Abstract
Lys-γ3-MSH is a melanocortin peptide derived from the C-terminal of the 16 kDa fragment of POMC. The physiological role of Lys-γ3-MSH is unclear, although it has previously been shown that, although not directly steroidogenic, it can act to potentiate the steroidogenic response of adrenal cortical cells to ACTH. This synergistic effect appears to be correlated with an ability to increase the activity of hormone sensitive lipase (HSL) and therefore the rate of cholesterol ester hydrolysis. Ligand binding studies have suggested that high-affinity binding sites for Lys-γ3-MSH exist in the adrenal gland and a number of other rat tissues that express HSL, including adipose, skeletal muscle and testes. To investigate the hypothesis that Lys-γ3-MSH may play a wider role in cholesterol and lipid metabolism, we tested the effect of Lys-γ3-MSH on lipolysis, an HSL-mediated process, in 3T3-L1 adipocytes. In comparison with other melanocortin peptides, Lys-γ3-MSH was found to be a potent stimulator of lipolysis. It was also able to phosphorylate HSL at key serine residues and stimulate the hyperphosphorylation of perilipin A. The receptor through which the lipolytic actions of Lys-γ3-MSH are being mediated is not clear. Attempts to characterise this receptor suggest that either the pharmacology of the melanocortin receptor 5 in 3T3-L1 adipocytes is different from that described when expressed in heterologous systems or the possibility that a further, as yet uncharacterised, receptor exists.
Introduction
The α-, β- and γ-melanocyte-stimulating hormones (MSHs) are melanocortin peptides that are derived from the adrenocorticotrophin (ACTH) precursor protein pro opiomelanocortin (POMC). Unlike α-MSH, which was purified from pituitary extracts, the sequence of γ-MSH was predicted from the gene sequence of POMC (Nakanishi et al. 1979). Based on the location of the putative dibasic residue cleavage sites, three related peptides (γ1, γ2 and γ3-MSH) have been described.
Subsequent studies where the peptides have been isolated from pituitary extracts have shown that the Arg-Lys site at the N-terminal of γ-MSH is, in fact, cleaved after the arginine residue resulting in all the peptides carrying an additional lysine at their N-terminus (Bohlen et al. 1981, Browne et al. 1981). These studies have also shown that γ2-MSH does not exist and that the bulk of γ-MSH in the anterior pituitary consists of pro-γ-MSH (corresponding to N-POMC 1–74 in rodents), whilst the intermediate lobe processes POMC to mainly Lys-γ3-MSH (corresponding to N-POMC 50–74) and small amounts of Lys-γ1-MSH (Zhou et al. 1993). In addition, unlike the other melanocortin peptides, both pro-γ-MSH and Lys-γ3-MSH carry an N-linked glycan at their C-terminus (corresponding to residue 65 of POMC).
In comparison with α-MSH and ACTH, the γ-MSH peptides have received relatively little attention and their biological role remains unclear. γ-MSH peptides have been shown to have effects on the heart (Veersteeg et al. 1998), kidney (Humpreys 2004), pituitary (Denef et al. 2003) and adrenal gland (Pedersen & Brownie 1980, Pedersen et al. 1980, Al-Dujaili et al. 1981). Studies performed in the early 1980s have shown that both pro-γ-MSH and Lys-γ3-MSH, although not directly steroidogenic, are able to potentiate the steroidogenic response of rat adrenal cells to ACTH (Pedersen & Brownie 1980, Pedersen et al. 1980, Al-Dujaili et al. 1981). This synergistic effect appears to be correlated with an ability to increase the activity of the enzyme hormone sensitive lipase (HSL; Pedersen et al. 1980). HSL acts to catalyse the hydrolysis of cholesterol esters and triacylglycerides, with the hydrolysis of cholesterol esters vital for steroidogenesis in the adrenal cortex, ovary and testes and the hydrolysis of triacylglycerides central to lipolysis in adipose tissue (Yeaman 2004). HSL has also been shown to regulate the hydrolysis of cholesterol esters and triacylglycerides in the heart, pancreas, skeletal muscle and macrophage cells (Small et al. 1989, 1991, Langfort et al. 1999, Mulder et al. 1999).
In 1983, before the identification of the five members of the melanocortin receptor (MC-R) family, Pedersen & Brownie used ligand binding to investigate the existence of a γ-MSH receptor expressed by the adrenal gland. They showed that rat adrenal cortex membrane preparations possessed high-affinity binding sites for radio-labelled synthetic Lys-γ3-MSH that are distinct from ACTH binding sites (Pedersen & Brownie 1983). To their surprise, they also found that binding was not confined to the adrenal gland. Indeed, in comparison with the adrenal gland, they observed higher levels of binding in rat adipose tissue, skeletal muscle and testes membrane preparations and lower levels of binding in rat cardiac muscle, ovary and spleen membrane preparations (Pedersen & Brownie 1983). These observations led Pedersen & Brownie 1983 to hypothesise that the actions of γ-MSH peptides on HSL activity, and therefore cholesterol and lipid utilisation, may not be limited to the adrenal gland.
The high level of specific binding sites for Lys-γ3-MSH on adipose tissue (Pedersen & Brownie 1983) suggests that the γ-MSH peptides may be able to regulate HSL activity in this tissue and therefore be lipolytic. The melanocortin peptides, ACTH, α-MSH and β-lipotrophin, have long been known to promote lipolysis, to varying degrees, in the adipocytes of various mammalian species (White & Engel 1958, Ramachandran & Lee 1976, Ramachandran et al. 1976, Boston 1999). However, upon an extensive literature review, only one study (Ng 1990) can be found showing that γ1-MSH (without the N-terminal lysine) is very weakly lipolytic in rabbit adipocytes. In this present study, we present results that support the hypothesis that the actions of γ-MSH peptides are not limited to the adrenal gland by demonstrating that Lys-γ3-MSH potently stimulates lipolysis in 3T3-L1 adipocytes. Although the lipolytic activity of Lys-γ3-MSH suggests that HSL is being activated, we also directly investigated the actions of this peptide, and other melanocortin peptides, on HSL phosphorylation and another protein central to the lipolytic process, perilipin A.
