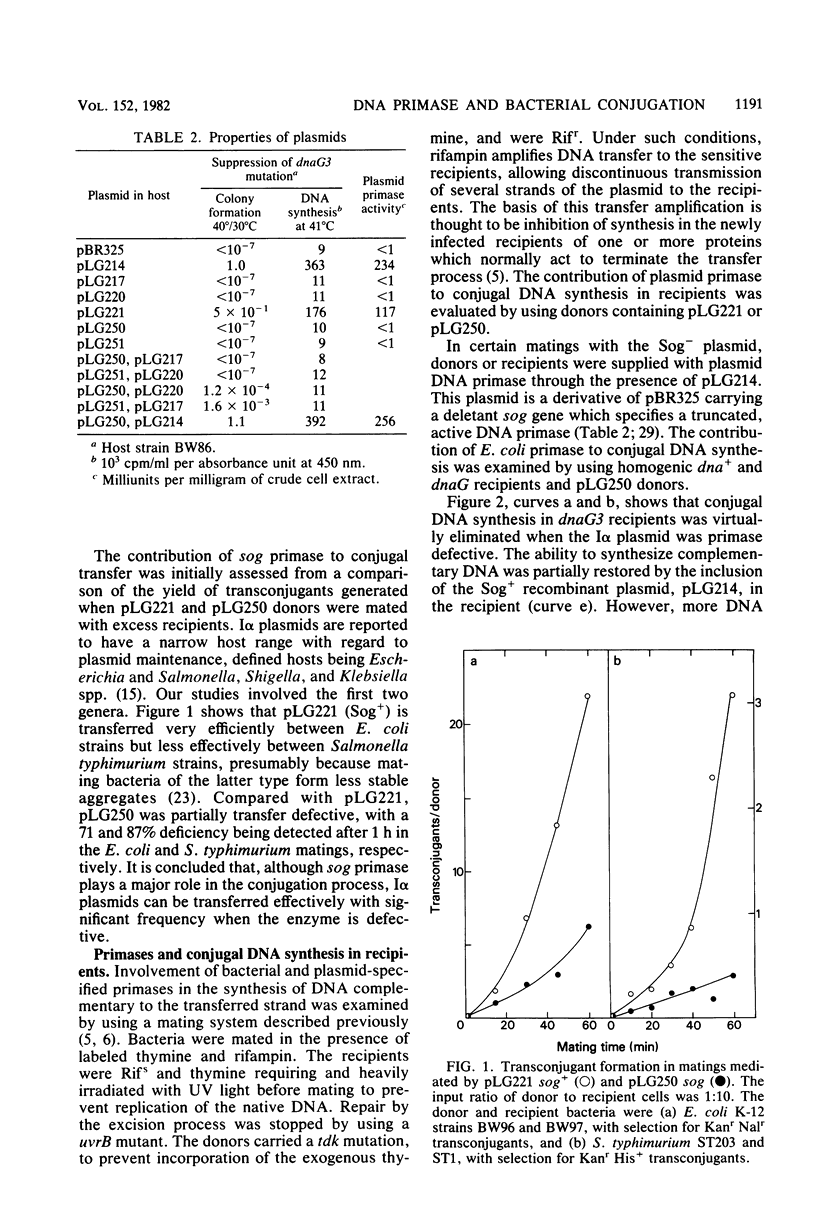
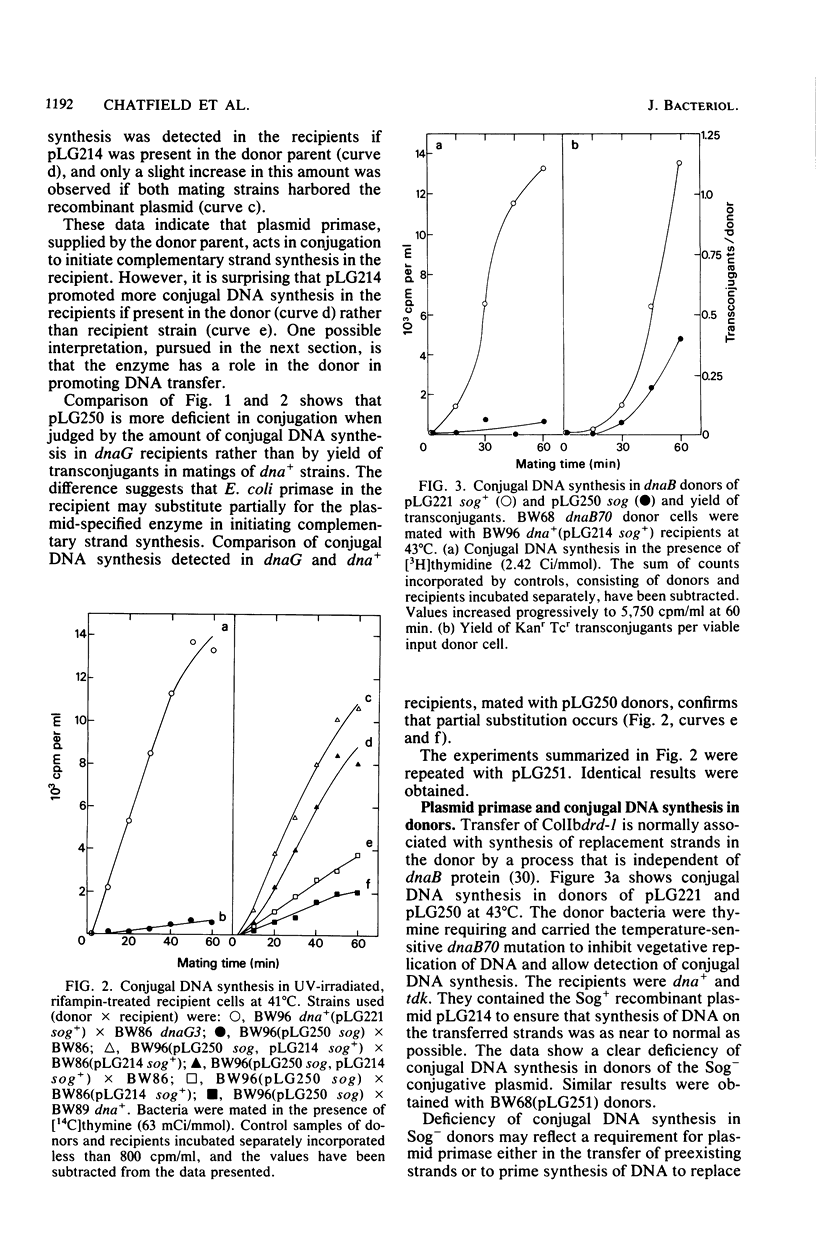
The sog gene of the IncI alpha group plasmid ColIb is known to encode a DNA primase that can substitute for defective host primase in dnaG mutants of Escherichia coli during discontinuous DNA replication. The biological significance of this enzyme was investigated by using sog mutants, constructed from a derivative of ColIb by in vivo recombination of previously defined mutations in a cloned sog gene. The resultant Sog- plasmids failed to specify detectable primase activity and were unable to suppress a dnaG lesion. These mutants were maintained stably in E. coli, implying that the enzyme is not involved in vegetative replication of ColIb. However, the Sog- plasmids were partially transfer deficient in E. coli and Salmonella typhimurium matings, consistent with the hypothesis that the normal physiological role of this enzyme is in conjugation. This was confirmed by measurements of conjugal DNA synthesis. Studies of recipient cells have indicated that plasmid primase is required to initiate efficient synthesis of DNA complementary to the transferred strand, with the protein being supplied by the donor parent and probably transmitted between the mating cells. Primase specified by the dnaG gene of the recipient can substitute partially for the mutant enzyme, thus providing an explanation for the partial transfer proficiency of the mutant plasmids. Conjugal DNA synthesis in dnaB donor cells was deficient in the absence of plasmid primase, implying that the enzyme also initiates synthesis of DNA to replace the transferred material.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Beddoes M. J., Wilkins B. M. Rifampin disrupts conjugal and chromosomal deoxyribonucleic acid metabolism in Escherichia coli K-12 carrying some IncIalpha plasmids. J Bacteriol. 