Finally, the specific receptor for Lys-γ3-MSH implicated in the binding study by Pedersen & Brownie (1983) remains unidentified. With the subsequent identification of the MC-Rs, it has been shown that 3T3-L1 adipocytes express only the MC2-R and MC5-R (Boston & Cone 1996) for which γ-MSH has no significant or limited affinity respectively (Griffon et al. 1994, Schioth et al. 1995, 1996). The limited affinity of γ-MSH for the MC2-R and MC5-R has led us to investigate the properties of the receptor that mediates the lipolytic action of Lys-γ3-MSH on 3T3-L1 adipocytes.
Materials and Methods
Growth and differentiation of the 3T3-L1 cell line
Cryopreserved 3T3-L1 pre-adipocytes were obtained from the ECACC. The cells were maintained in a humidified incubator at 37 °C with 5% CO2 in growth media consisting of Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 10% (v/v) calf serum (Invitrogen), l-glutamine (2 mM) and penicillin/streptomycin (100 U/ml; Invitrogen) and sub-cultured every 2–3 days. Stocks of 3T3-L1 pre-adipocytes from early passage were stored at −70 °C in calf serum supplemented with 10% DMSO (Sigma).
To differentiate 3T3-L1 pre-adipocytes into adipocytes, cells were grown to confluence in six-well plates (∼6·3×105 cells), and 2 days later differentiation was induced (day 0) by the addition of growth media containing 10% foetal bovine serum (FBS; Invitrogen) in place of calf serum supplemented with 3-isobutyl-1-methylxanthine (0·5 mM; Sigma), insulin (1·7 μM; Sigma) and dexamethasone (1 μM; Sigma). After 2 days, the media was changed to FBS growth media supplemented with just insulin (1·7 μM) and maintained for a further 2 days. On day 4, the media was changed to just FBS growth media and subsequently changed every second day. Differentiation from pre-adipocyte to adipocyte occurred generally 1–2 days after the insulin was removed. On day 8, the cells were serum starved overnight in DMEM supplemented with l-glutamine (2 mM) and penicillin/streptomycin (100 U/ml; Invitrogen) and experiments conducted on day 9.
Investigating the actions of various melanocortin peptides on glycerol release from 3T3-L1 adipocytes
On day 9, serum starved 3T3-L1 adipocytes (∼6·3×105) were washed thrice with phenol red-free Hank's balanced salt solution (HBSS; Sigma). Peptides of interest were prepared in Glycerol Assay Solution (phenol red-free HBSS supplemented with 0·5% BSA (Sigma) and penicillin/streptomycin (100 U/ml; Invitrogen)) and added to the adipocytes in 2 ml volumes. After the addition of peptides, the cells were incubated for 4 h in a humidified incubator at 37 °C with 5% CO2. Following incubation, the media was harvested and glycerol content measured using a sequential enzymatic assay based on that described in Sharma et al. (1987) except that N-ethyl-N-(2-hydroxy-3-sulphopropyl)-3-methylaniline (Dojindo, NBS, Biologicals, Huntingdon, UK) was used instead of sodium 2-hydroxy-3,5-dichlorobenzenesulphonate.
In each experiment, the lipolytic response of 3T3-L1 adipocytes to test peptides was determined in triplicate for each dose tested and compared with the control level within that experiment. The control level was determined in each experiment from three responses that did not receive any peptide treatment. Cell number was determined from six random wells per experiment using a haemocytometer after the removal of the Glycerol Assay Solution. The lipolytic response of 3T3-L1 adipocytes to test peptides is expressed as glycerol release (fold above control), per 4-h incubation, per 6·3×105 cells. Results were analysed using GraphPad Prism (Graphpad Software, San Diego, CA, USA) and are presented as fitted dose–response curves showing means±s.e.m. of the number of responses tested.
Source of peptides
Lys-γ3-MSH was purified to homogeneity from bovine pituitary tissue (Pel-Freez Biologicals, Rogers, AZ, USA) using ion exchange and reversed-phase HPLC as previously described (Bennett 1986) and quantified using amino acid analysis. NDP-α-MSH, α-MSH HS024 and ACTH1–24 were obtained from Bachem (St Helens, UK).
Raising and purification of an HSL-specific antibody
The C-terminal fragment of mouse HSL corresponding to residues 536–739 was amplified by PCR and cloned into the pET32a vector (Novagen, Nottingham, UK) to generate a fusion protein with thioredoxin at the N-terminal and a 6× His tag between the two protein partners. After expression in Origami (DE3) pLysS cells (Novagen), recombinant HSL fusion protein was harvested in 8 M Urea and subsequently purified to homogeneity using immobilised metal-ion affinity chromatography and anion exchange chromatography. The purified HSL fusion protein (50 μg per injection) was mixed with Freund's incomplete adjuvant and injected (1 ml total per animal) into three different female rabbits (New Zealand White; Charles River, Kent, UK). Animals were injected and bled as previously described (Bicknell et al. 2001). All procedures were carried out under the UK Animals (Scientific Procedures) Act (1986). The antibody was then affinity purified from serum by affinity chromatography using the HSL fusion protein covalently linked to CnBr-activated sepharose resin (Sigma). The bound antibody was eluted using a stepwise protocol as previously described in Hodgkinson & Lowry (1982). Purified antibody amount was determined by spectrophotometric analysis, and the specificity of the HSL antibody generated was assessed by western blot of various rat and mouse tissues (data not shown).
Evaluation of HSL and perilipin A phosphorylation
On day 9, serum-starved 3T3-L1 adipocytes that had been differentiated in six-well plates were incubated for 20 min with Glycerol Assay Solution containing either Lys-γ3-MSH, ACTH, α-MSH or NDP-α-MSH at a dose of 1 pM, 10 pM, 100 pM, 1 nM, 10 nM and 100 nM.
After incubation, protein was harvested by lysing the cells directly in 200 μl of 1× Reducing Sample Treatment Buffer (50 mM Tris–HCl (pH 6·8), 0·1 M dithiothreitol (Sigma), 2% (w/v) SDS (Sigma), 0·01% bromophenol blue (Sigma) and 10% (v/v) glycerol (Fisher, Loughborough, UK)). After brief sonication, and boiling at 94 °C for 5 min, 5 μl of each sample was separated on a 6·5% (perilipin A) or 10% (HSL) SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membrane (Bio-Rad). The membrane was blocked by incubating with Tris-buffered saline (10 mM Tris–HCl (pH 7·4), 150 mM sodium chloride) containing 5% skimmed milk powder and 0·1% Tween 20 (Sigma) for 2 h at room temperature. After blocking, the membranes were probed with either a perilipin-specific antibody (Abcam, Cambridge, UK) at a 1:2000 dilution, the HSL-specific antibody described above, at a 1:2000 dilution, or the phospho-HSL-specific antibodies: HSLphos660, HSLphos563 and HSLphos565 at a 1:2000 dilution (Cell Signalling Technology (NEB), Hitchin, UK). All primary antibodies were then detected using a horse radish peroxidase (HRP)-conjugated secondary goat anti-rabbit antibody (Dako, Ely, UK) and visualised using an ECL system, as per manufacturer's instructions (Amersham). All blots were carried out on a minimum of three independent samples.
After immunoblotting, bands were analysed by densitometry using a GS-710 calibrated imaging densitometer (Bio-Rad).
Statistical analysis of results
Statistical analysis was performed using one-way ANOVA and Fisher's PLSD test. Results were considered statistically significant if the P value was <0·05. Statistical analysis was performed using StatView software version 5.0.1 SAS Institute Inc., Cary, NC, USA).
Results
Comparison of the lipolytic actions of melanocortin peptides on 3T3-L1 adipocytes
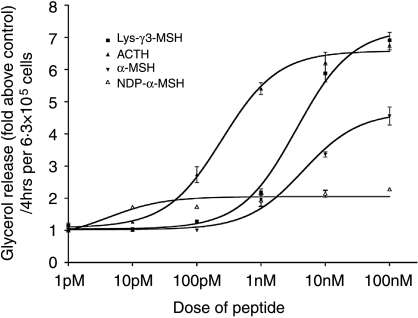
To investigate the hypothesis proposed by Pedersen & Brownie (1983) that γ-MSH peptides may be able to regulate the activity of HSL in tissues other than the adrenal gland, we compared the lipolytic activity of Lys-γ3-MSH, α-MSH, NDP-α-MSH and ACTH on 3T3-L1 adipocytes. As can be seen in Fig. 1, all the peptides stimulated an increase in lipolysis in a dose-dependent manner with ACTH (BMax 6·58±0·16×control values) and Lys-γ3-MSH (BMax 7·26±18×control values) producing the largest maximal responses, although ACTH was more potent (EC50 248±45 pM) than Lys-γ3-MSH (EC50 3·56±0·47 nM). α-MSH was not as potent (EC50 4·50±1·35 nM) nor able to induce the levels of lipolysis (BMax 4·68±0·22×control values) seen in response to a maximal dose of Lys-γ3-MSH or ACTH. Surprisingly, NDP-α-MSH, a peptide that has been shown to be a potent agonist at all of the MC-Rs (with the exception of the MC2-R; Schioth et al. 1996, Haskell-Luevano et al. 2001) produced only a modest increase in lipolysis (BMax 2·05±0·07×control values), but was more potent than any of the other peptides tested (EC50 4·19±1·69 pM).
Figure 1.
Lys-γ3-MSH, ACTH, α-MSH and NDP-α-MSH are able to increase lipolysis in 3T3-L1 adipocytes. Results are presented as glycerol release (fold above control), per 4 h, per 6·3×105 cells. Each data point is the mean of the number of responses tested and the error bars represent mean±s.e.m. Curves were fitted using GraphPad Prism and used to calculate EC50 and BMax values for each peptide.
The effect of Lys-γ3-MSH on the phosphorylation state of HSL
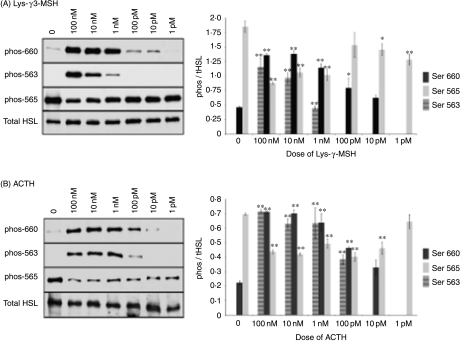
The ability of Lys-γ3-MSH to increase the rate of liploysis suggests that this peptide is able to regulate the activity of HSL. The activity of HSL is primarily regulated by reversible phosphorylation mechanisms (Holm 2003). We therefore investigated the ability of Lys-γ3-MSH and ACTH to regulate the phosphorylation state of HSL at three specific phosphorylation sites using immunoblotting and phospho-specific antibodies. Based on densitometry of the bands, we found that Lys-γ3-MSH was able to significantly phosphorylate HSL at serine residues 660 at doses of 100 pM (P<0·05) or above (P<0·01) and 563 at a dose of 1 nM or above (P<0·01), while ACTH phosphorylated serine 660 at a dose of 100 pM or above (P<0·01) and 563 at doses of 100 pM or above (P<0·01). Both Lys-γ3-MSH and ACTH resulted in a significant (P<0·01) reduction in the basal phosphorylation state of serine 565 (Fig. 2). NDP-α-MSH and α-MSH were not able to stimulate phosphorylation of serine 660 or 563 at any of the doses tested, nor did they have any effect on the basal phosphorylation of serine 565 (results not shown).
Figure 2.
Representative immunoblots showing the effects of (A) Lys-γ3-MSH and (B) ACTH on the phosphorylation state of HSL at specific serine residues in 3T3-L1 adipocytes. The phosphorylation state of HSL was determined, after a 20-min stimulation in the presence or absence of Lys-γ3-MSH or ACTH at increasing dose from 1 pM to 100 nM, by analysing the degree of phosphorylation of serine residues 563, 565 and 660 using antibodies specific for each phosphorylated serine site. (0), no peptide added. Densitometric analysis was performed on the results of three independent experiments and is presented as the means of the phosphorylated HSL content divided by the mean of the total HSL content for the same sample. The error bars represent the mean±s.e.m. *P<0·05 and **P<0·01 indicate a significant difference from the corresponding untreated value.