1979 May;138(2):324–332. doi: 10.1128/jb.138.2.324-332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J. Colicin Ib does not cause plasmid-promoted abortive phage infection of Escherichia coli K-12. Mol Gen Genet. 1981;182(3):508–510. doi: 10.1007/BF00293944. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A colI-specified product, synthesized in newly infected recipients, limits the amount of DNA transferred during conjugation of Escherichia coli K-12. J Bacteriol. 1978 Jan;133(1):1–9. doi: 10.1128/jb.133.1.1-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A novel priming system for conjugal synthesis of an IncI alpha plasmid in recipients. Mol Gen Genet. 1979 Oct 1;175(3):275–279. doi: 10.1007/BF00397227. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M., Lanka E. Overlapping genes at the DNA primase locus of the large plasmid ColI. Nucleic Acids Res. 1982 Feb 11;10(3):855–869. doi: 10.1093/nar/10.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Sirgel F. A., Lecatsas G. Properties of a filamentous phage which adsorbs to pili coded by plasmids of the IncI complex. J Gen Microbiol. 1980 Apr;117(2):547–551. doi: 10.1099/00221287-117-2-547. [DOI] [PubMed] [Google Scholar]
- Dalrymple B. P., Boulnois G. J., Wilkins B. M., Orr E., Williams P. H. Evidence for two genetically distinct DNA primase activities specified by plasmids of the B and I incompatibility groups. J Bacteriol. 1982 Jul;151(1):1–7. doi: 10.1128/jb.151.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Excherichia coli K-12: effect of chloramphenicol and rifampin. J Bacteriol. 1973 Dec;116(3):1224–1235. doi: 10.1128/jb.116.3.1224-1235.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- Lanka E., Barth P. T. Plasmid RP4 specifies a deoxyribonucleic acid primase involved in its conjugal transfer and maintenance. J Bacteriol. 