The effect of melanocortin peptides on the phosphorylation state of perilipin A
Within the last 10 years, it has become clear that activation of HSL on its own is not enough for maximal lipolysis to occur (Tansey et al. 2001, 2003, Souza et al. 2002). A further protein, perilipin A, must also be activated (Souza et al. 2002, Sztalryd et al. 2003, Tansey et al. 2003). In its unstimulated state, perilipin A acts to guard the lipid droplet from the activity of HSL (Souza et al. 2002, Tansey et al. 2003) and upon stimulation, becomes hyperphosphorylated and moves away from the lipid droplet, allowing HSL access to its substrate (Tansey et al. 2003). In the unphosphorylated state, perilipin A migrates under reducing conditions on an SDS-PAGE gel as a 62 kDa protein and upon hyperphosphorylation, it migrates as a 67 kDa protein (Souza et al. 2002, Tansey et al. 2003).
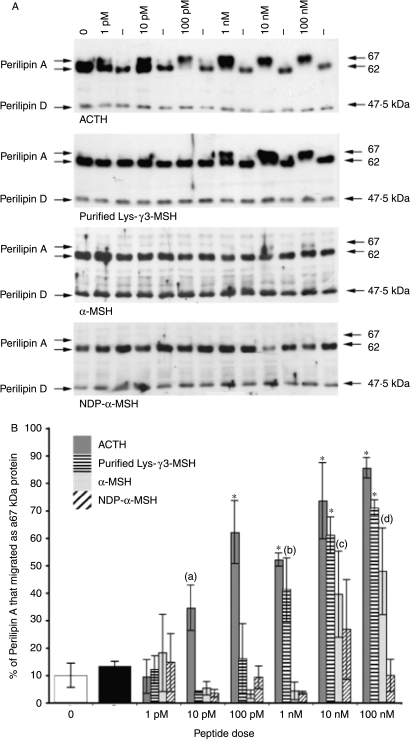
We investigated the actions of Lys-γ3-MSH, ACTH, α-MSH and NDP-α-MSH on the phosphorylation status of perilipin A in 3T3-L1 adipocytes. In unstimulated cells, perilipin was detected at 62 and 47·5 kDa (Fig. 3A). The 62 kDa band represented perilipin A and the 47·5 kDa band most likely represented perilipin D (an isoform of perilipin generated by alternative splicing; Servetnick et al. 1995). Treatment of 3T3-L1 adipocytes with doses of ACTH at 10 pM or above resulted in a significant shift (P=0·0194) in the migratory rates of perilipin A from 62 to 67 kDa (Fig. 3A and B). Lys-γ3-MSH caused a significant (P=0·0022) shift at a dose of 1 nM or above (Fig. 3A and B), while α-MSH caused a significant (P=0·0061) shift at a dose of 10 nM or above (Fig. 3A and B). NDP-α-MSH was not able to cause a significant shift in the migratory rate of perilipin A at any dose tested (Fig. 3A and B).
Figure 3.
(A) Analysis of the effect of Lys-γ3-MSH, ACTH, α-MSH and NDP-α-MSH on the phosphorylation state of perilipin A in 3T3-L1 adipocytes. The phosphorylation state of perilipin A was determined by analysing the migratory rate of perilipin A, on 6·5% SDS-PAGE gels, either 62 kDa (unstimulated) or 67 kDa (stimulated, hyperphosphorylated). (−), no peptide added. (0), time point zero. (B) Densitometric analysis of the effect of Lys-γ3-MSH, ACTH, α-MSH and NDP-α-MSH on the phosphorylation state of perilipin A in 3T3-L1 adipocytes. The phosphorylation state of perilipin A was determined by analysing the migratory rate of perilipin A, on 6·5% SDS-PAGE gels, either 62 kDa (unstimulated) or 67 kDa (stimulated, hyperphosphorylated). All results presented, except those at time point 0 (0) and (−) (no peptide added), are the average of three responses from independent experiments. Results presented at time point (0) and (−) are the average of 12 responses from three independent experiments. The error bars represent the mean ±s.e.m. (a) (P=0·0194) is significantly different from the control (−) value. (b) (P=0·0022) is significantly different from the control (−) value. (c) (P=0·0061) is significantly different from the control (−) value. (d) (P=0·004) is significantly different from the control (−) value. *(P<0·0001) indicates that values are significantly different from the control (−) value.
Antagonistic action of NDP-α-MSH on α-MSH but not Lys-γ3-MSH or ACTH stimulated lipolysis
The observation that Lys-γ3-MSH stimulates lipolysis in 3T3-L1 adipocytes indicates the presence of a receptor for the peptide in this cell line. These cells have been shown to only express the MC2-R and MC5-R (Boston & Cone 1996) for which γ-MSH peptides have no significant or limited affinity respectively (Griffon et al. 1994, Schioth et al. 1995, 1996). The limited affinity of these receptors for γ-MSH peptides suggests that they are unlikely to play a role in mediating the lipolytic actions of Lys-γ3-MSH.
In 1996, Boston & Cone (1996) attempted to characterise the roles played by the MC2-R and MC5-R in mediating the actions of α-MSH and ACTH on cAMP accumulation in 3T3-L1 adipocytes. They observed that NDP-α-MSH, although binding with high affinity, was unable to stimulate cAMP accumulation. They also found that it could act as an antagonist of α-MSH, but not ACTH, induced cAMP accumulation (Boston & Cone 1996). In a similar manner, our results show that NDP-α-MSH is a weak, but potent, stimulator of lipolysis (Fig. 1) indicating that NDP-α-MSH has high affinity for a receptor site, presumably the MC5-R, but cannot act to fully stimulate lipolysis.
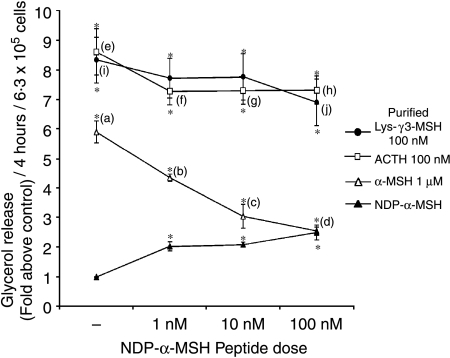
In an attempt to characterise the receptor that mediates the actions of Lys-γ3-MSH, we tested the ability of NDP-α-MSH to act as an antagonist to the lipolytic actions of Lys-γ3-MSH and other melanocortin peptides. 3T3-L1 adipocytes were stimulated with a maximal dose of ACTH (100 nM), Lys-γ3-MSH, (100 nM) or α-MSH (1 μM), in the presence or absence of an increasing dose of NDP-α-MSH (Fig. 4). NDP-α-MSH at all doses caused a slight, but significant (P=0·0061, 0·0068, and 0·0077), reduction in the lipolytic activity of ACTH (Fig. 4). In comparison, NDP-α-MSH at 1 or 10 nM had no effect on the lipolytic activity of Lys-γ3-MSH but at 100 nM did cause a slight, but significant (P=0·0265), reduction in lipolytic activity (Fig. 4). In direct contrast, NDP-α-MSH had a much more profound effect on the lipolytic actions of α-MSH (Fig. 4). NDP-α-MSH at all doses tested caused significant (P<0·0001) reductions in the lipolytic action of α-MSH (Fig. 4). NDP-α-MSH at 100 nM was able to reduce the lipolytic activities of α-MSH, but not ACTH or Lys-γ3-MSH, to the level seen in response to NDP-α-MSH at 100 nM alone (Fig. 4).
Figure 4.
The potent melanocortin receptor agonist NDP-α-MSH can reduce the lipolytic activity of α-MSH but not ACTH or Lys-γ3-MSH in 3T3-L1 adipocytes when compared with the lipolytic activity seen in response to NDP-α-MSH alone. The lipolytic activity of ACTH, Lys-γ3-MSH and α-MSH were determined in the presence or absence of an increasing dose of NDP-α-MSH. Results are presented as glycerol release (fold above control), per 4-h incubation, per 6·3×105 cells. The error bars represent mean±s.e.m. *(P<0·0001) significant difference from the control level. (b), (c) and (d) are (P<0·0001) significantly different from (a). (f), (g) and (h) are (P<0·05) significantly different from (e). (j) is (P<0·05) significantly different from (i).
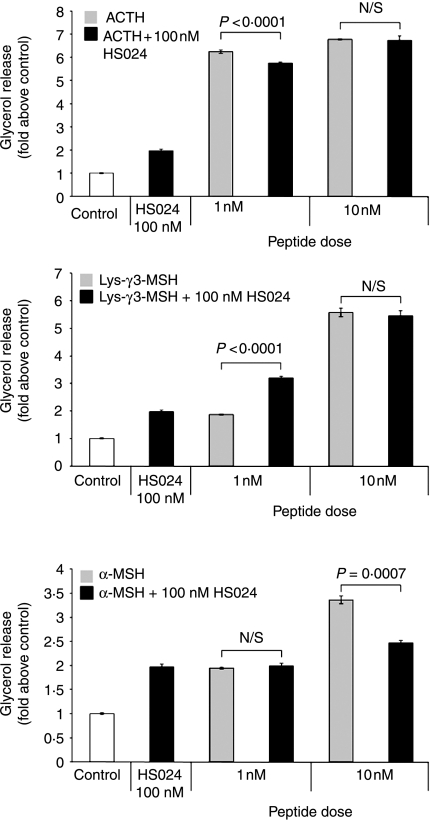
Antagonistic action of HS024, an MC1, MC3, MC4 and MC5 receptor antagonist on α-MSH but not Lys-γ3-MSH or ACTH stimulated lipolysis
Since NDP-α-MSH was able to act as an antagonist of the actions of α-MSH, we decided to investigate the effects on lipolysis of HS024, another antagonist that has potent actions on the MC1, MC3, MC4 and MC5-R receptors (Kask et al. 1998). In a similar manner to NDP-α-MSH, HS024 at a dose of 100 nM was found to weakly, but significantly (P<0·0001), stimulate lipolysis (Fig. 5). When HS024 was administered with 1 nM α-MSH, there was no change in lipolysis when compared with administration of either α-MSH or HS024 alone. However, co-administration of HS024 with 10 nM α-MSH had the effect of reducing significantly (P<0·0001) lipolytic activity when compared with 10 nM α-MSH alone.
Figure 5.
The MC1, MC3, MC4 and MC5 receptor antagonist HS024 can reduce the lipolytic activity of α-MSH, but increases the lipolytic response to Lys-γ3-MSH in 3T3-L1 adipocytes when compared with the lipolytic activity seen in response to HS024 alone. The lipolytic activity of two sub-maximal doses of ACTH, Lys-γ3-MSH and α-MSH were determined in the presence or 100 nM HS024. Results are presented as glycerol release (fold above control), per 4-h incubation, per 6·3×105 cells. The error bars represent mean±s.e.m. All treatments resulted in a significant (P<0·0001) increase in lipolysis when compared with control cells. Between-treatment differences (with their respective P values) are shown on the figure.
Interestingly, when HS024 was administered in conjunction with 1 nM purified Lys-γ3-MSH, it was found that there was an additive effect resulting in a significant (P<0·0001) increase in lipolytic activity compared with that observed with either HS024 or Lys-γ3-MSH alone. However, HS024 had no effect on the lipolysis induced by 10 nM Lys-γ3-MSH.
Administration of HS024 together with 1 nM ACTH resulted in a small but significant (P=0·0033) decrease in lipolysis when compared with that induced by 1 nM ACTH alone, although HS024 had no effect on lipolysis induced by 10 nM ACTH.