1981 Dec;148(3):769–781. doi: 10.1128/jb.148.3.769-781.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanka E., Scherzinger E., Günther E., Schuster H. A DNA primase specified by I-like plasmids. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3632–3636. doi: 10.1073/pnas.76.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturin L. J., Sr, Curtiss R., 3rd Role of ribonucleic acid synthesis in conjugational transfer of chromosomal and plasmid deoxyribonucleic acids. J Bacteriol. 1981 May;146(2):552–563. doi: 10.1128/jb.146.2.552-563.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y., Akimoto S. I-like R plasmids promote degradation of stable ribonucleic acid in Escherichia coli. J Bacteriol. 1980 Nov;144(2):833–835. doi: 10.1128/jb.144.2.833-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sanderson K. E., Janzer J., Head J. Influence of lipopolysaccharide and protein in the cell envelope on recipient capacity in conjugation of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):283–293. doi: 10.1128/jb.148.1.283-293.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. DNA or RNA priming of bacteriophage G4 DNA synthesis by Escherichia coli dnaG protein. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2815–2819. doi: 10.1073/pnas.74.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Hollom S. E. Conjugational synthesis of F lac+ and Col I DNA in the presence of rifampicin and in Escherichia coli K12 mutants defective in DNA synthesis. Mol Gen Genet. 1974;134(2):143–156. doi: 10.1007/BF00268416. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M. Partial suppression of the phenotype of Escherichia coli K-12 dnaG mutants by some I-like conjugative plasmids. J Bacteriol. 1975 Jun;122(3):899–904. doi: 10.1128/jb.122.3.899-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