Discussion
Elegant studies carried out by Pedersen & Brownie (1980) and Al-Dujaili et al. (1981) demonstrated that γ-MSH peptides have the ability to potentiate the steroidogenic activity of ACTH at the adrenal gland. It was demonstrated that this ability was the result of an increase in the activity of HSL – the enzyme that converts cholesterol esters to free cholesterol (Pedersen & Brownie 1980). Subsequent ligand binding studies showed the existence of binding sites for Lys-γ3-MSH on a number of other tissues leading to the hypothesis that γ-MSH peptides may have an extra-adrenal activity (Pedersen & Brownie 1983). Since the highest binding levels were observed on adipocyte membrane preparations (Pedersen & Brownie 1983), we investigated this hypothesis by comparing the ability of Lys-γ3-MSH with several other melanocortin peptides to stimulate lipolysis in 3T3-L1 adipocytes. Using glycerol release as a marker of lipolysis, 3T3-L1 adipocytes were found to be responsive to all of the melanocortin peptides tested. As previously described, ACTH and α-MSH to a lesser extent were both able to stimulate lipolysis with an EC50 value of 248±45 pM and 4·5±1·35 nM respectively. NDP-α-MSH, a potent ligand of the MC1, MC3, MC4 and MC5 receptors (Haskell-Luevano et al. 2001), was observed to be only weakly lipolytic, although was relatively potent with an EC50 of 4·19±1·69 pM. The weak activity of NDP-α-MSH is perhaps not surprising since work by Boston & Cone (1996) has shown that NDP-α-MSH, although able to bind with high affinity, does not stimulate an increase in cAMP accumulation in 3T3-L1 adipocytes. Lys-γ3-MSH produced a maximal response comparable with that of ACTH, but was not as potent with an EC50 value of 3·56±0·47 nM. The synergistic activity of Lys-γ3-MSH in the adrenal gland is linked to the regulation of HSL activity (Pedersen & Brownie 1980). The activity of HSL, in adipocytes, has been shown to be regulated predominantly by the reversible phosphorylation of key serine residues (Fredrikson et al. 1981, Garton et al. 1988, 1989, Garton & Yeaman 1990, Anthonsen et al. 1998). We therefore attempted to investigate the actions of Lys-γ3-MSH, ACTH, NDP-α-MSH and α-MSH on the phosphorylation state of several specific serine residues in HSL. Only Lys-γ3-MSH and ACTH were able to phosphorylate serine residues 563 and 660 at doses that were compatible with their lipolytic activities. Both serine 563 and 660 are phosphorylated by protein kinase A in response to elevated levels of cAMP resulting in an increase in the activity of HSL by approximately twofold (Fredrikson et al. 1981). Both Lys-γ3-MSH and ACTH reduced the phosphorylation of serine 565. This residue is phosphorylated in its basal state and it has been postulated that when phosphorylated this site acts to prevent phosphorylation of serine 563 (Garton et al. 1988, 1989, Garton & Yeaman 1990). In this study, the basal phosphorylation state of serine 565 appears to be reduced by both Lys-γ3-MSH and ACTH perhaps providing evidence that basal phosphorylation of this residue may prevent the phosphorylation of serine 563.
Although lipolysis has traditionally been regarded to be the result of an up-regulation of HSL activity, in the last 10 years it has become clear that activation of HSL is not, on its own, sufficient for maximal lipolysis to occur (Tansey et al. 2001, 2003, Souza et al. 2002, Sztalryd et al. 2003). A further protein, perilipin A, which is expressed in the adipocyte and steroidogenic cells of the adrenal gland, testis and ovary (Servetnick et al. 1995), must also be activated (Tansey et al. 2001, 2003, Souza et al. 2002, Sztalryd et al. 2003). In its unstimulated state, perilipin A acts to guard the lipid droplet from the hydrolytic actions of HSL, but when stimulated it becomes hyperphosphorylated and moves away from the lipid droplet surface to allow HSL access to its substrate (Tansey et al. 2003). We therefore felt that it was important to investigate the effects of the melanocortin peptides on perilipin A. Both ACTH and Lys-γ3-MSH caused a significant increase in perilipin A phosphorylation, whereas α-MSH could only promote a minor increase in phosphorylation and NDP-α-MSH was incapable of causing perilipin A phosphorylation. Although α-MSH and NDP-α-MSH are both potent stimulators of MC5-R mediated cAMP accumulation in heterologous systems (Haskell-Luevano et al. 2001), it has previously been shown in 3T3-L1 adipocytes that α-MSH only weakly stimulates, and NDP-α-MSH is unable to stimulate cAMP accumulation (Boston & Cone 1996). Since the phosphorylation of perilipin A is believed to be primarily a cAMP-dependent mediated process (Tansey et al. 2003), the limited ability and inability of these peptides to stimulate cAMP accumulation may explain their effects on both the phosphorylation of HSL and perilipin A. Interestingly, the ability of the melanocortin peptides to phosphorylate perilipin A correlates well with their lipolytic activities, which may suggest that the ability to move perilipin A away from the lipid droplet surface is as important, if not more important than, as the activation of HSL during the lipolytic process.
Since NDP-α-MSH can bind with high affinity to 3T3-L1 adipocytes but not stimulate cAMP accumulation, Boston & Cone (1996) used NDP-α-MSH as a receptor antagonist in an attempt to determine the respective roles played by the MC2-R and MC5-R in mediating the actions of α-MSH and ACTH. They observed that NDP-α-MSH was able to block the actions of α-MSH but not ACTH (Boston & Cone 1996). Since NDP-α-MSH has poor affinity for the MC2-R and high affinity for the MC5-R, they concluded that α-MSH signals through the MC5-R and that the actions of ACTH are mediated through the MC2-R (Boston & Cone 1996, Schioth et al. 1996). Although we found NDP-α-MSH to be weakly lipolytic, we reasoned that it would still be useful as an antagonist to dissect the melanocortin receptor responsible for mediating the lipolytic actions of Lys-γ3-MSH.
We found that NDP-α-MSH was able to reduce the lipolytic activity of α-MSH, but not Lys-γ3-MSH or ACTH, to the level seen in response to NDP-α-MSH alone. Although our results agree with those of Boston & Cone suggesting that α-MSH appears to act though the MC5-R and ACTH through the MC2-R (Boston & Cone 1996), our results do not clearly support a role for the MC5-R mediating the actions of Lys-γ3-MSH.
In an attempt to clarify these observations, we investigated the effects of HS024, a known antagonist in the low nanomolar range of the MC1, MC3, MC4 and MC5 receptors. In a manner similar to NDP-α-MSH, HS024 was found to be weakly lipolytic and able to antagonise the lipolytic effects of α-MSH. The compound had a small but significant effect on the lipolytic effects of a low dose (1 nM) of ACTH but had no effect on lipolysis stimulated by ACTH at a dose of 10 nM. Since ACTH has a binding affinity for the MC5-R of around half that of α-MSH (Schioth et al. 1995), these results raise the possibility that at least some of the lipolytic actions of ACTH are mediated by the MC5-R.
Interestingly, cells treated with both HS024 and Lys-γ3-MSH at a dose of 1 nM showed an additive effect on lipolysis when compared with each peptide alone, although HS024 had no effect on the lipolysis stimulated by 10 nM Lys-γ3-MSH. This result could possibly suggest that the two peptides do not bind to the same site, again, not fully supporting a role for the MC5-R in mediating the actions of Lys-γ3-MSH.
The results from these studies are not easy to interpret; especially since 3T3-L1 cells constitute a mixed melanocortin receptor environment and thus the possibility that more than one receptor has been activated. The simplest explanation is that the pharmacological properties of MC5-R expressed by 3T3-L1 adipocytes are different from when expressed in heterologous systems. This hypothesis is indirectly supported by the observations of Boston & Cone (1996) who found that NDP-α-MSH, a potent activator of the MC5-R when expressed in heterologous systems (Haskell-Luevano et al. 2001), does not promote cAMP accumulation (Boston & Cone 1996) in 3T3-L1 adipocytes. This, together supported by our results showing that this peptide is only very weakly lipolytic, clearly demonstrates that the pharmacology of endogenously expressed MC5-R is different and it is thus not inconceivable that it could have a higher affinity for γ-MSH peptides than previously reported.
Another explanation is that the lipolytic actions of Lys-γ3-MSH are being mediated through an uncharacterised melanocortin receptor. Although this seems unlikely, other studies have provided pharmacological evidence to support the idea that a further MC-R exists. Experiments analysing the actions of γ-MSH peptides in the pituitary suggest the existence of a G-protein-coupled receptor that is pharmacologically distinct from any member of the melanocortin receptor family in this tissue (Lorsignol et al. 1999, Langouche et al. 2001, 2002). Li et al. (1996) have shown that the centrally mediated pressor and tachycardic actions of γ-MSH do not appear to be mediated through any of the five known melanocortin receptors.
Although the effects of Lys-γ3-MSH in the adrenal gland have been clearly demonstrated and our results suggest that its actions are not limited to the adrenal gland, the physiological relation of Lys-γ3-MSH to the other peptides derived from POMC in vivo in both rodents and humans is still unclear. However, our results do suggest that it may play a wider peripheral role than has previously been thought. In addition, the receptor through which these actions are mediated, and whether Lys-γ3-MSH can exert actions on other tissues that express HSL and possess binding sites, remains to be determined and warrants further investigation.
Acknowledgements
We are grateful the BBSRC and The Wellcome Trust and for funding. A B B is a Wellcome Trust Research Career Development Fellow. The authors declare that there is no conflict of interest that would prejudice the impartiality of the work described in this manuscript.
References
- Al-Dujaili EA, Hope J, Estivariz FE, Lowry PJ, Edwards CR. Circulating human pituitary pro-gamma-melanotropin enhances the adrenal response to ACTH. Nature. 1981;29:156–159. doi: 10.1038/291156a0. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response isoproterenol and govern activation properties in vitro. Journal of Biological Chemistry. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Bennett HPJ. Use of ion-exchange Sep-Pak cartridges in the batch fractionation of pituitary peptides. Journal of Chromatography. 1986;359:383–390. doi: 10.1016/0021-9673(86)80092-9. [DOI] [PubMed] [Google Scholar]
- Bicknell AB, Lomthaisong K, Woods RJ, Hutchinson EG, Bennett HP, Gladwell RT, Lowry PJ. Characterisation of a serine protease that cleaves pro-gamma-melanotropin at the adrenal to stimulate growth. Cell. 2001;105:903–912. doi: 10.1016/s0092-8674(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Bohlen P, Esch F, Shibasaki T, Baird A, Ling N, Guillemin R. Isolation and characterization of γ1-melanotropin-like peptide from bovine neurointermediate pituitary. FEBS Letters. 1981;128:67–70. doi: 10.1016/0014-5793(81)81081-2. [DOI] [PubMed] [Google Scholar]
- Boston BA. The role of melanocortins in adipocyte function. Annals of the New York Academy of Sciences. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Boston BA, Cone RD. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- Browne CA, Bennett HP, Solomon S. The isolation of characterization of gamma 3-melanotropin from the neurointermediary lobe of the rat pituitary. Biochemical and Biophysical Research Communications. 1981;100:336–343. doi: 10.1016/s0006-291x(81)80101-5. [DOI] [PubMed] [Google Scholar]
- Denef C, Lu J, Swinnen E. Gamma-MSH peptides in the pituitary: effects, target cells, and receptors. Annals of the New York Academy of Sciences. 2003;994:123–132. doi: 10.1111/j.1749-6632.2003.tb03171.x. [DOI] [PubMed] [Google Scholar]
- Fredrikson G, Strålfors P, Nilsson NO, Belfrage P. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. Journal of Biological Chemistry. 1981;256:6311–6320. [PubMed] [Google Scholar]
- Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. European Journal of Biochemistry. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Cohen P, Yeaman SJ. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Letters. 1988;229:68–72. doi: 10.1016/0014-5793(88)80799-3. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. European Journal of Biochemistry. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochemical and Biophysical Research Communications. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Holder JR, Monck EK, Bauzo RM. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. Journal of Medicinal Chemistry. 2001;44:2247–2252. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- Hodgkinson SC, Lowry PJ. Selective elution of immunoadsorbed anti-(human prolactin) immunoglobulins with enhanced immunochemical properties. Biochemical Journal. 1982;205:535–541. doi: 10.1042/bj2050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochemical Society Transactions. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- Humpreys MH. Gamma-MSH, sodium metabolism, and salt-sensitive hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;286:417–430. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- Kask A, Mutulis F, Muceniece R, Pahkla R, Mutule I, Wikberg JE, Rago L, Schioth HB. Discovery of a novel superpotent and selective melanocortin-4 receptor antagonist (HS024): evaluation in vitro and in vivo. Endocrinology. 1998;139:5006–5014. doi: 10.1210/endo.139.12.6352. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochemical Journal. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Langouche L, Roudbaraki M, Pals K, Denef C. Stimulation of intracellular free calcium in GH3 cells by gamma3-melanocyte-stimulating hormone. Involvement of a novel melanocortin receptor? Endocrinology. 2001;142:257–266. doi: 10.1210/endo.142.1.7878. [DOI] [PubMed] [Google Scholar]
- Langouche L, Pals K, Denef C. Structure–activity relationship and signal transduction of gamma-MSH peptides in GH3 cells: further evidence for a new melanocortin receptor. Peptides. 2002;23:1077–1086. doi: 10.1016/s0196-9781(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. Journal of Neuroscience. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsignol A, Vande Vijver V, Ramaekers D, Vankelecom H, Denef C. Detection of melanocortin-3 receptor mRNA in immature rat pituitary: functional relation to gamma3-MSH-induced changes in intracellular Ca2+ concentration? Journal of Neuroendocrinology. 1999;11:171–179. doi: 10.1046/j.1365-2826.1999.00305.x. [DOI] [PubMed] [Google Scholar]
- Mulder H, Holst LS, Svensson H, Degerman E, Sundler F, Ahren B, Rorsman P, Holm C. Hormone-sensitive lipase, the rate-limiting enzyme in triglyceride hydrolysis, is expressed and active in beta-cells. Diabetes. 1999;48:228–232. doi: 10.2337/diabetes.48.1.228. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Ng TB. Studies on hormonal regulation of lipolysis and lipogenesis in fat cells of various mammalian species. Comparative Biochemistry and Physiology-Part B: Biochemistry and Molecular Biology. 1990;97:441–446. doi: 10.1016/0305-0491(90)90141-f. [DOI] [PubMed] [Google Scholar]
- Pedersen RC, Brownie AC. Adrenocortical response to corticotropin is potentiated by part of the amino-terminal region of pro-corticotropin/endorphin. PNAS. 1980;77:2249–2253. doi: 10.1073/pnas.77.4.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen RC, Brownie AC. Lys-γ3-melanotropin binds with high affinity to the rat adrenal cortex. Endocrinology. 1983;112:1279–1287. doi: 10.1210/endo-112-4-1279. [DOI] [PubMed] [Google Scholar]
- Pedersen RC, Brownie AC, Ling N. Pro-adrenocorticotropin/endorphin-derived peptides: coordinate action on adrenal steroidogenesis. Science. 1980;208:1044–1066. doi: 10.1126/science.6246578. [DOI] [PubMed] [Google Scholar]
- Ramachandran J, Lee V. Divergent effects of adrenocorticotropin and melanotropin on isolated rat and rabbit adipocytes. Biochimica et Biophysica Acta. 1976;428:339–346. doi: 10.1016/0304-4165(76)90041-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran J, Farmer SW, Liles S, Li CH. Comparison of the steroidogenic and melanotropic activities of corticotropin, alpha-melanotropin and analogs with their lipolytic activities in rat and rabbit adipocytes. Biochimica et Biophysica Acta. 1976;428:347–354. doi: 10.1016/0304-4165(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Muceniece R, Wikberg JES, Chhajlani V. Characterisation of melanocortin receptor subtypes by radioligand binding analysis. European Journal of Pharmacology. 1995;288:311–317. doi: 10.1016/0922-4106(95)90043-8. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sciences. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- Servetnick DA, Brasaemle DL, Gruia-Gray J, Kimmel AR, Wolff J, Londos C. Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. Journal of Biological Chemistry. 1995;270:16970–16973. doi: 10.1074/jbc.270.28.16970. [DOI] [PubMed] [Google Scholar]
- Sharma A, Artiss JD, Zak B. A method for the sequential colorimetric determination of serum triglycerides and cholesterol. Clinical Biochemistry. 1987;20:167–172. doi: 10.1016/s0009-9120(87)80115-7. [DOI] [PubMed] [Google Scholar]
- Small CA, Garton AJ, Yeaman SJ. The presence and role of hormone-sensitive lipase in heart muscle. Biochemical Journal. 1989;258:67–72. doi: 10.1042/bj2580067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CA, Rogers MP, Goodacre JA, Yeaman SJ. Phosphorylation and activation of hormone-sensitive lipase in isolated macrophages. FEBS Letters. 1991;279:323–326. doi: 10.1016/0014-5793(91)80179-7. [DOI] [PubMed] [Google Scholar]
- Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, et al. Modulation of hormone-sensitive Lipase and Protein Kinase A-mediated Lipolysis by Perilipin A in an Adenoviral Reconstituted System. Journal of Biological Chemistry. 2002;277:8267–8272. doi: 10.1074/jbc.M108329200. [DOI] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. Journal of Cell Biology. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. PNAS. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. Journal of Biological Chemistry. 2003;278:8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- Veersteeg DH, Van Bergen P, Adan RA, De Wildt DJ. Melanocortins and cardiovascular regulation. European Journal of Pharmacology. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- White JE, Engel FL. Lipolytic action of corticotropin on rat adipose tissue in vitro. Journal of Clinical Investigation. 1958;37:1556–1563. doi: 10.1172/JCI103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase – new roles for an old enzyme. Biochemical Journal. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. Journal of Biological Chemistry. 1993;268:1763–1769. [PubMed] [Google Scholar]